 Found 112 hits with Last Name = 'roomans' and Initial = 's'
Found 112 hits with Last Name = 'roomans' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM13216

(BMS-354825 | CHEMBL1421 | DASATINIB | N-(2-Chloro-...)Show SMILES Cc1nc(Nc2ncc(s2)C(=O)Nc2c(C)cccc2Cl)cc(n1)N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26ClN7O2S/c1-14-4-3-5-16(23)20(14)28-21(32)17-13-24-22(33-17)27-18-12-19(26-15(2)25-18)30-8-6-29(7-9-30)10-11-31/h3-5,12-13,31H,6-11H2,1-2H3,(H,28,32)(H,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416380

(CHEMBL1210561)Show InChI InChI=1S/C15H15ClF3N3O/c1-8-11(9(2)22-21-8)6-13(23)20-7-10-4-3-5-12(14(10)16)15(17,18)19/h3-5H,6-7H2,1-2H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416375

(CHEMBL1210623)Show InChI InChI=1S/C15H14ClF3N2O2/c1-8-11(9(2)23-21-8)6-13(22)20-7-10-4-3-5-12(14(10)16)15(17,18)19/h3-5H,6-7H2,1-2H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416378
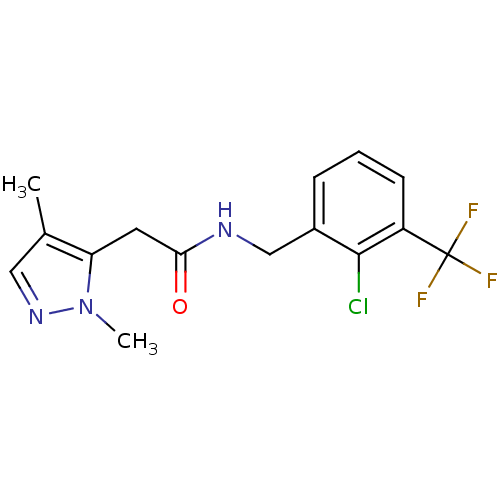
(CHEMBL1210563)Show InChI InChI=1S/C15H15ClF3N3O/c1-9-7-21-22(2)12(9)6-13(23)20-8-10-4-3-5-11(14(10)16)15(17,18)19/h3-5,7H,6,8H2,1-2H3,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112617
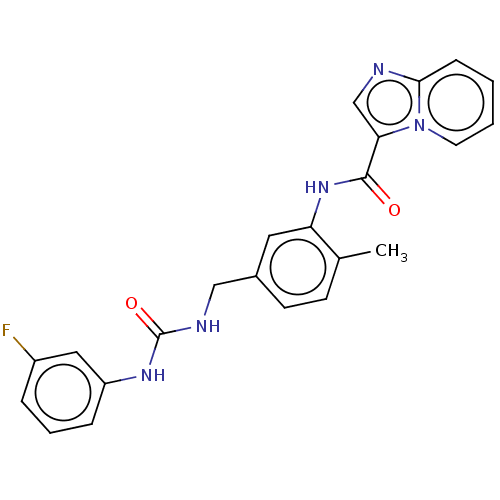
(CHEMBL3608786)Show SMILES Cc1ccc(CNC(=O)Nc2cccc(F)c2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H20FN5O2/c1-15-8-9-16(13-26-23(31)27-18-6-4-5-17(24)12-18)11-19(15)28-22(30)20-14-25-21-7-2-3-10-29(20)21/h2-12,14H,13H2,1H3,(H,28,30)(H2,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112628
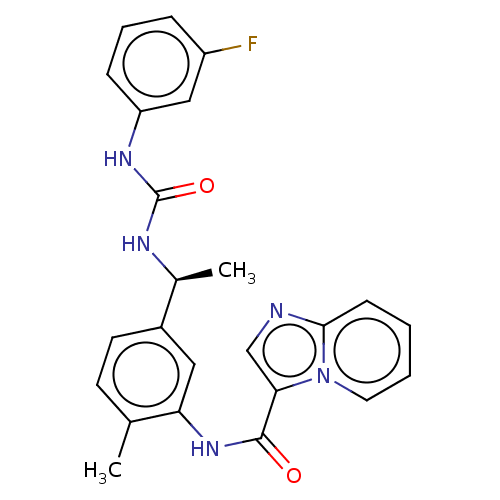
(CHEMBL3608789)Show SMILES C[C@H](NC(=O)Nc1cccc(F)c1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-9-10-17(16(2)27-24(32)28-19-7-5-6-18(25)13-19)12-20(15)29-23(31)21-14-26-22-8-3-4-11-30(21)22/h3-14,16H,1-2H3,(H,29,31)(H2,27,28,32)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112631
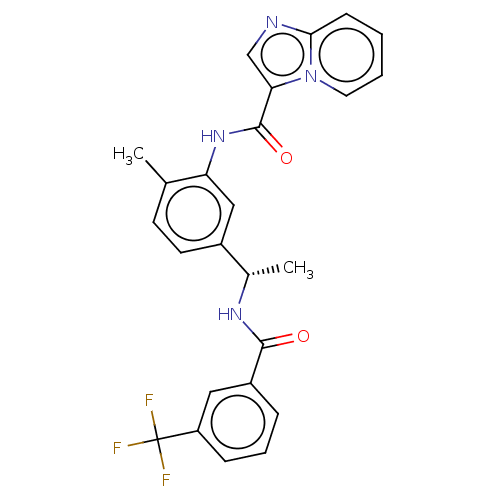
(CHEMBL3608790)Show SMILES C[C@H](NC(=O)c1cccc(c1)C(F)(F)F)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C25H21F3N4O2/c1-15-9-10-17(16(2)30-23(33)18-6-5-7-19(12-18)25(26,27)28)13-20(15)31-24(34)21-14-29-22-8-3-4-11-32(21)22/h3-14,16H,1-2H3,(H,30,33)(H,31,34)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50112618

(CHEMBL3608787)Show SMILES C[C@H](NC(=O)Nc1ccccc1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-11-12-18(17(2)26-24(31)27-19-8-4-3-5-9-19)14-20(16)28-23(30)21-15-25-22-10-6-7-13-29(21)22/h3-15,17H,1-2H3,(H,28,30)(H2,26,27,31)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR1 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112634
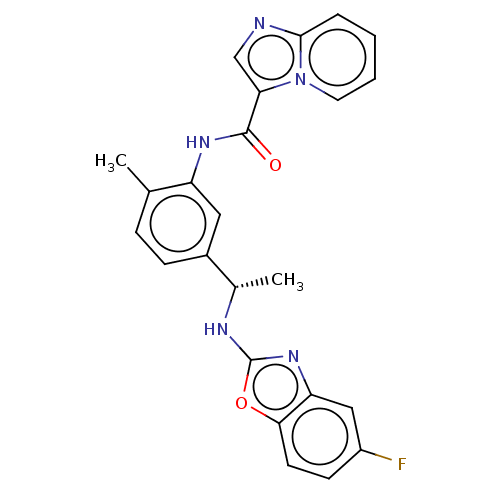
(CHEMBL3608791)Show SMILES C[C@H](Nc1nc2cc(F)ccc2o1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H20FN5O2/c1-14-6-7-16(15(2)27-24-29-19-12-17(25)8-9-21(19)32-24)11-18(14)28-23(31)20-13-26-22-5-3-4-10-30(20)22/h3-13,15H,1-2H3,(H,27,29)(H,28,31)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416383
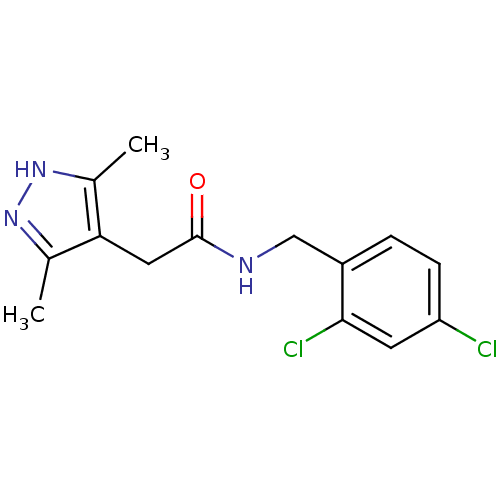
(CHEMBL1210558)Show InChI InChI=1S/C14H15Cl2N3O/c1-8-12(9(2)19-18-8)6-14(20)17-7-10-3-4-11(15)5-13(10)16/h3-5H,6-7H2,1-2H3,(H,17,20)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112618

(CHEMBL3608787)Show SMILES C[C@H](NC(=O)Nc1ccccc1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-11-12-18(17(2)26-24(31)27-19-8-4-3-5-9-19)14-20(16)28-23(30)21-15-25-22-10-6-7-13-29(21)22/h3-15,17H,1-2H3,(H,28,30)(H2,26,27,31)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112615

(CHEMBL3608785)Show SMILES Cc1ccc(CNC(=O)Nc2ccccc2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H21N5O2/c1-16-10-11-17(14-25-23(30)26-18-7-3-2-4-8-18)13-19(16)27-22(29)20-15-24-21-9-5-6-12-28(20)21/h2-13,15H,14H2,1H3,(H,27,29)(H2,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50112615

(CHEMBL3608785)Show SMILES Cc1ccc(CNC(=O)Nc2ccccc2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H21N5O2/c1-16-10-11-17(14-25-23(30)26-18-7-3-2-4-8-18)13-19(16)27-22(29)20-15-24-21-9-5-6-12-28(20)21/h2-13,15H,14H2,1H3,(H,27,29)(H2,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) using biotinylated HER2 peptide as substrate by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416377
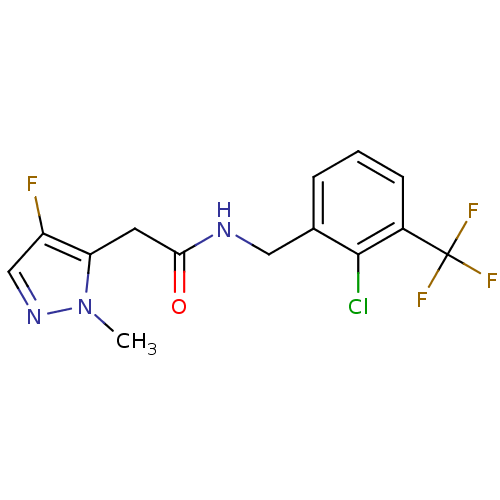
(CHEMBL1210621)Show InChI InChI=1S/C14H12ClF4N3O/c1-22-11(10(16)7-21-22)5-12(23)20-6-8-3-2-4-9(13(8)15)14(17,18)19/h2-4,7H,5-6H2,1H3,(H,20,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416379

(CHEMBL1210562)Show InChI InChI=1S/C14H15Cl2N3O/c1-9-7-18-19(2)13(9)6-14(20)17-8-10-3-4-11(15)5-12(10)16/h3-5,7H,6,8H2,1-2H3,(H,17,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416395

(CHEMBL1210369)Show InChI InChI=1S/C17H21ClFN3O/c1-3-15-13(16(4-2)22-21-15)7-8-17(23)20-10-11-5-6-12(19)9-14(11)18/h5-6,9H,3-4,7-8,10H2,1-2H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416390
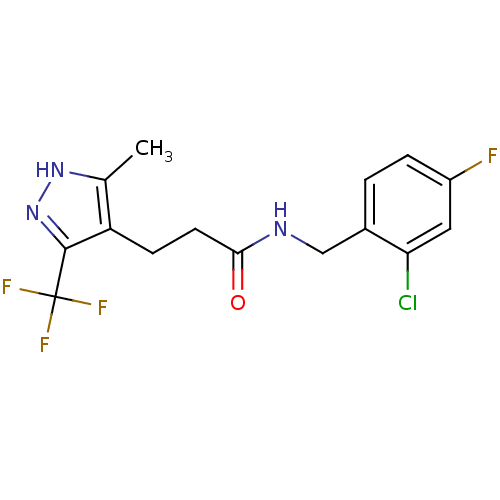
(CHEMBL1210439)Show SMILES Cc1[nH]nc(c1CCC(=O)NCc1ccc(F)cc1Cl)C(F)(F)F Show InChI InChI=1S/C15H14ClF4N3O/c1-8-11(14(23-22-8)15(18,19)20)4-5-13(24)21-7-9-2-3-10(17)6-12(9)16/h2-3,6H,4-5,7H2,1H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416376
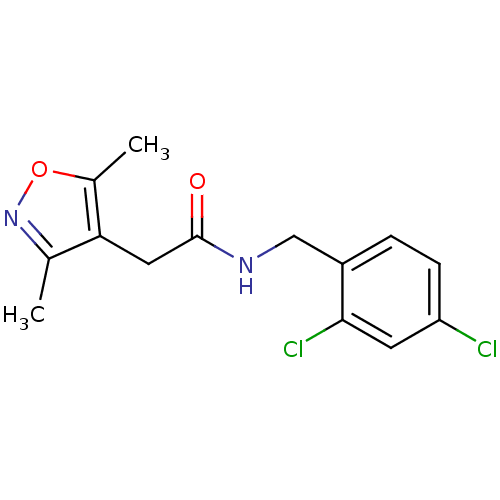
(CHEMBL1210622)Show InChI InChI=1S/C14H14Cl2N2O2/c1-8-12(9(2)20-18-8)6-14(19)17-7-10-3-4-11(15)5-13(10)16/h3-5H,6-7H2,1-2H3,(H,17,19) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50112617

(CHEMBL3608786)Show SMILES Cc1ccc(CNC(=O)Nc2cccc(F)c2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H20FN5O2/c1-15-8-9-16(13-26-23(31)27-18-6-4-5-17(24)12-18)11-19(15)28-22(30)20-14-25-21-7-2-3-10-29(20)21/h2-12,14H,13H2,1H3,(H,28,30)(H2,26,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) using biotinylated HER2 peptide as substrate by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50415882
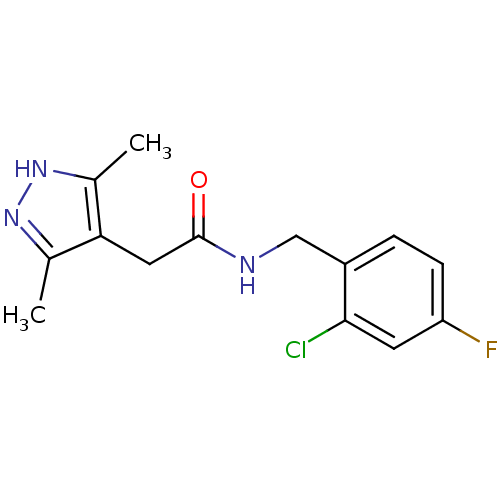
(CHEMBL1094106)Show InChI InChI=1S/C14H15ClFN3O/c1-8-12(9(2)19-18-8)6-14(20)17-7-10-3-4-11(16)5-13(10)15/h3-5H,6-7H2,1-2H3,(H,17,20)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416382

(CHEMBL1210559)Show InChI InChI=1S/C15H15F4N3O/c1-8-12(9(2)22-21-8)6-14(23)20-7-10-3-4-11(16)5-13(10)15(17,18)19/h3-5H,6-7H2,1-2H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416386

(CHEMBL1210500)Show InChI InChI=1S/C15H16ClFN2O2/c1-9-13(10(2)21-19-9)5-6-15(20)18-8-11-3-4-12(17)7-14(11)16/h3-4,7H,5-6,8H2,1-2H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416381

(CHEMBL1210560)Show SMILES Cc1n[nH]c(C)c1CC(=O)NCc1ccc(Cl)c(c1)C(F)(F)F Show InChI InChI=1S/C15H15ClF3N3O/c1-8-11(9(2)22-21-8)6-14(23)20-7-10-3-4-13(16)12(5-10)15(17,18)19/h3-5H,6-7H2,1-2H3,(H,20,23)(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Rattus norvegicus (Rat)) | BDBM50416383

(CHEMBL1210558)Show InChI InChI=1S/C14H15Cl2N3O/c1-8-12(9(2)19-18-8)6-14(20)17-7-10-3-4-11(15)5-13(10)16/h3-5H,6-7H2,1-2H3,(H,17,20)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416393

(CHEMBL1210436)Show InChI InChI=1S/C18H23ClFN3O/c1-11(2)8-17-15(12(3)22-23-17)6-7-18(24)21-10-13-4-5-14(20)9-16(13)19/h4-5,9,11H,6-8,10H2,1-3H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416394
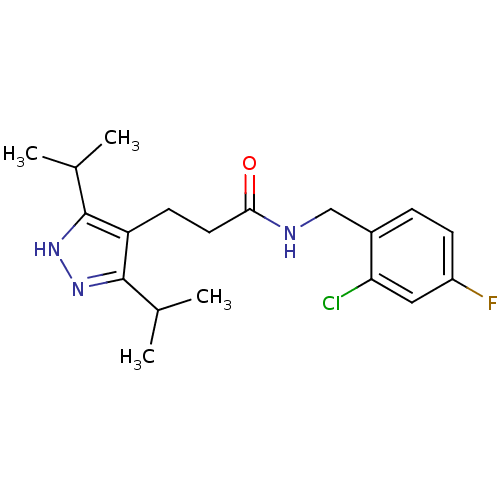
(CHEMBL1210435)Show SMILES CC(C)c1n[nH]c(C(C)C)c1CCC(=O)NCc1ccc(F)cc1Cl Show InChI InChI=1S/C19H25ClFN3O/c1-11(2)18-15(19(12(3)4)24-23-18)7-8-17(25)22-10-13-5-6-14(21)9-16(13)20/h5-6,9,11-12H,7-8,10H2,1-4H3,(H,22,25)(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50112628

(CHEMBL3608789)Show SMILES C[C@H](NC(=O)Nc1cccc(F)c1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H22FN5O2/c1-15-9-10-17(16(2)27-24(32)28-19-7-5-6-18(25)13-19)12-20(15)29-23(31)21-14-26-22-8-3-4-11-30(21)22/h3-14,16H,1-2H3,(H,29,31)(H2,27,28,32)/t16-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) using biotinylated HER2 peptide as substrate by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50112613

(CHEMBL3608784)Show SMILES O=C(NCc1cccc(NC(=O)c2cnc3ccccn23)c1)Nc1ccccc1 Show InChI InChI=1S/C22H19N5O2/c28-21(19-15-23-20-11-4-5-12-27(19)20)25-18-10-6-7-16(13-18)14-24-22(29)26-17-8-2-1-3-9-17/h1-13,15H,14H2,(H,25,28)(H2,24,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR1 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416374
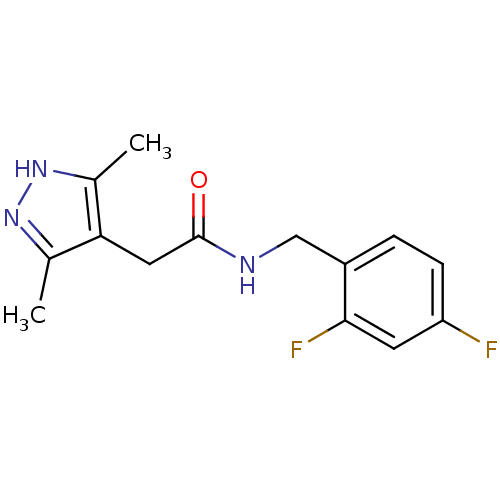
(CHEMBL1210501)Show InChI InChI=1S/C14H15F2N3O/c1-8-12(9(2)19-18-8)6-14(20)17-7-10-3-4-11(15)5-13(10)16/h3-5H,6-7H2,1-2H3,(H,17,20)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416392

(CHEMBL1210437)Show SMILES Cc1[nH]nc(c1CCC(=O)NCc1ccc(F)cc1Cl)C(C)(C)C Show InChI InChI=1S/C18H23ClFN3O/c1-11-14(17(23-22-11)18(2,3)4)7-8-16(24)21-10-12-5-6-13(20)9-15(12)19/h5-6,9H,7-8,10H2,1-4H3,(H,21,24)(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416388
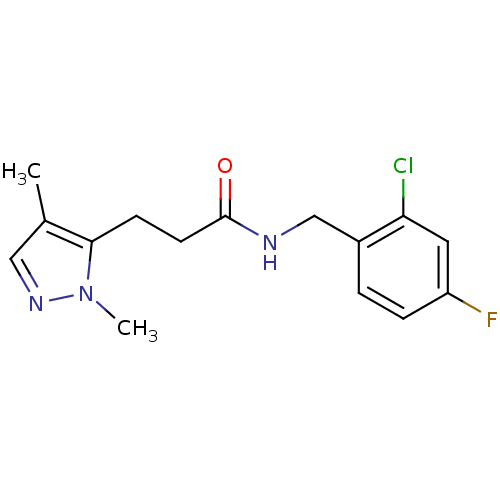
(CHEMBL1210498)Show InChI InChI=1S/C15H17ClFN3O/c1-10-8-19-20(2)14(10)5-6-15(21)18-9-11-3-4-12(17)7-13(11)16/h3-4,7-8H,5-6,9H2,1-2H3,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416385
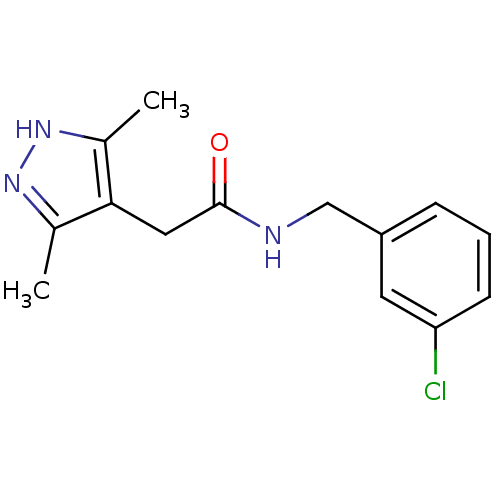
(CHEMBL1210503)Show InChI InChI=1S/C14H16ClN3O/c1-9-13(10(2)18-17-9)7-14(19)16-8-11-4-3-5-12(15)6-11/h3-6H,7-8H2,1-2H3,(H,16,19)(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Rattus norvegicus (Rat)) | BDBM50415882

(CHEMBL1094106)Show InChI InChI=1S/C14H15ClFN3O/c1-8-12(9(2)19-18-8)6-14(20)17-7-10-3-4-11(16)5-13(10)15/h3-5H,6-7H2,1-2H3,(H,17,20)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at rat P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50112634

(CHEMBL3608791)Show SMILES C[C@H](Nc1nc2cc(F)ccc2o1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H20FN5O2/c1-14-6-7-16(15(2)27-24-29-19-12-17(25)8-9-21(19)32-24)11-18(14)28-23(31)20-13-26-22-5-3-4-10-30(20)22/h3-13,15H,1-2H3,(H,27,29)(H,28,31)/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) using biotinylated HER2 peptide as substrate by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50112613

(CHEMBL3608784)Show SMILES O=C(NCc1cccc(NC(=O)c2cnc3ccccn23)c1)Nc1ccccc1 Show InChI InChI=1S/C22H19N5O2/c28-21(19-15-23-20-11-4-5-12-27(19)20)25-18-10-6-7-16(13-18)14-24-22(29)26-17-8-2-1-3-9-17/h1-13,15H,14H2,(H,25,28)(H2,24,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) using biotinylated HER2 peptide as substrate by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416373
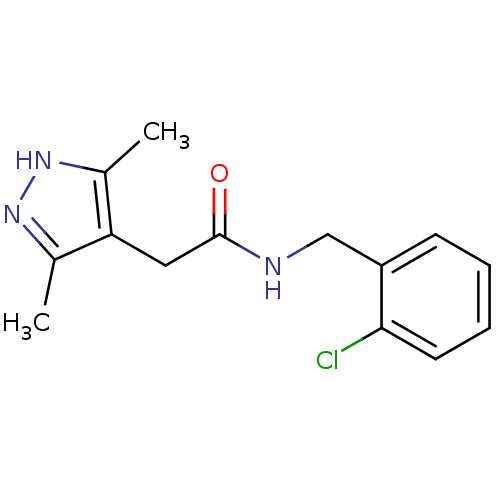
(CHEMBL1210502)Show InChI InChI=1S/C14H16ClN3O/c1-9-12(10(2)18-17-9)7-14(19)16-8-11-5-3-4-6-13(11)15/h3-6H,7-8H2,1-2H3,(H,16,19)(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112613

(CHEMBL3608784)Show SMILES O=C(NCc1cccc(NC(=O)c2cnc3ccccn23)c1)Nc1ccccc1 Show InChI InChI=1S/C22H19N5O2/c28-21(19-15-23-20-11-4-5-12-27(19)20)25-18-10-6-7-16(13-18)14-24-22(29)26-17-8-2-1-3-9-17/h1-13,15H,14H2,(H,25,28)(H2,24,26,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50112618

(CHEMBL3608787)Show SMILES C[C@H](NC(=O)Nc1ccccc1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-11-12-18(17(2)26-24(31)27-19-8-4-3-5-9-19)14-20(16)28-23(30)21-15-25-22-10-6-7-13-29(21)22/h3-15,17H,1-2H3,(H,28,30)(H2,26,27,31)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of c-Kit (unknown origin) using biotinylated HER2 peptide as substrate by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416387

(CHEMBL1210499)Show InChI InChI=1S/C15H17ClFN3O/c1-10-7-13(20(2)19-10)5-6-15(21)18-9-11-3-4-12(17)8-14(11)16/h3-4,7-8H,5-6,9H2,1-2H3,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416389

(CHEMBL1210440)Show InChI InChI=1S/C14H15ClFN3O/c1-9-7-18-19-13(9)4-5-14(20)17-8-10-2-3-11(16)6-12(10)15/h2-3,6-7H,4-5,8H2,1H3,(H,17,20)(H,18,19) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416384
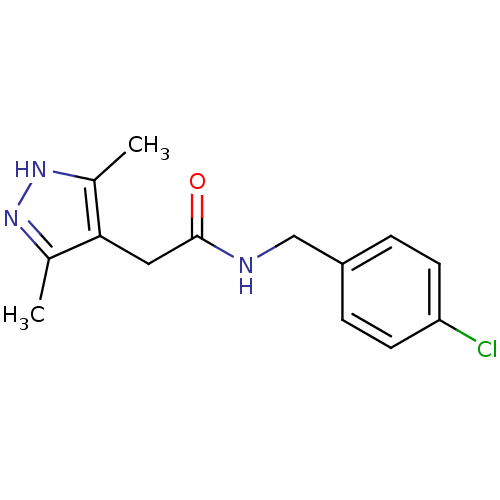
(CHEMBL1210504)Show InChI InChI=1S/C14H16ClN3O/c1-9-13(10(2)18-17-9)7-14(19)16-8-11-3-5-12(15)6-4-11/h3-6H,7-8H2,1-2H3,(H,16,19)(H,17,18) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
P2X purinoceptor 7
(Homo sapiens (Human)) | BDBM50416391
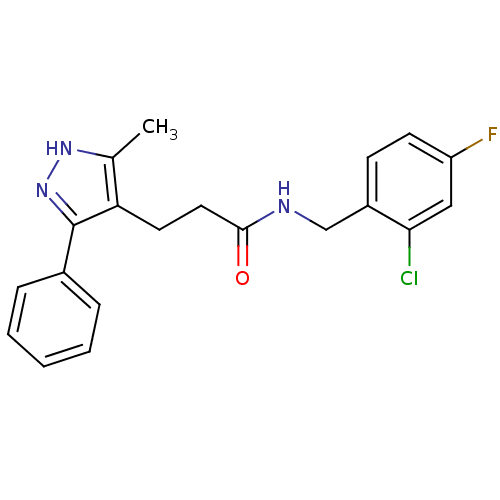
(CHEMBL1210438)Show SMILES Cc1[nH]nc(c1CCC(=O)NCc1ccc(F)cc1Cl)-c1ccccc1 Show InChI InChI=1S/C20H19ClFN3O/c1-13-17(20(25-24-13)14-5-3-2-4-6-14)9-10-19(26)23-12-15-7-8-16(22)11-18(15)21/h2-8,11H,9-10,12H2,1H3,(H,23,26)(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Antagonist activity at human P2X7 receptor ethidium bromide release assay |
Bioorg Med Chem Lett 20: 4653-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.05.107
BindingDB Entry DOI: 10.7270/Q2DZ09HF |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112624
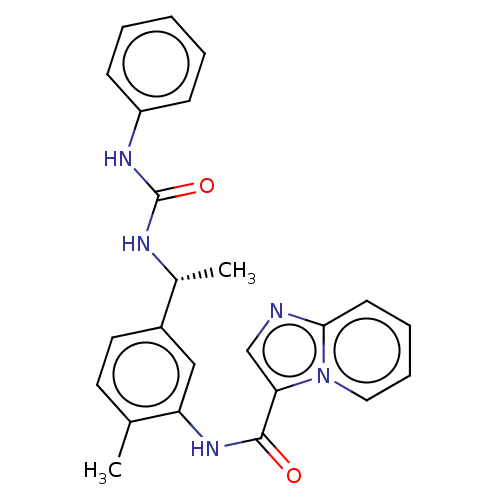
(CHEMBL3608788)Show SMILES C[C@@H](NC(=O)Nc1ccccc1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-11-12-18(17(2)26-24(31)27-19-8-4-3-5-9-19)14-20(16)28-23(30)21-15-25-22-10-6-7-13-29(21)22/h3-15,17H,1-2H3,(H,28,30)(H2,26,27,31)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50112624

(CHEMBL3608788)Show SMILES C[C@@H](NC(=O)Nc1ccccc1)c1ccc(C)c(NC(=O)c2cnc3ccccn23)c1 |r| Show InChI InChI=1S/C24H23N5O2/c1-16-11-12-18(17(2)26-24(31)27-19-8-4-3-5-9-19)14-20(16)28-23(30)21-15-25-22-10-6-7-13-29(21)22/h3-15,17H,1-2H3,(H,28,30)(H2,26,27,31)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR1 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | |
Epithelial discoidin domain-containing receptor 1
(Homo sapiens (Human)) | BDBM50112637
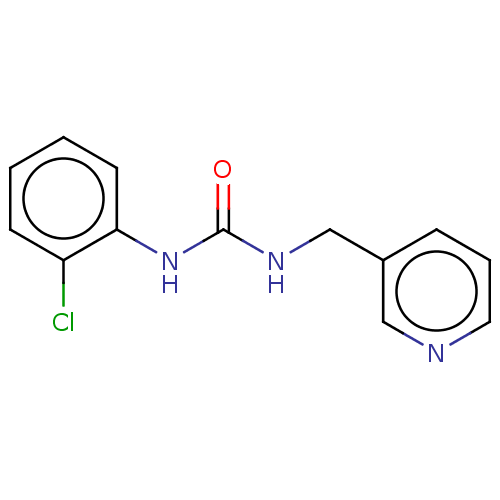
(CHEMBL1299308)Show InChI InChI=1S/C13H12ClN3O/c14-11-5-1-2-6-12(11)17-13(18)16-9-10-4-3-7-15-8-10/h1-8H,9H2,(H2,16,17,18) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of DDR1 (unknown origin) after 1 hr by time resolved fluorescence method |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50415331
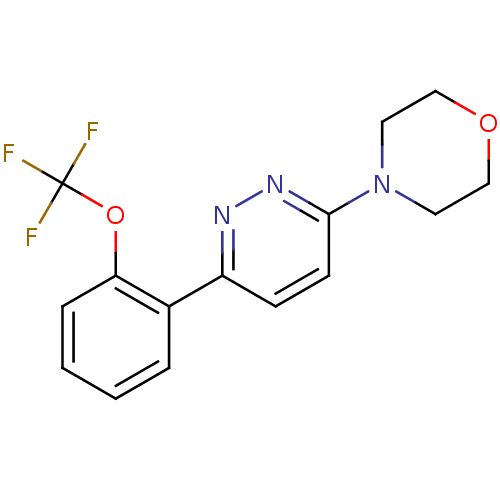
(CHEMBL593131)Show InChI InChI=1S/C15H14F3N3O2/c16-15(17,18)23-13-4-2-1-3-11(13)12-5-6-14(20-19-12)21-7-9-22-10-8-21/h1-6H,7-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor |
Bioorg Med Chem Lett 20: 465-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.117
BindingDB Entry DOI: 10.7270/Q27M096D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50415332

(CHEMBL611321)Show InChI InChI=1S/C15H14F3N3O/c16-15(17,18)12-3-1-2-11(10-12)13-4-5-14(20-19-13)21-6-8-22-9-7-21/h1-5,10H,6-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor |
Bioorg Med Chem Lett 20: 465-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.117
BindingDB Entry DOI: 10.7270/Q27M096D |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50415333
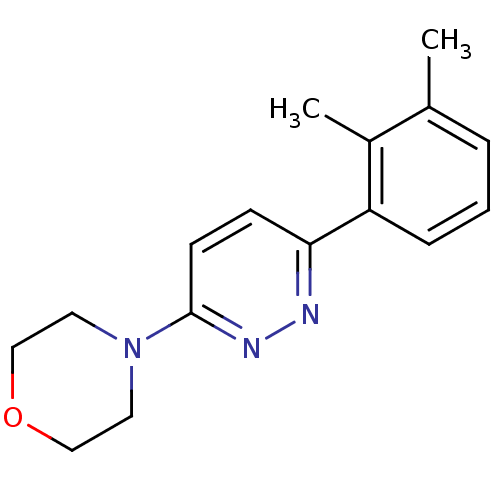
(CHEMBL596355)Show InChI InChI=1S/C16H19N3O/c1-12-4-3-5-14(13(12)2)15-6-7-16(18-17-15)19-8-10-20-11-9-19/h3-7H,8-11H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor |
Bioorg Med Chem Lett 20: 465-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.117
BindingDB Entry DOI: 10.7270/Q27M096D |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50112617

(CHEMBL3608786)Show SMILES Cc1ccc(CNC(=O)Nc2cccc(F)c2)cc1NC(=O)c1cnc2ccccn12 Show InChI InChI=1S/C23H20FN5O2/c1-15-8-9-16(13-26-23(31)27-18-6-4-5-17(24)12-18)11-19(15)28-22(30)20-14-25-21-7-2-3-10-29(20)21/h2-12,14H,13H2,1H3,(H,28,30)(H2,26,27,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a |
Astex Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of collagen I-induced DDR2 (unknown origin) phosphorylation expressed in HEK293 cells after 16 hrs by mesoscale discovery assay |
ACS Med Chem Lett 6: 798-803 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00143
BindingDB Entry DOI: 10.7270/Q2SQ925G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50415334

(CHEMBL610489)Show SMILES FC(F)(F)c1cccc(-c2ccc(nn2)N2CCOCC2)c1C(F)(F)F Show InChI InChI=1S/C16H13F6N3O/c17-15(18,19)11-3-1-2-10(14(11)16(20,21)22)12-4-5-13(24-23-12)25-6-8-26-9-7-25/h1-5H,6-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor |
Bioorg Med Chem Lett 20: 465-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.117
BindingDB Entry DOI: 10.7270/Q27M096D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data