

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM719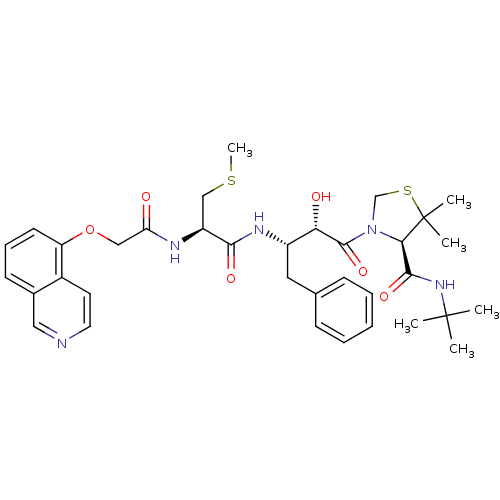 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0880 | -59.7 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002099 (CHEMBL366965 | Dimethyl-[3-(2-methyl-6H-dibenzo[b,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM718 ((4R)-3-[(2S,3S)-3-[(2-ethyl-3-hydroxyphenyl)formam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.160 | -58.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM717 ((4R)-N-[(2-chlorophenyl)methyl]-3-[(2S,3S)-2-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.290 | -56.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002087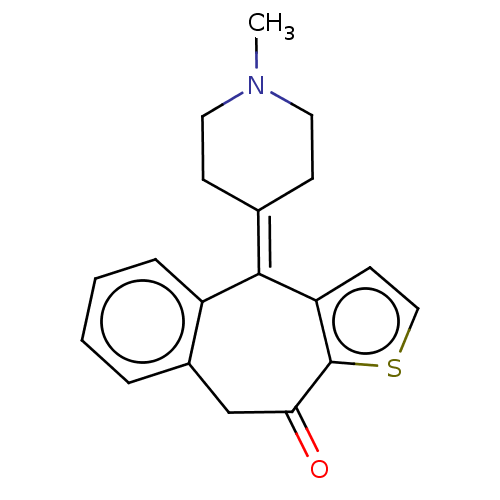 (4-(1-Methyl-piperidin-4-ylidene)-4,9-dihydro-1-thi...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity against histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand a... | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.330 | -56.3 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002083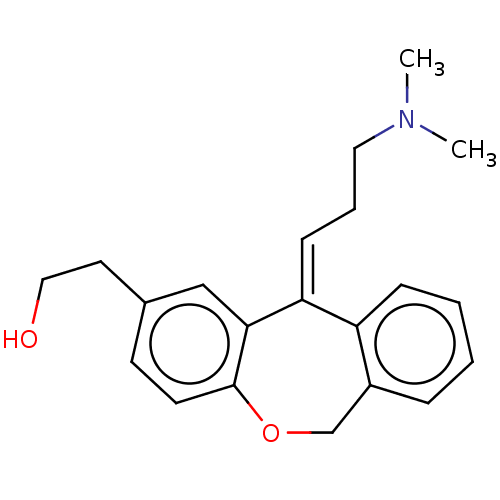 (2-[11-(3-Dimethylamino-propylidene)-6,11-dihydro-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002102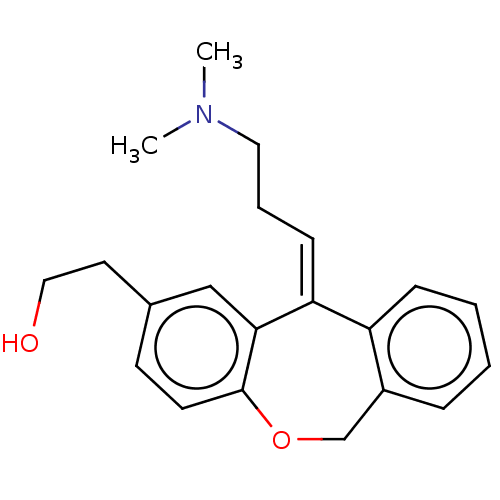 (2-[11-(3-Dimethylamino-propylidene)-6,11-dihydro-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity against histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand a... | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM579 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(2R)-2-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.740 | -54.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002792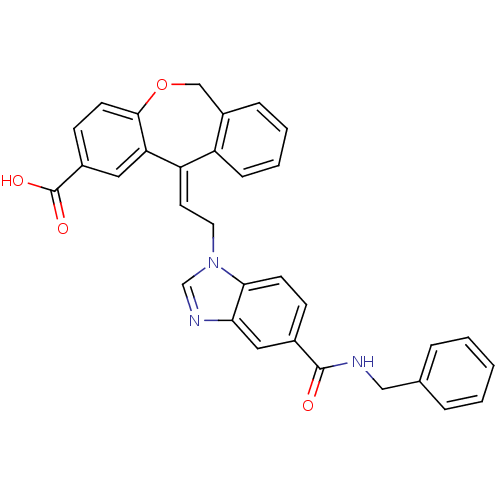 (11-[2-(5-Benzylcarbamoyl-benzoimidazol-1-yl)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM712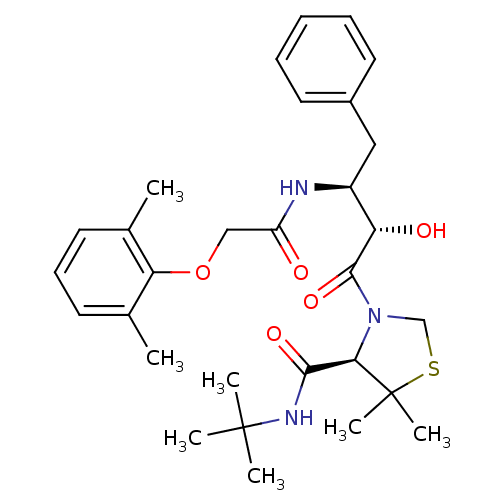 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[2-(2,6-dimethylphe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | -52.6 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002778 (11-[2-(5-Nitro-benzoimidazol-1-yl)-ethylidene]-6,1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002784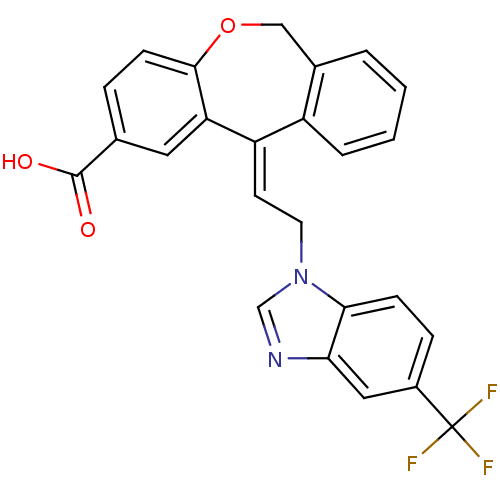 (11-[2-(5-Trifluoromethyl-benzoimidazol-1-yl)-ethyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM715 ((4R)-N-tert-butyl-3-[(2S,3S)-3-[(2-ethyl-3-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.24 | -51.4 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002787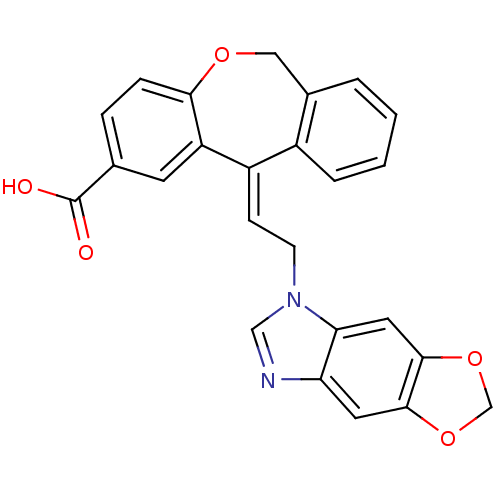 (11-(2-[1,3]Dioxolo[4',5':4,5]benzo[1,2-d]imidazol-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002785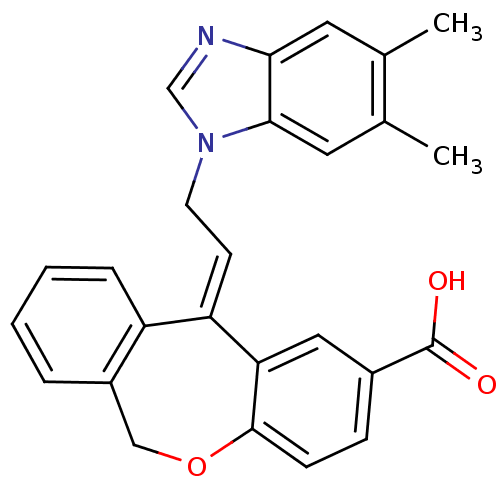 (11-[2-(5,6-Dimethyl-benzoimidazol-1-yl)-ethylidene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002796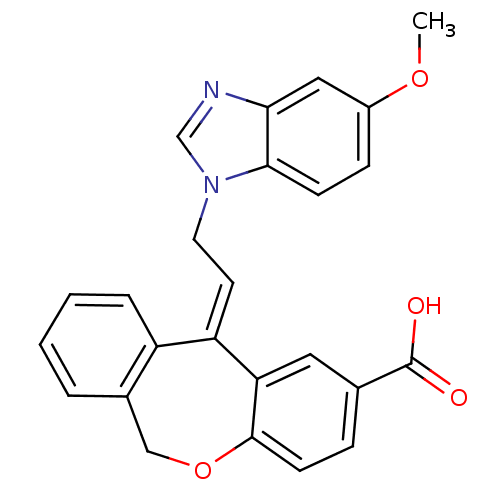 (11-[2-(5-Methoxy-benzoimidazol-1-yl)-ethylidene]-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002808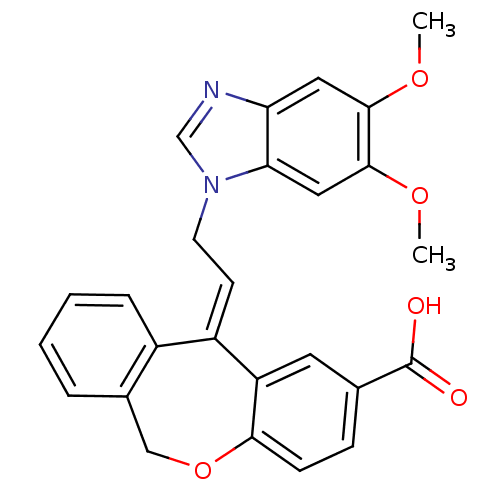 (11-[2-(5,6-Dimethoxy-benzoimidazol-1-yl)-ethyliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002814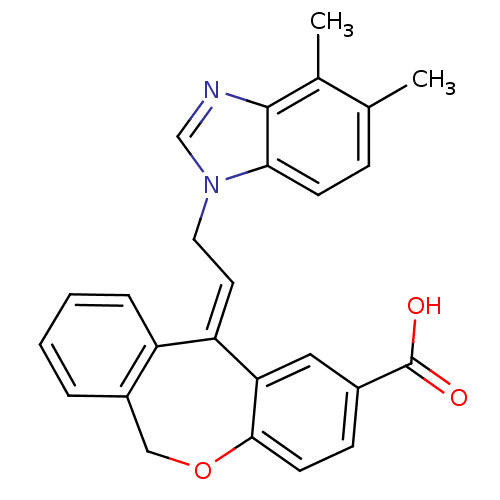 (11-[2-(4,5-Dimethyl-benzoimidazol-1-yl)-ethylidene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002759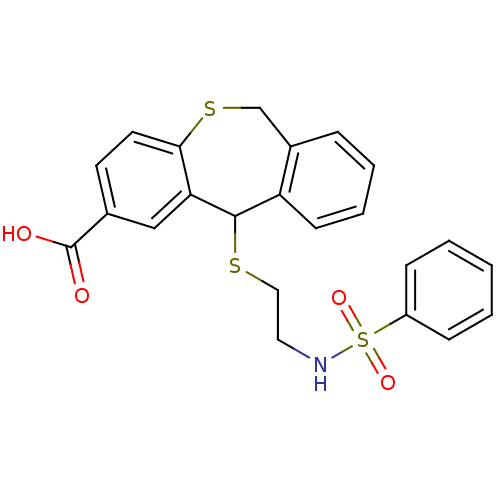 (5-(2-Benzenesulfonylamino-ethylsulfanyl)-5,11-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002759 (5-(2-Benzenesulfonylamino-ethylsulfanyl)-5,11-dihy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of Shh signaling in mouse Shh-light2 cells by Gli-dependent firefly luciferase reporter gene assay | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002783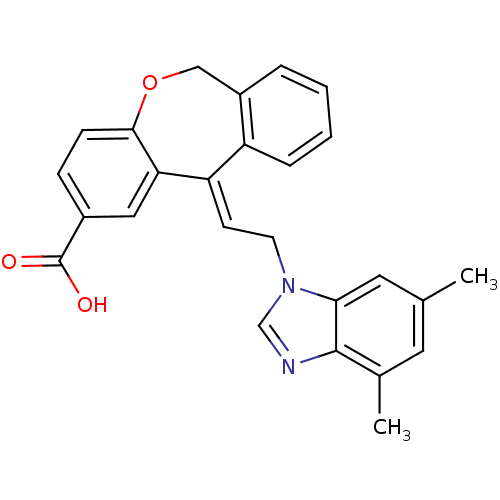 (11-[2-(4,6-Dimethyl-benzoimidazol-1-yl)-ethylidene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50164606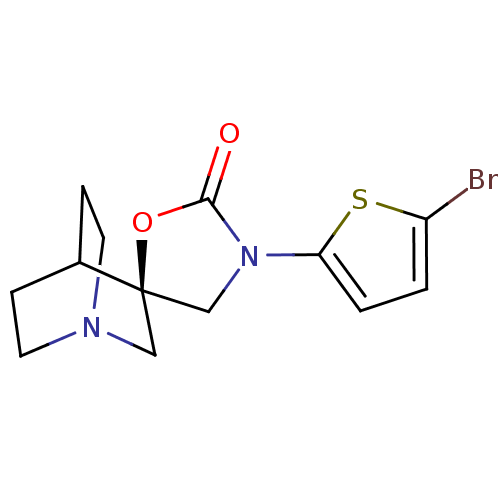 ((2R)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to rat nicotinic acetylcholine receptor alpha7 | J Med Chem 48: 2678-86 (2005) Article DOI: 10.1021/jm049188d BindingDB Entry DOI: 10.7270/Q2M0466W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190677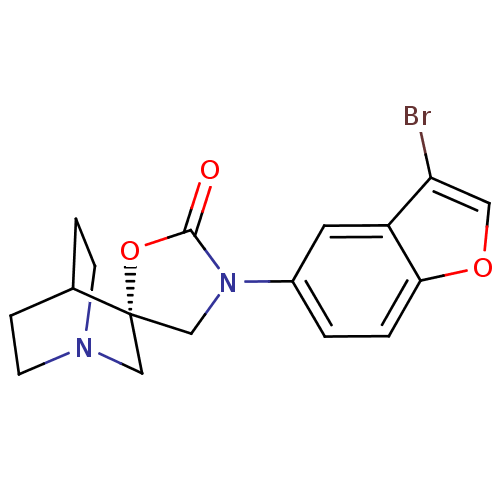 ((R)-3'-(3-bromobenzo[b]thiophen-5-yl)spiro[1-azabi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190686 ((R)-3'-(2,3-dimethylbenzo[b]thiophen-5-yl)spiro[1-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002820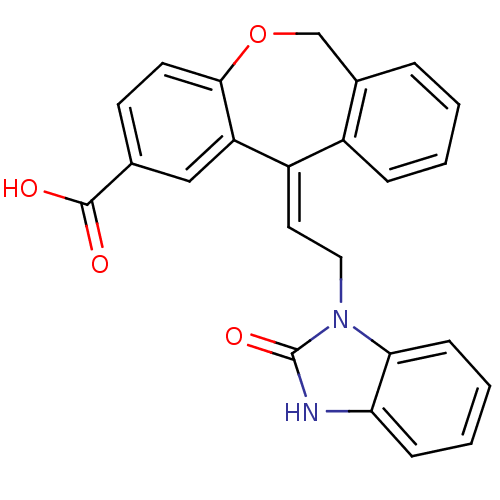 (11-[2-(2-Hydroxy-benzoimidazol-1-yl)-ethylidene]-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50190696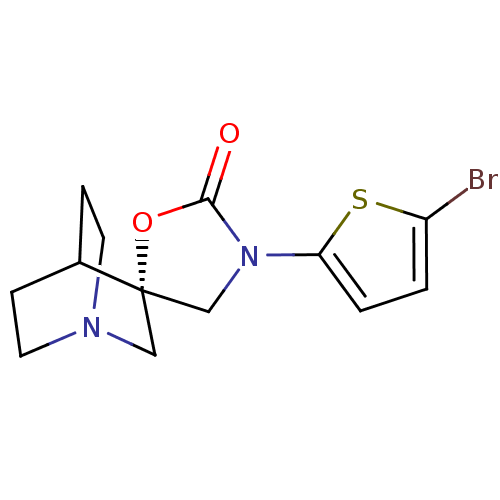 ((2S)-3'-(5-bromothien-2-yl)-2'H-spiro[4-azabicyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Mitsubishi Pharma Corporation Curated by ChEMBL | Assay Description Displacement of [125I]alpha-BTX from alpha-7 nAChR in rat hippocampus membrane | J Med Chem 49: 4374-83 (2006) Article DOI: 10.1021/jm060249c BindingDB Entry DOI: 10.7270/Q2BR8T08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002095 (11-(3-Dimethylamino-propylidene)-6,11-dihydro-dibe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002103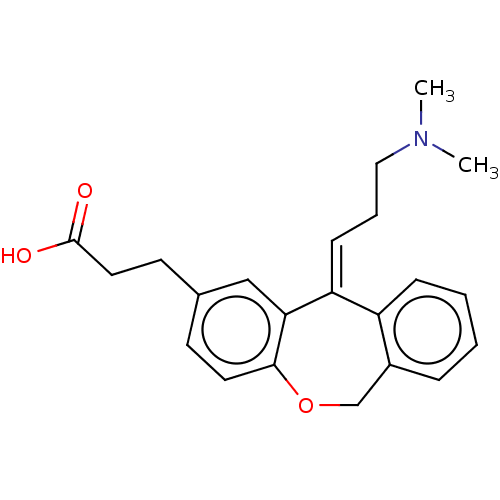 (3-[11-(3-Dimethylamino-propylidene)-6,11-dihydro-d...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002085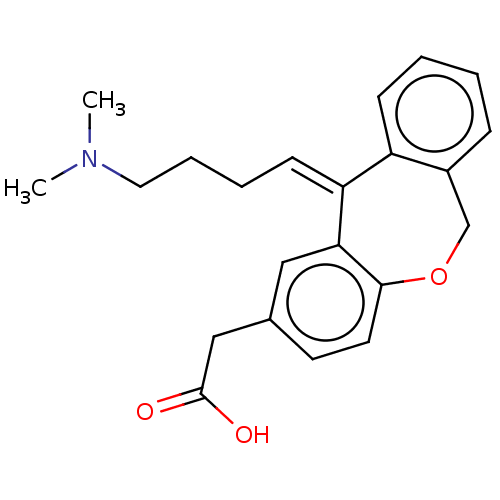 (CHEMBL304185 | [11-(4-Dimethylamino-butylidene)-6,...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002086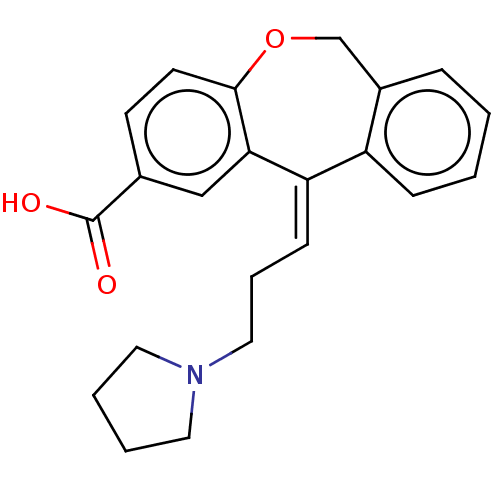 (11-(3-Pyrrolidin-1-yl-propylidene)-6,11-dihydro-di...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002772 (11-[2-(2-Hydroxy-benzoylamino)-ethylsulfanyl]-6,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of the thromboxane A2 receptor assayed by binding to guinea pig platelets using [3H]-U-46,619 as radioligand | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002772 (11-[2-(2-Hydroxy-benzoylamino)-ethylsulfanyl]-6,11...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM714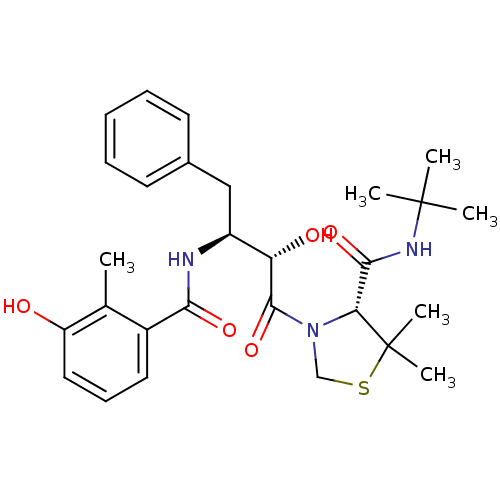 ((4R)-N-tert-butyl-3-[(2S,3S)-2-hydroxy-3-[(3-hydro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5.14 | -49.2 | n/a | n/a | n/a | n/a | n/a | 6.0 | 37 |
Japan Energy Corporation | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Substrates and cleavage fragments... | J Med Chem 42: 1789-802 (1999) Article DOI: 10.1021/jm980637h BindingDB Entry DOI: 10.7270/Q2MG7MP3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002093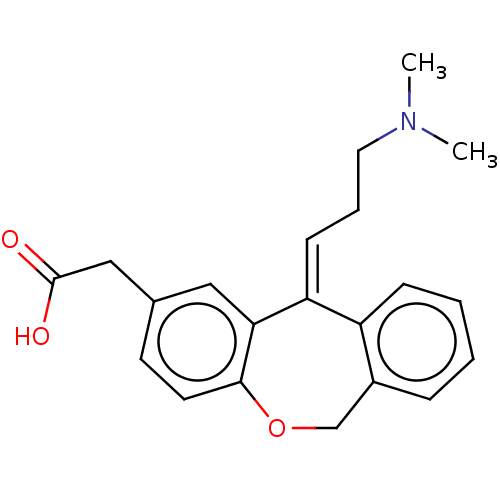 (CHEMBL65699 | [11-(3-Dimethylamino-propylidene)-6,...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002757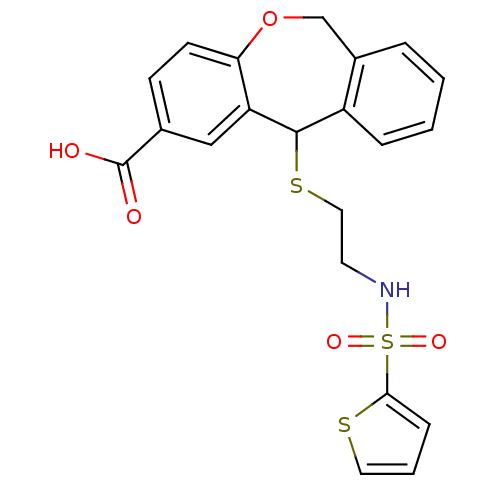 (11-[2-(Thiophene-2-sulfonylamino)-ethylsulfanyl]-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for its binding affinity at Thromboxane A2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets ... | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002757 (11-[2-(Thiophene-2-sulfonylamino)-ethylsulfanyl]-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002097 (11-(4-Dimethylamino-butylidene)-6,11-dihydro-diben...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description The compound was tested in vitro for binding affinity against histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand a... | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002782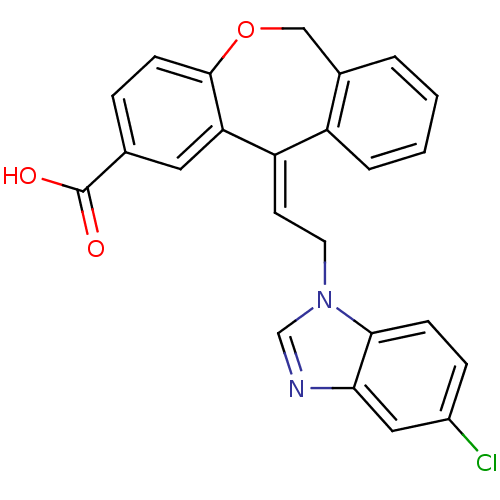 (11-[2-(5-Chloro-benzoimidazol-1-yl)-ethylidene]-6,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002770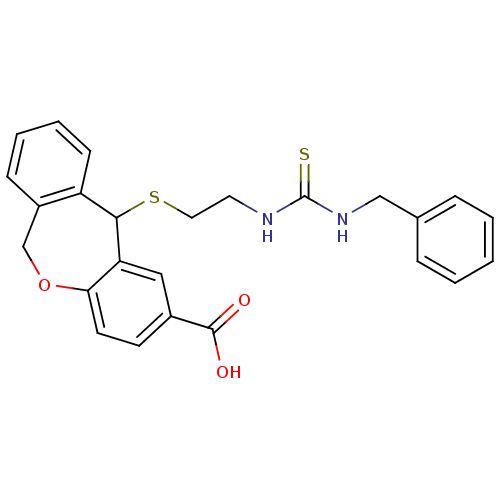 (11-[2-(3-Benzyl-thioureido)-ethylsulfanyl]-6,11-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of the thromboxane A2 receptor assayed by binding to guinea pig platelets using [3H]-U-46,619 as radioligand | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002818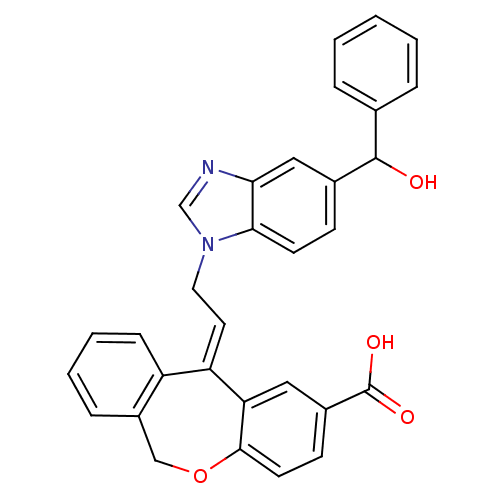 (11-{2-[5-(Hydroxy-phenyl-methyl)-benzoimidazol-1-y...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002801 (11-[2-(5,7-Dimethoxy-benzoimidazol-1-yl)-ethyliden...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2/PGH2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3402-13 (1992) BindingDB Entry DOI: 10.7270/Q2J38RG1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002770 (11-[2-(3-Benzyl-thioureido)-ethylsulfanyl]-6,11-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of the thromboxane A2 receptor assayed by binding to guinea pig platelets using [3H]-U-46,619 as radioligand | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50002098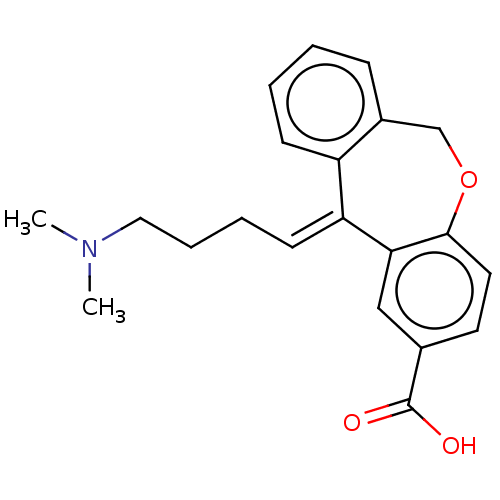 (11-(4-Dimethylamino-butylidene)-6,11-dihydro-diben...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co., Ltd. Curated by ChEMBL | Assay Description Binding affinity for histamine H1 receptor from guinea pig cerebellum, using [3H]pyrilamine as radioligand at 0.1 uM | J Med Chem 35: 2074-84 (1992) BindingDB Entry DOI: 10.7270/Q2N87BC0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for its binding affinity at Thromboxane A2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets ... | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002751 (11-[2-(2-Phenyl-ethenesulfonylamino)-ethylsulfanyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Compound was tested for its binding affinity at Thromboxane A2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002758 (11-(2-Benzenesulfonylamino-ethylsulfanyl)-9-bromo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Inhibition of the thromboxane A2 receptor assayed by binding to guinea pig platelets using [3H]-U-46,619 as radioligand | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50002758 (11-(2-Benzenesulfonylamino-ethylsulfanyl)-9-bromo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Company, Ltd. Curated by ChEMBL | Assay Description Binding affinity at TXA2 receptor by measuring its ability to displace [3H]-U-46,619 from guinea pig platelets | J Med Chem 35: 3394-402 (1992) BindingDB Entry DOI: 10.7270/Q2NS0SV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50040108 (11-[2-(3,4-Dihydro-1H-isoquinolin-2-yl)-ethylsulfa...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyowa Hakko Kogyo Co. Curated by ChEMBL | Assay Description Inhibition of the specific binding of [3H]-pyrilamine to guinea pig cerebellum histamine H1 receptor | J Med Chem 36: 417-20 (1993) BindingDB Entry DOI: 10.7270/Q2Q23Z9K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1503 total ) | Next | Last >> |