

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017434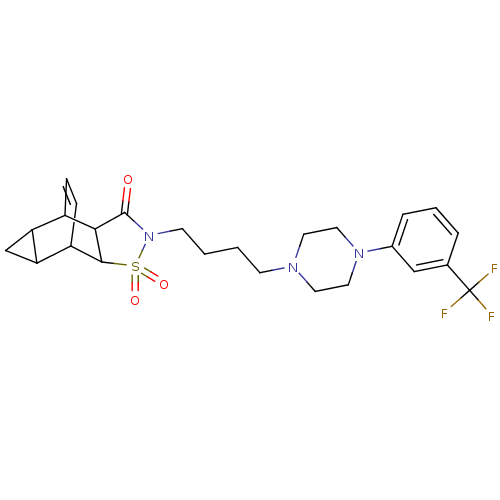 (4-{4-[4-(3-trifluoromethylphenyl)hexahydro-1-pyraz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and the value ranges from 3 -5 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017437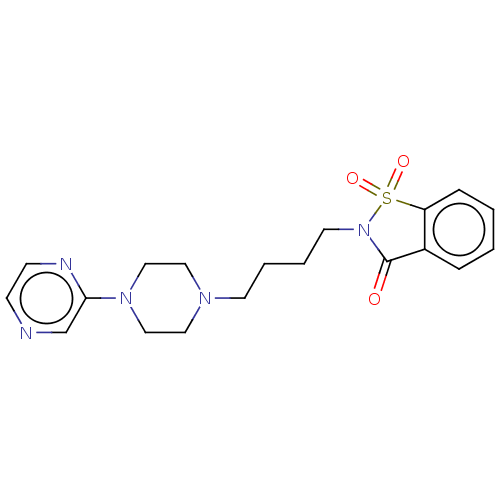 (1,1-Dioxo-2-[4-(2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and value ranges from 6 - 13 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017432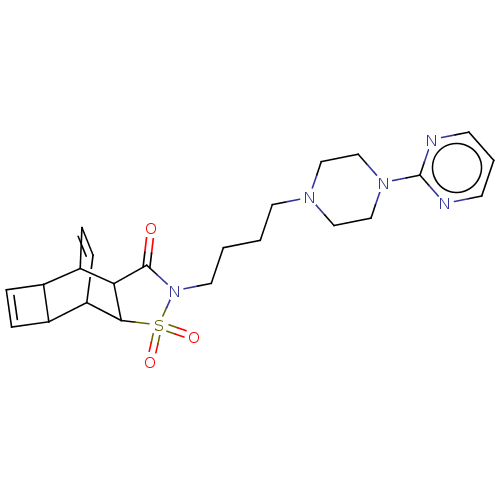 (4-{4-[4-(2-pyrimidinyl)hexahydro-1-pyrazinyl]butyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-HT1A receptor of rat hippocampus and the value ranges from 9 - 12 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50005127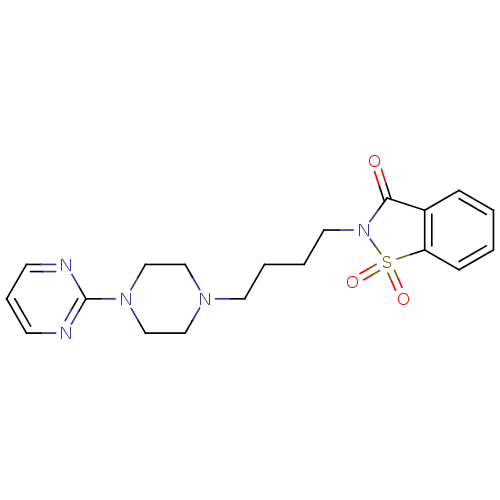 (1,1-Dioxo-2-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017432 (4-{4-[4-(2-pyrimidinyl)hexahydro-1-pyrazinyl]butyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-HT1A receptor of rat hippocampus and the value ranges from 9 - 12 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50001859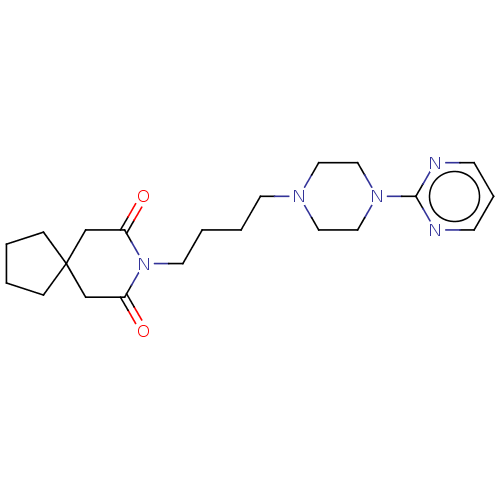 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017431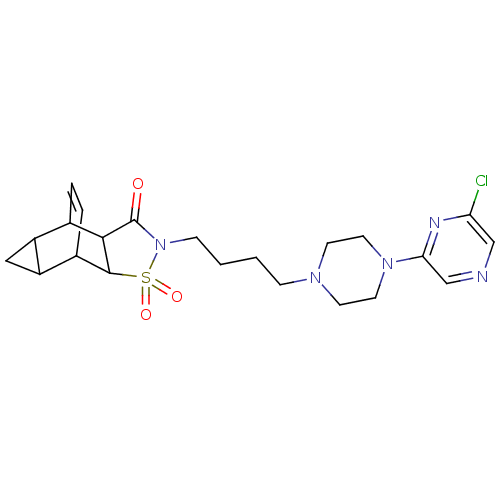 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus (94%CI) from 12 to 17 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017443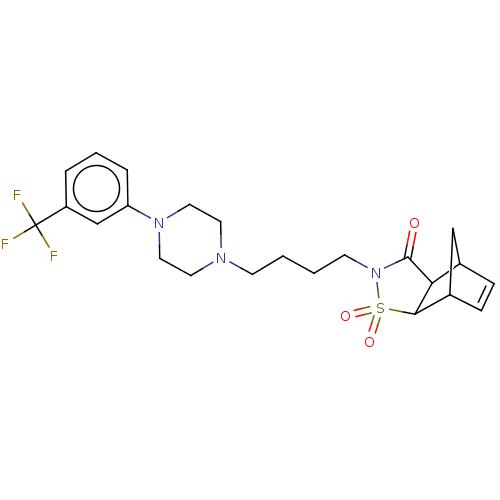 (3,3-Dioxo-4-{4-[4-(3-trifluoromethyl-phenyl)-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and the value ranges from 12 to 17. | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017447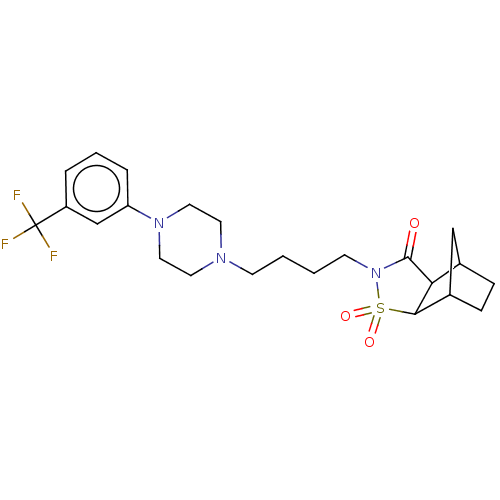 (3,3-Dioxo-4-{4-[4-(3-trifluoromethyl-phenyl)-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and the value ranges from 12 to 20. | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017435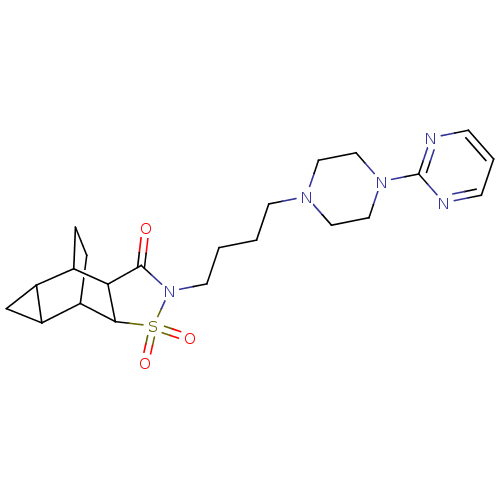 (4-{4-[4-(2-pyrimidinyl)hexahydro-1-pyrazinyl]butyl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus. and the value ranges from 16 - 27 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017442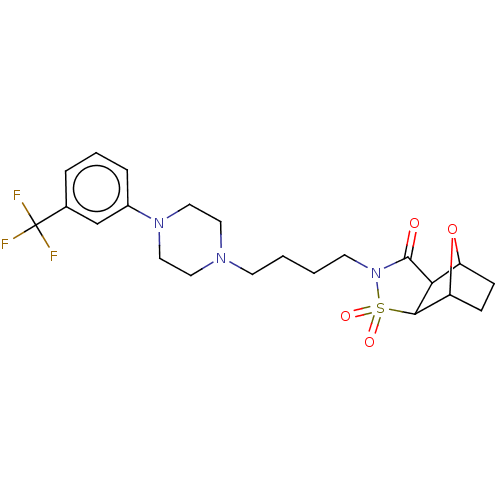 (3,3-Dioxo-4-{4-[4-(3-trifluoromethyl-phenyl)-piper...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]-8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and value ranges from 15 - 31 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017445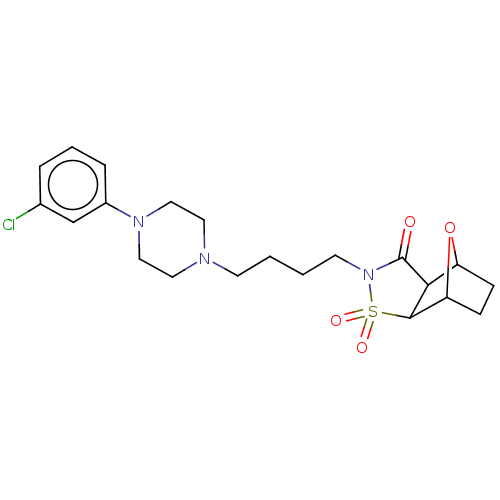 (4-{4-[4-(3-Chloro-phenyl)-piperazin-1-yl]-butyl}-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and value ranges from 27 - 42 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017444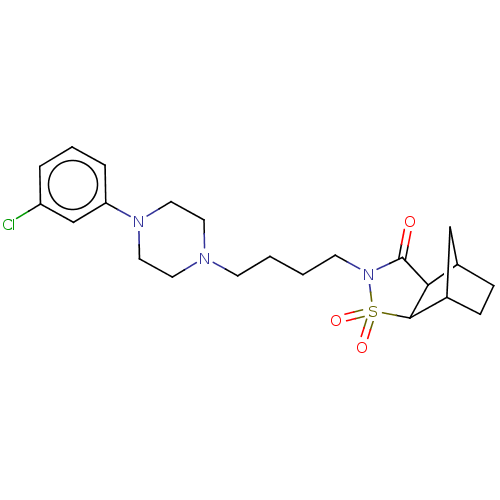 (4-{4-[4-(3-Chloro-phenyl)-piperazin-1-yl]-butyl}-3...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and the value ranges from 25 - 84 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017433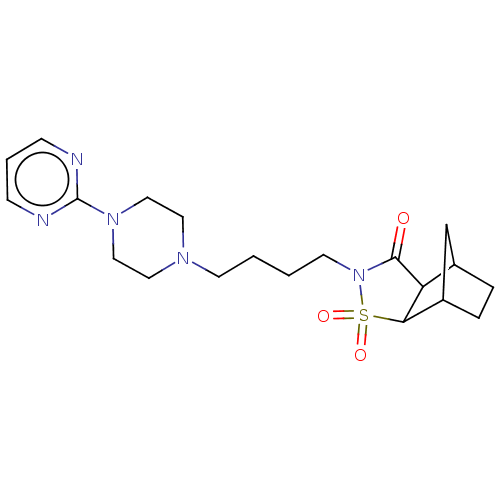 (3,3-Dioxo-4-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and the value ranges from 48 to 59 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017443 (3,3-Dioxo-4-{4-[4-(3-trifluoromethyl-phenyl)-piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures. | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001859 ((buspirone) 8-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to dopamine receptor D2 of rat limbic structures | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017432 (4-{4-[4-(2-pyrimidinyl)hexahydro-1-pyrazinyl]butyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 136 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures and the value ranges from 116 - 159. | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017436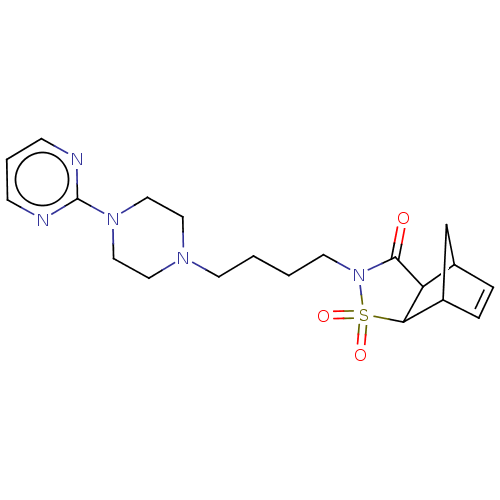 (3,3-Dioxo-4-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 434 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus and the value ranges from 245 to 736 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017441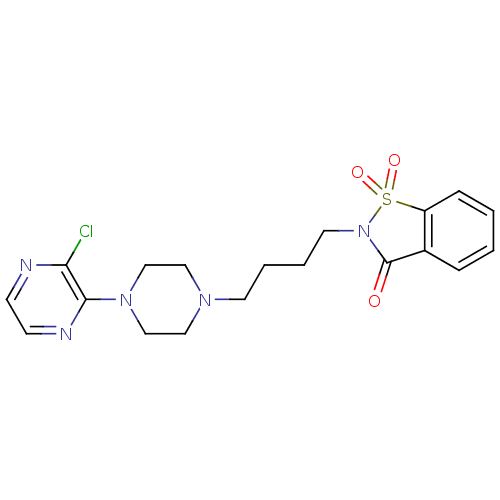 (2-[4-(3'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures (95%CI) from 375-661 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50017440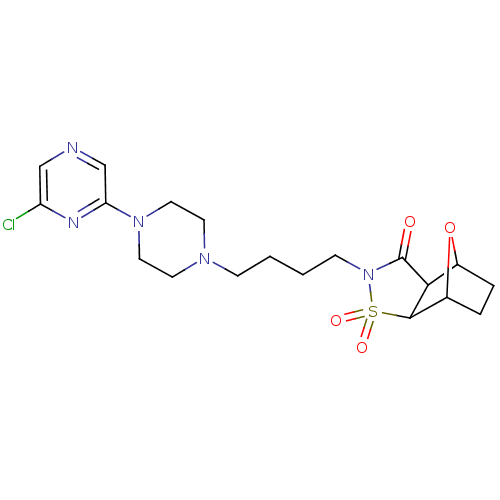 (4-[4-(6'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor of rat hippocampus (94%CI) from 391-841 | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017431 (4-{4-[4-(6-chloro-2-pyrazinyl)hexahydro-1-pyraziny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures. | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017436 (3,3-Dioxo-4-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017438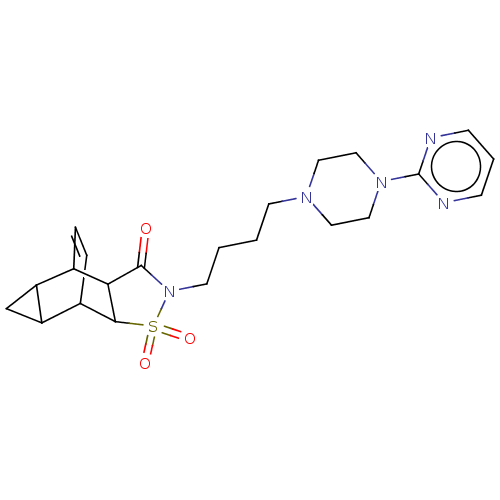 (4-{4-[4-(2-pyrimidinyl)hexahydro-1-pyrazinyl]butyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures. | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017439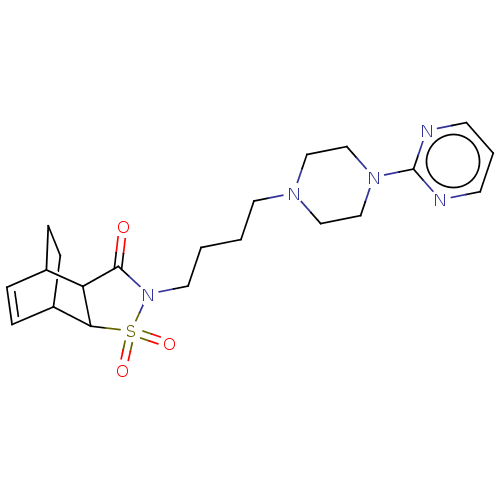 (3,3-Dioxo-4-[4-(4-pyrimidin-2-yl-piperazin-1-yl)-b...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50017446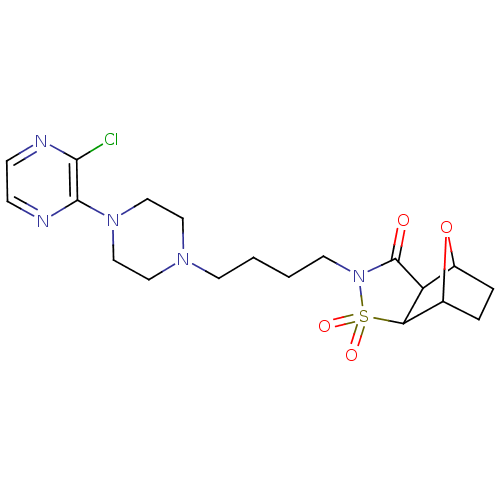 (4-[4-(3'-Chloro-2,3,5,6-tetrahydro-[1,2']bipyrazin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro ability to inhibit [3H]spiperone binding to Dopamine receptor D2 of rat limbic structures. | J Med Chem 32: 1024-33 (1989) BindingDB Entry DOI: 10.7270/Q21G0MVD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25418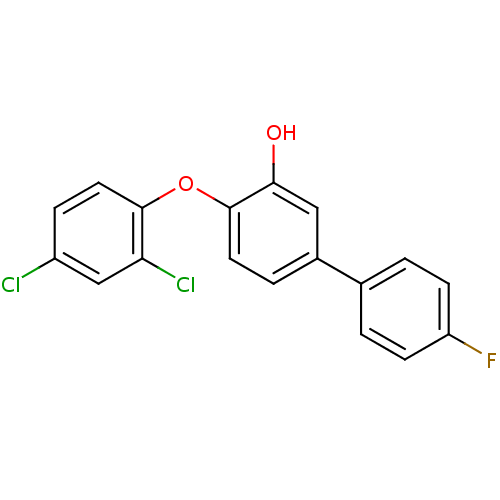 (2-(2,4-dichlorophenoxy)-5-(4-fluorophenyl)phenol |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25403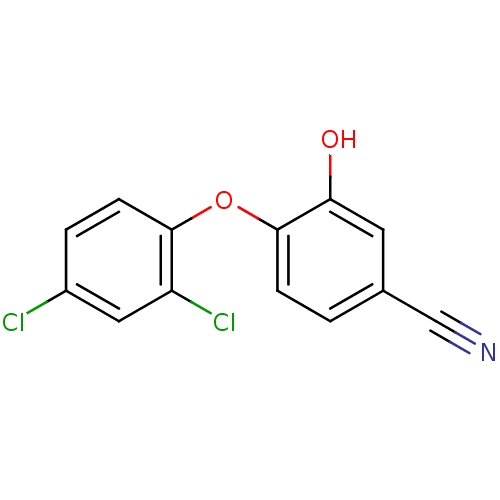 (4‐(2,4‐dichlorophenoxy)‐3‐...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174772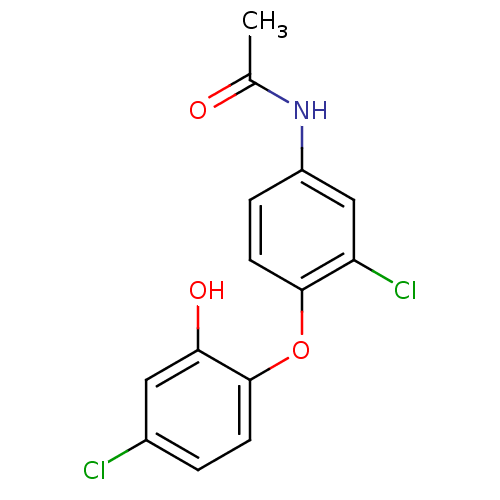 (CHEMBL200658 | N-(3-chloro-4-(4-chloro-2-hydroxyph...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25419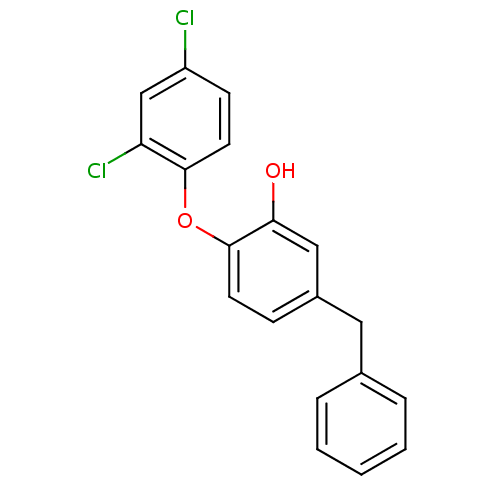 (5-benzyl-2-(2,4-dichlorophenoxy)phenol | Triclosan...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM8726 (5-chloro-2-(2,4-dichlorophenoxy)phenol | CHEMBL849...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ENR enzymatic activity | Bioorg Med Chem Lett 16: 2163-9 (2006) Article DOI: 10.1016/j.bmcl.2006.01.051 BindingDB Entry DOI: 10.7270/Q2154GNG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25420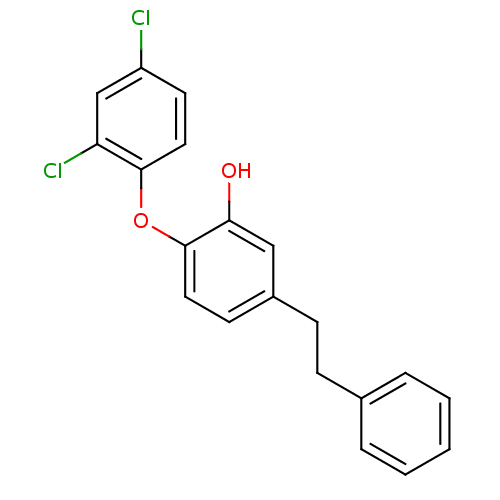 (2-(2,4-dichlorophenoxy)-5-(2-phenylethyl)phenol | ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25407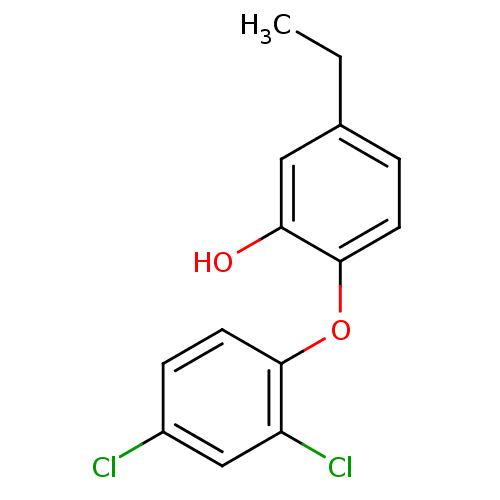 (2‐(2,4‐dichlorophenoxy)‐5‐...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174766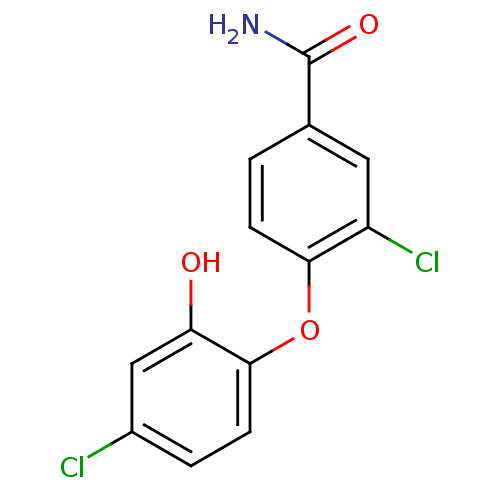 (3-chloro-4-(4-chloro-2-hydroxyphenoxy)benzamide | ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174775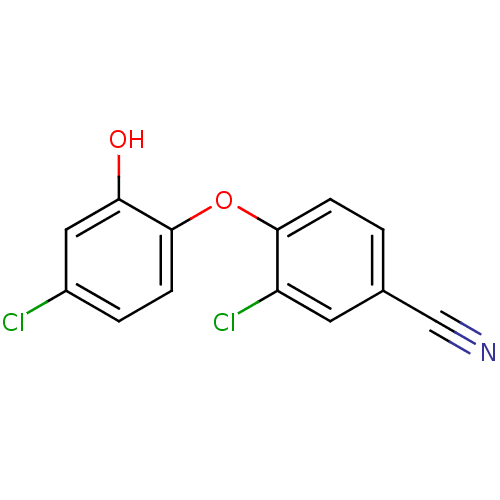 (3-chloro-4-(4-chloro-2-hydroxyphenoxy)benzonitrile...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25412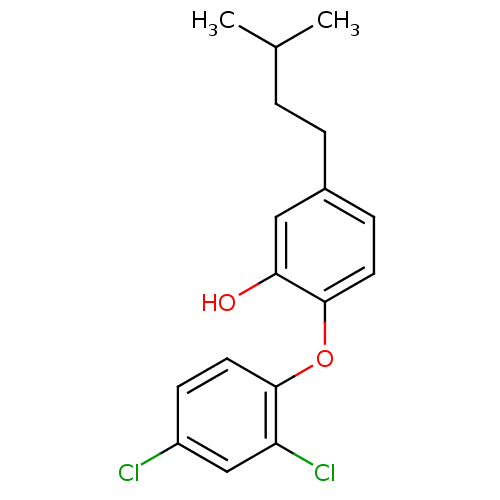 (2-(2,4-dichlorophenoxy)-5-(3-methylbutyl)phenol | ...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25402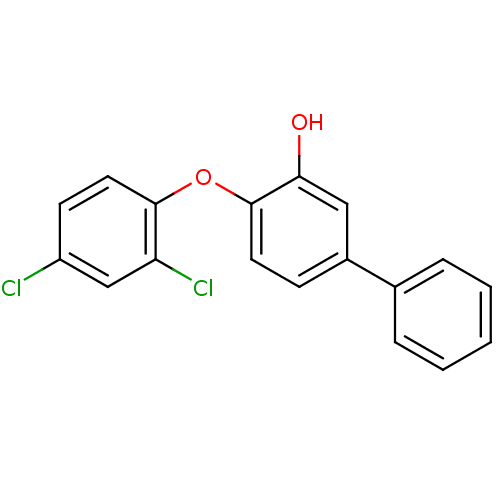 (2-(2,4-dichlorophenoxy)-5-phenylphenol | Triclosan...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174760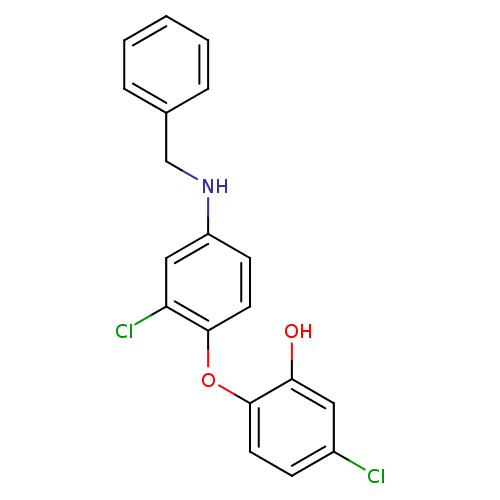 (2‐[4‐(benzylamino)‐2‐chlor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174769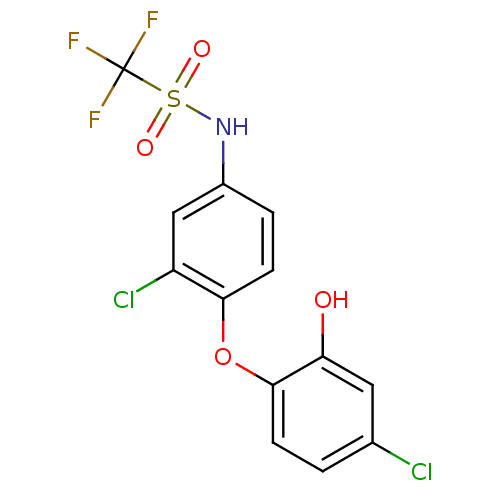 (CHEMBL198781 | N-(3-chloro-4-(4-chloro-2-hydroxyph...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174778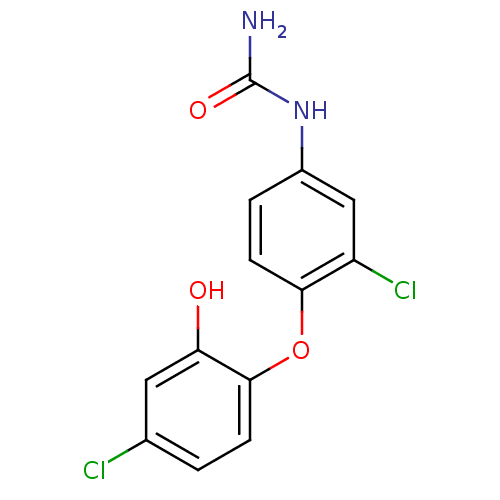 (1-(3-chloro-4-(4-chloro-2-hydroxyphenoxy)phenyl)ur...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174776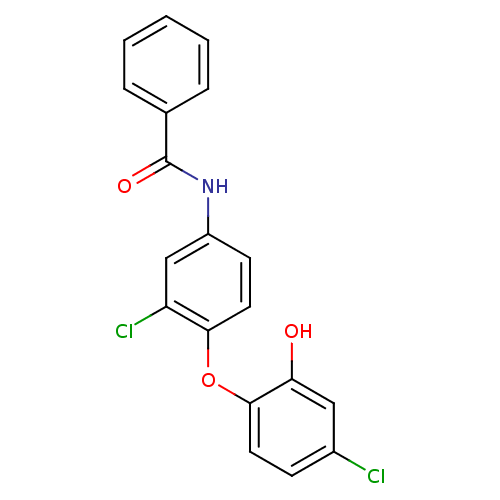 (CHEMBL200871 | N-(3-chloro-4-(4-chloro-2-hydroxyph...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25410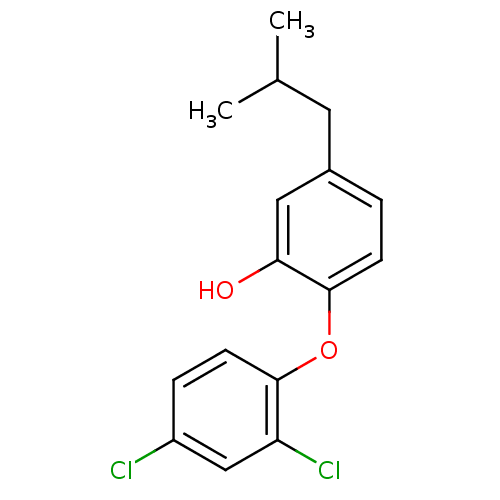 (2-(2,4-dichlorophenoxy)-5-(2-methylpropyl)phenol |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174770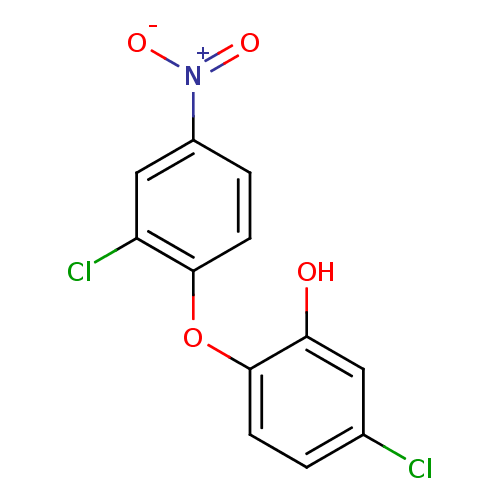 (5-chloro-2-(2-chloro-4-nitrophenoxy)phenol | CHEMB...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25417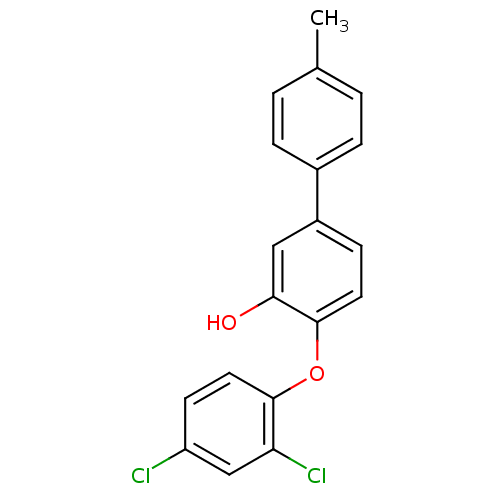 (2-(2,4-dichlorophenoxy)-5-(4-methylphenyl)phenol |...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174767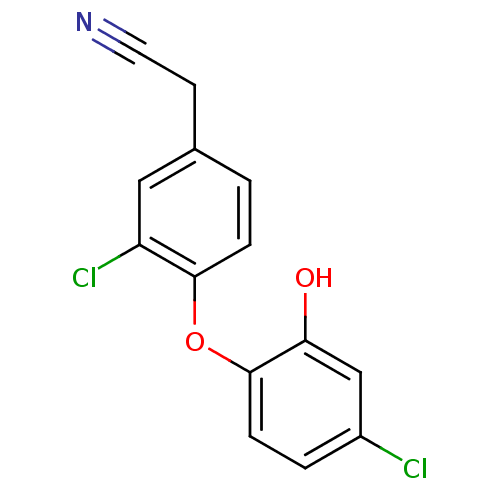 (2-(3-chloro-4-(4-chloro-2-hydroxyphenoxy)phenyl)ac...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174754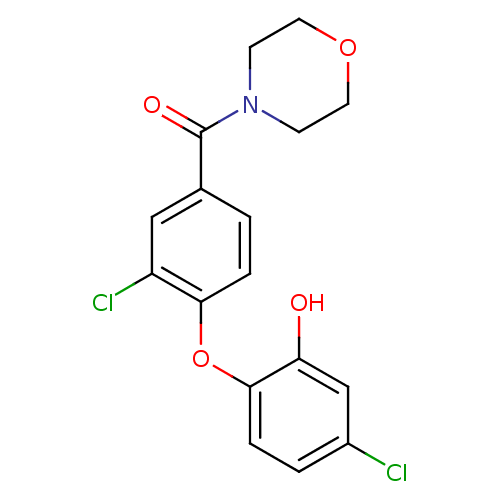 ((3-chloro-4-(4-chloro-2-hydroxyphenoxy)phenyl)(mor...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25401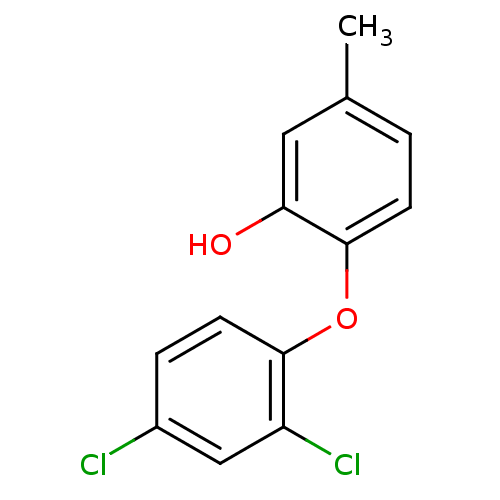 (2-(2,4-dichlorophenoxy)-5-methylphenol | Triclosan...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-ACP reductase (Plasmodium falciparum) | BDBM25408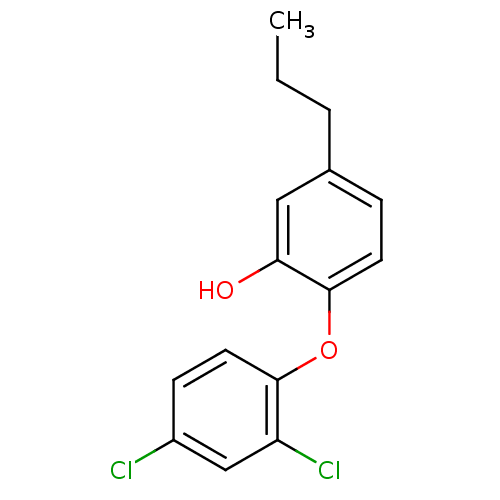 (2-(2,4-dichlorophenoxy)-5-propylphenol | Triclosan...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Jacobus Pharmaceutical Company | Assay Description PfENR inhibition assays were carried out on a Cary 100 Bio Spectrophotometer or POLARstar Optima by monitoring oxidation of NADH at 340 nm. The IC50 ... | J Biol Chem 282: 25436-44 (2007) Article DOI: 10.1074/jbc.M701813200 BindingDB Entry DOI: 10.7270/Q22V2DFD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Enoyl-acyl-carrier protein reductase (Plasmodium falciparum) | BDBM50174768 (5‐chloro‐2‐(2‐chloro‐...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibition of PfENR enzymatic activity | Bioorg Med Chem Lett 15: 5247-52 (2005) Article DOI: 10.1016/j.bmcl.2005.08.044 BindingDB Entry DOI: 10.7270/Q2NC60RF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Displayed 1 to 50 (of 110 total ) | Next | Last >> |