 Found 147 hits with Last Name = 'sciotti' and Initial = 'rj'
Found 147 hits with Last Name = 'sciotti' and Initial = 'rj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447108

(CHEMBL3112881)Show InChI InChI=1S/C11H7ClFNO2S/c12-11-7-2-1-6(13)5-9(7)17-8(11)3-4-10(15)14-16/h1-5,16H,(H,14,15)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013677
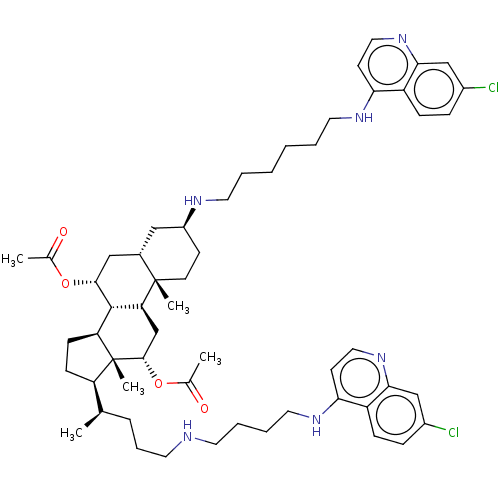
(CHEMBL3264512)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)NCCCCCCNc1ccnc2cc(Cl)ccc12)OC(C)=O)[C@H](C)CCCNCCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C56H78Cl2N6O4/c1-36(13-12-25-59-24-10-11-28-62-49-22-30-64-51-34-41(58)15-17-44(49)51)45-18-19-46-54-47(35-53(56(45,46)5)68-38(3)66)55(4)23-20-42(31-39(55)32-52(54)67-37(2)65)60-26-8-6-7-9-27-61-48-21-29-63-50-33-40(57)14-16-43(48)50/h14-17,21-22,29-30,33-34,36,39,42,45-47,52-54,59-60H,6-13,18-20,23-28,31-32,35H2,1-5H3,(H,61,63)(H,62,64)/t36-,39+,42+,45-,46+,47+,52-,53+,54+,55+,56-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013673

(CHEMBL3264509)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])CC(CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)NCCCNc1ccnc2cc(Cl)ccc12)OC(C)=O)[C@H](C)CCCNCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C52H70Cl2N6O4/c1-32(9-6-20-55-21-7-23-57-44-17-25-59-46-29-36(53)10-12-39(44)46)41-14-15-42-50-43(31-49(52(41,42)5)64-34(3)62)51(4)19-16-38(27-35(51)28-48(50)63-33(2)61)56-22-8-24-58-45-18-26-60-47-30-37(54)11-13-40(45)47/h10-13,17-18,25-26,29-30,32,35,38,41-43,48-50,55-56H,6-9,14-16,19-24,27-28,31H2,1-5H3,(H,57,59)(H,58,60)/t32-,35+,38?,41-,42+,43+,48-,49+,50+,51+,52-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013676
(CHEMBL3264511)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)NCCCCCCNc1ccnc2cc(Cl)ccc12)OC(C)=O)[C@H](C)CCCNCCCCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C58H82Cl2N6O4/c1-38(15-14-27-61-26-10-6-7-12-29-63-50-23-31-65-52-35-42(59)16-18-45(50)52)47-20-21-48-56-49(37-55(58(47,48)5)70-40(3)68)57(4)25-22-44(33-41(57)34-54(56)69-39(2)67)62-28-11-8-9-13-30-64-51-24-32-66-53-36-43(60)17-19-46(51)53/h16-19,23-24,31-32,35-36,38,41,44,47-49,54-56,61-62H,6-15,20-22,25-30,33-34,37H2,1-5H3,(H,63,65)(H,64,66)/t38-,41+,44-,47-,48+,49+,54-,55+,56+,57+,58-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 285 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013674

(CHEMBL3264510)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)NCCCCNc1ccnc2cc(Cl)ccc12)OC(C)=O)[C@H](C)CCCNCCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C54H74Cl2N6O4/c1-34(11-10-23-57-22-6-7-25-59-46-19-27-61-48-31-38(55)12-14-41(46)48)43-16-17-44-52-45(33-51(54(43,44)5)66-36(3)64)53(4)21-18-40(29-37(53)30-50(52)65-35(2)63)58-24-8-9-26-60-47-20-28-62-49-32-39(56)13-15-42(47)49/h12-15,19-20,27-28,31-32,34,37,40,43-45,50-52,57-58H,6-11,16-18,21-26,29-30,33H2,1-5H3,(H,59,61)(H,60,62)/t34-,37+,40+,43-,44+,45+,50-,51+,52+,53+,54-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013678
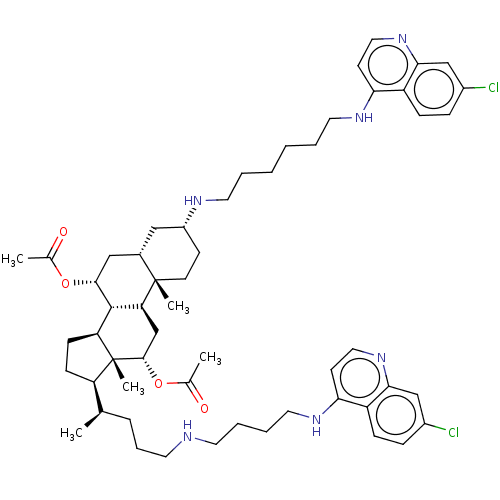
(CHEMBL3264513)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)NCCCCCCNc1ccnc2cc(Cl)ccc12)OC(C)=O)[C@H](C)CCCNCCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C56H78Cl2N6O4/c1-36(13-12-25-59-24-10-11-28-62-49-22-30-64-51-34-41(58)15-17-44(49)51)45-18-19-46-54-47(35-53(56(45,46)5)68-38(3)66)55(4)23-20-42(31-39(55)32-52(54)67-37(2)65)60-26-8-6-7-9-27-61-48-21-29-63-50-33-40(57)14-16-43(48)50/h14-17,21-22,29-30,33-34,36,39,42,45-47,52-54,59-60H,6-13,18-20,23-28,31-32,35H2,1-5H3,(H,61,63)(H,62,64)/t36-,39+,42-,45-,46+,47+,52-,53+,54+,55+,56-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50384950

(CHEMBL2037386)Show SMILES Clc1ccc2c(NCCCNCc3ccc(cc3)-c3ccc(s3)-c3ccc(CNCCCNc4ccnc5cc(Cl)ccc45)cc3)ccnc2c1 Show InChI InChI=1S/C42H40Cl2N6S/c43-33-11-13-35-37(17-23-49-39(35)25-33)47-21-1-19-45-27-29-3-7-31(8-4-29)41-15-16-42(51-41)32-9-5-30(6-10-32)28-46-20-2-22-48-38-18-24-50-40-26-34(44)12-14-36(38)40/h3-18,23-26,45-46H,1-2,19-22,27-28H2,(H,47,49)(H,48,50) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013672
(CHEMBL3264508)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])CC(CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)NCCNc1ccnc2cc(Cl)ccc12)OC(C)=O)[C@H](C)CCCNCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C50H66Cl2N6O4/c1-30(7-6-18-53-21-22-57-42-15-19-55-44-27-34(51)8-10-37(42)44)39-12-13-40-48-41(29-47(50(39,40)5)62-32(3)60)49(4)17-14-36(25-33(49)26-46(48)61-31(2)59)54-23-24-58-43-16-20-56-45-28-35(52)9-11-38(43)45/h8-11,15-16,19-20,27-28,30,33,36,39-41,46-48,53-54H,6-7,12-14,17-18,21-26,29H2,1-5H3,(H,55,57)(H,56,58)/t30-,33+,36?,39-,40+,41+,46-,47+,48+,49+,50-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013675
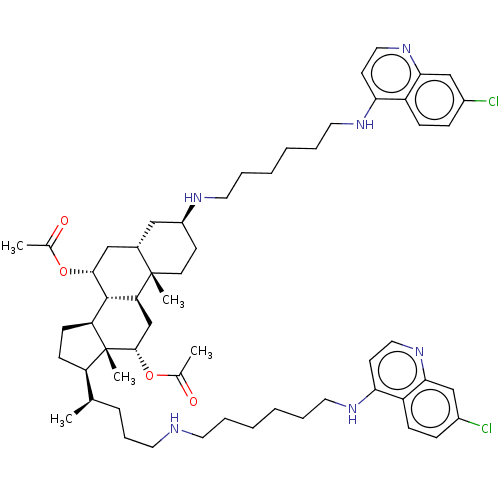
(CHEMBL3259867)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)NCCCCCCNc1ccnc2cc(Cl)ccc12)OC(C)=O)[C@H](C)CCCNCCCCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C58H82Cl2N6O4/c1-38(15-14-27-61-26-10-6-7-12-29-63-50-23-31-65-52-35-42(59)16-18-45(50)52)47-20-21-48-56-49(37-55(58(47,48)5)70-40(3)68)57(4)25-22-44(33-41(57)34-54(56)69-39(2)67)62-28-11-8-9-13-30-64-51-24-32-66-53-36-43(60)17-19-46(51)53/h16-19,23-24,31-32,35-36,38,41,44,47-49,54-56,61-62H,6-15,20-22,25-30,33-34,37H2,1-5H3,(H,63,65)(H,64,66)/t38-,41+,44+,47-,48+,49+,54-,55+,56+,57+,58-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM23274

((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...)Show InChI InChI=1S/C9H7Cl2NO2/c10-7-3-1-6(8(11)5-7)2-4-9(13)12-14/h1-5,14H,(H,12,13)/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013658
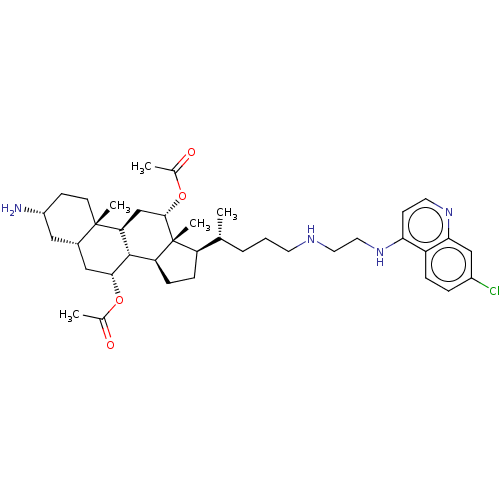
(CHEMBL3264499)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](N)CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)[C@H](C)CCCNCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C39H57ClN4O4/c1-23(7-6-15-42-17-18-44-33-13-16-43-34-21-27(40)8-9-29(33)34)30-10-11-31-37-32(22-36(39(30,31)5)48-25(3)46)38(4)14-12-28(41)19-26(38)20-35(37)47-24(2)45/h8-9,13,16,21,23,26,28,30-32,35-37,42H,6-7,10-12,14-15,17-20,22,41H2,1-5H3,(H,43,44)/t23-,26+,28-,30-,31+,32+,35-,36+,37+,38+,39-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013659
(CHEMBL3264500)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@H](N)CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)[C@H](C)CCCNCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C40H59ClN4O4/c1-24(8-6-16-43-17-7-18-44-34-14-19-45-35-22-28(41)9-10-30(34)35)31-11-12-32-38-33(23-37(40(31,32)5)49-26(3)47)39(4)15-13-29(42)20-27(39)21-36(38)48-25(2)46/h9-10,14,19,22,24,27,29,31-33,36-38,43H,6-8,11-13,15-18,20-21,23,42H2,1-5H3,(H,44,45)/t24-,27+,29-,31-,32+,33+,36-,37+,38+,39+,40-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013668
(CHEMBL3264171)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)OC(C)=O)[C@H](C)CCCNCCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C42H60ClN3O6/c1-25(9-7-17-44-18-8-19-45-36-15-20-46-37-23-30(43)10-11-32(36)37)33-12-13-34-40-35(24-39(42(33,34)6)52-28(4)49)41(5)16-14-31(50-26(2)47)21-29(41)22-38(40)51-27(3)48/h10-11,15,20,23,25,29,31,33-35,38-40,44H,7-9,12-14,16-19,21-22,24H2,1-6H3,(H,45,46)/t25-,29+,31-,33-,34+,35+,38-,39+,40+,41+,42-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013667

(CHEMBL450398)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)OC(C)=O)[C@H](C)CCCNCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C41H58ClN3O6/c1-24(8-7-16-43-18-19-45-35-14-17-44-36-22-29(42)9-10-31(35)36)32-11-12-33-39-34(23-38(41(32,33)6)51-27(4)48)40(5)15-13-30(49-25(2)46)20-28(40)21-37(39)50-26(3)47/h9-10,14,17,22,24,28,30,32-34,37-39,43H,7-8,11-13,15-16,18-21,23H2,1-6H3,(H,44,45)/t24-,28+,30-,32-,33+,34+,37-,38+,39+,40+,41-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013645
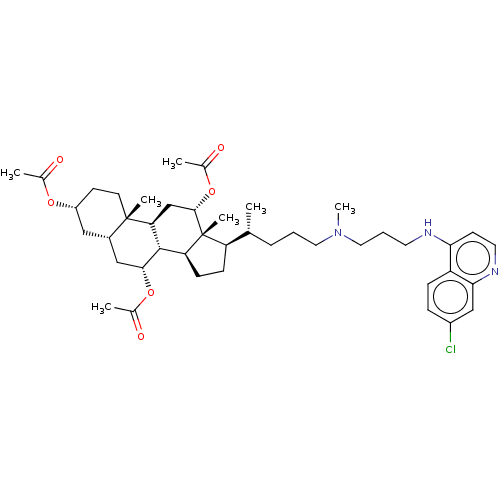
(CHEMBL3264183)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)OC(C)=O)[C@H](C)CCCN(C)CCCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C43H62ClN3O6/c1-26(10-8-20-47(7)21-9-18-45-37-16-19-46-38-24-31(44)11-12-33(37)38)34-13-14-35-41-36(25-40(43(34,35)6)53-29(4)50)42(5)17-15-32(51-27(2)48)22-30(42)23-39(41)52-28(3)49/h11-12,16,19,24,26,30,32,34-36,39-41H,8-10,13-15,17-18,20-23,25H2,1-7H3,(H,45,46)/t26-,30+,32-,34-,35+,36+,39-,40+,41+,42+,43-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50343187

(CHEMBL1773155 | N,N'-Bis(3-aminopropyl)-3,9-dimeth...)Show SMILES Cc1cc(NCCCN)c2ccc3c(ccc4c(NCCCN)cc(C)nc34)c2n1 Show InChI InChI=1S/C24H30N6/c1-15-13-21(27-11-3-9-25)19-7-6-18-17(23(19)29-15)5-8-20-22(28-12-4-10-26)14-16(2)30-24(18)20/h5-8,13-14H,3-4,9-12,25-26H2,1-2H3,(H,27,29)(H,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM |
J Med Chem 54: 1157-69 (2011)
Article DOI: 10.1021/jm100938u
BindingDB Entry DOI: 10.7270/Q26T0MZR |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50343188

(CHEMBL1773156 | N,N'-Bis(2-aminoethyl)-3,9-dimethy...)Show SMILES Cc1cc(NCCN)c2ccc3c(ccc4c(NCCN)cc(C)nc34)c2n1 Show InChI InChI=1S/C22H26N6/c1-13-11-19(25-9-7-23)17-5-4-16-15(21(17)27-13)3-6-18-20(26-10-8-24)12-14(2)28-22(16)18/h3-6,11-12H,7-10,23-24H2,1-2H3,(H,25,27)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of clostridium botulinum Botulinum neurotoxin type A light chain at 20 uM |
J Med Chem 54: 1157-69 (2011)
Article DOI: 10.1021/jm100938u
BindingDB Entry DOI: 10.7270/Q26T0MZR |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50013644
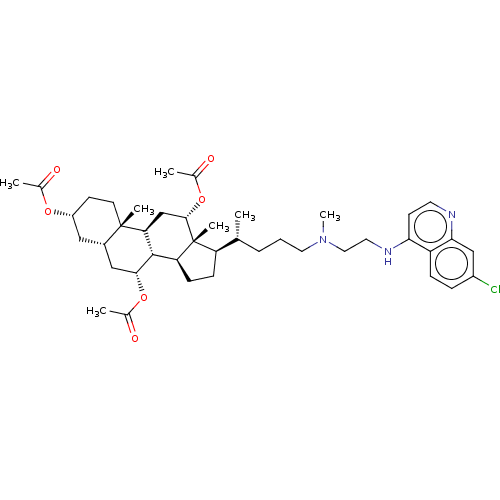
(CHEMBL3264182)Show SMILES [H][C@@]1(CC[C@@]2([H])[C@]3([H])[C@@H](C[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])C[C@H](OC(C)=O)[C@]12C)OC(C)=O)OC(C)=O)[C@H](C)CCCN(C)CCNc1ccnc2cc(Cl)ccc12 |r| Show InChI InChI=1S/C42H60ClN3O6/c1-25(9-8-19-46(7)20-18-45-36-15-17-44-37-23-30(43)10-11-32(36)37)33-12-13-34-40-35(24-39(42(33,34)6)52-28(4)49)41(5)16-14-31(50-26(2)47)21-29(41)22-38(40)51-27(3)48/h10-11,15,17,23,25,29,31,33-35,38-40H,8-9,12-14,16,18-22,24H2,1-7H3,(H,44,45)/t25-,29+,31-,33-,34+,35+,38-,39+,40+,41+,42-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50067697
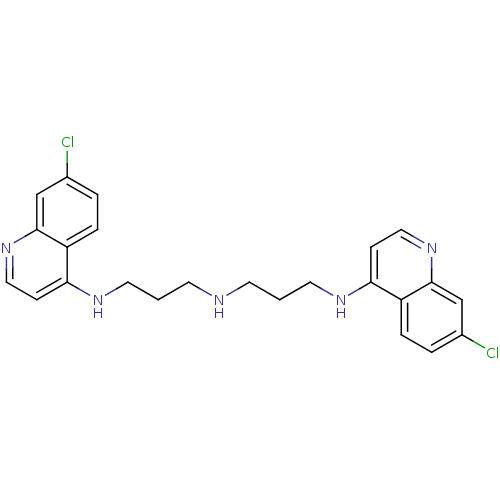
(7-chloro-N-(3-(3-(7-chloroquinolin-4-ylamino)propy...)Show SMILES Clc1ccc2c(NCCCNCCCNc3ccnc4cc(Cl)ccc34)ccnc2c1 Show InChI InChI=1S/C24H25Cl2N5/c25-17-3-5-19-21(7-13-30-23(19)15-17)28-11-1-9-27-10-2-12-29-22-8-14-31-24-16-18(26)4-6-20(22)24/h3-8,13-16,27H,1-2,9-12H2,(H,28,30)(H,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum BoNT/A light chain |
J Med Chem 56: 5860-71 (2014)
Article DOI: 10.1021/jm4006077
BindingDB Entry DOI: 10.7270/Q2QF8V8K |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378451
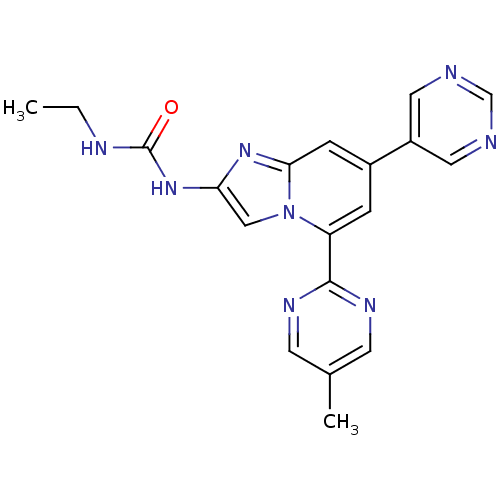
(CHEMBL578362)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cncnc1)-c1ncc(C)cn1 Show InChI InChI=1S/C19H18N8O/c1-3-22-19(28)26-16-10-27-15(18-23-6-12(2)7-24-18)4-13(5-17(27)25-16)14-8-20-11-21-9-14/h4-11H,3H2,1-2H3,(H2,22,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50423645
(Albamycin | Cathomycin | NOVOBIOCIN)Show SMILES [#6]-[#8]-[#6@@H]1-[#6@@H](-[#8]-[#6](-[#7])=O)-[#6@H](-[#8])-[#6@@H](-[#8]-c2ccc3c(-[#8])c(-[#7]-[#6](=O)-c4ccc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c4)c(=O)oc3c2-[#6])-[#8]C1([#6])[#6] Show InChI InChI=1S/C31H36N2O11/c1-14(2)7-8-16-13-17(9-11-19(16)34)27(37)33-21-22(35)18-10-12-20(15(3)24(18)42-28(21)38)41-29-23(36)25(43-30(32)39)26(40-6)31(4,5)44-29/h7,9-13,23,25-26,29,34-36H,8H2,1-6H3,(H2,32,39)(H,33,37)/t23-,25-,26+,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378443

(CHEMBL568020)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1ccc(C)nc1)-c1ncccn1 Show InChI InChI=1S/C20H19N7O/c1-3-21-20(28)26-17-12-27-16(19-22-7-4-8-23-19)9-15(10-18(27)25-17)14-6-5-13(2)24-11-14/h4-12H,3H2,1-2H3,(H2,21,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378425
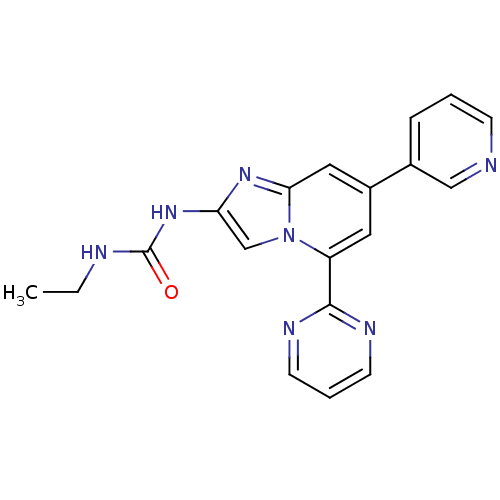
(CHEMBL565765)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncccn1 Show InChI InChI=1S/C19H17N7O/c1-2-21-19(27)25-16-12-26-15(18-22-7-4-8-23-18)9-14(10-17(26)24-16)13-5-3-6-20-11-13/h3-12H,2H2,1H3,(H2,21,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378420

(CHEMBL565750)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1ccn(CC)c(=O)c1)-c1ncc(C)cn1 Show InChI InChI=1S/C22H23N7O2/c1-4-23-22(31)27-18-13-29-17(21-24-11-14(3)12-25-21)8-16(9-19(29)26-18)15-6-7-28(5-2)20(30)10-15/h6-13H,4-5H2,1-3H3,(H2,23,27,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378433

(CHEMBL584265)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1nccc(n1)C(=O)OC Show InChI InChI=1S/C21H19N7O3/c1-3-23-21(30)27-17-12-28-16(19-24-8-6-15(25-19)20(29)31-2)9-14(10-18(28)26-17)13-5-4-7-22-11-13/h4-12H,3H2,1-2H3,(H2,23,27,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378426
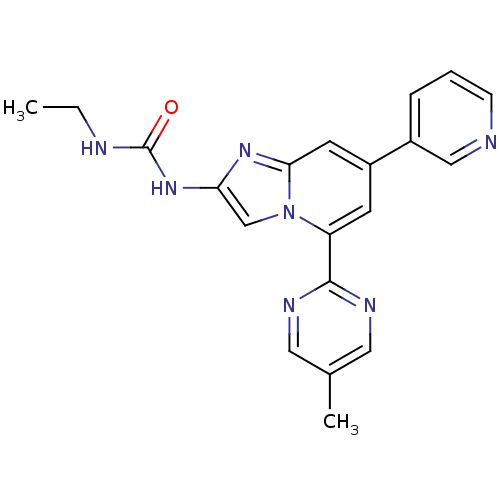
(CHEMBL577478)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(C)cn1 Show InChI InChI=1S/C20H19N7O/c1-3-22-20(28)26-17-12-27-16(19-23-9-13(2)10-24-19)7-15(8-18(27)25-17)14-5-4-6-21-11-14/h4-12H,3H2,1-2H3,(H2,22,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378429

(CHEMBL583049)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(F)cn1 Show InChI InChI=1S/C19H16FN7O/c1-2-22-19(28)26-16-11-27-15(18-23-9-14(20)10-24-18)6-13(7-17(27)25-16)12-4-3-5-21-8-12/h3-11H,2H2,1H3,(H2,22,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378450
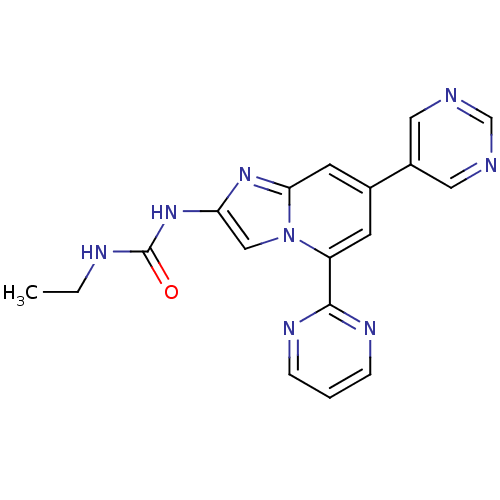
(CHEMBL578361)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cncnc1)-c1ncccn1 Show InChI InChI=1S/C18H16N8O/c1-2-21-18(27)25-15-10-26-14(17-22-4-3-5-23-17)6-12(7-16(26)24-15)13-8-19-11-20-9-13/h3-11H,2H2,1H3,(H2,21,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378438
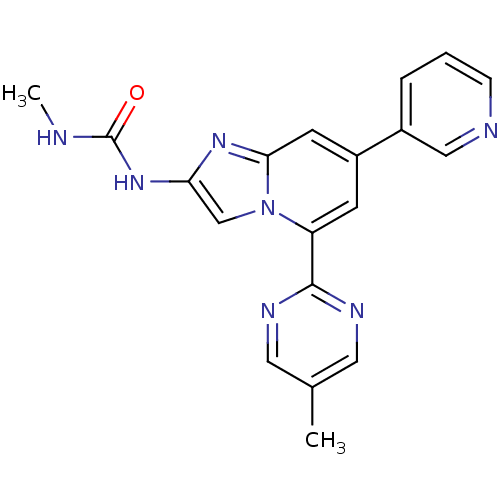
(CHEMBL577053)Show SMILES CNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(C)cn1 Show InChI InChI=1S/C19H17N7O/c1-12-8-22-18(23-9-12)15-6-14(13-4-3-5-21-10-13)7-17-24-16(11-26(15)17)25-19(27)20-2/h3-11H,1-2H3,(H2,20,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378444

(CHEMBL568021)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1ccc(C)nc1)-c1ncc(C)cn1 Show InChI InChI=1S/C21H21N7O/c1-4-22-21(29)27-18-12-28-17(20-24-9-13(2)10-25-20)7-16(8-19(28)26-18)15-6-5-14(3)23-11-15/h5-12H,4H2,1-3H3,(H2,22,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378421

(CHEMBL578149)Show SMILES CCCn1ccc(cc1=O)-c1cc(-c2ncc(C)cn2)n2cc(NC(=O)NCC)nc2c1 Show InChI InChI=1S/C23H25N7O2/c1-4-7-29-8-6-16(11-21(29)31)17-9-18(22-25-12-15(3)13-26-22)30-14-19(27-20(30)10-17)28-23(32)24-5-2/h6,8-14H,4-5,7H2,1-3H3,(H2,24,28,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378423

(CHEMBL583440)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1ccn(CC(O)=O)c(=O)c1)-c1ncc(C)cn1 Show InChI InChI=1S/C22H21N7O4/c1-3-23-22(33)27-17-11-29-16(21-24-9-13(2)10-25-21)6-15(7-18(29)26-17)14-4-5-28(12-20(31)32)19(30)8-14/h4-11H,3,12H2,1-2H3,(H,31,32)(H2,23,27,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378447
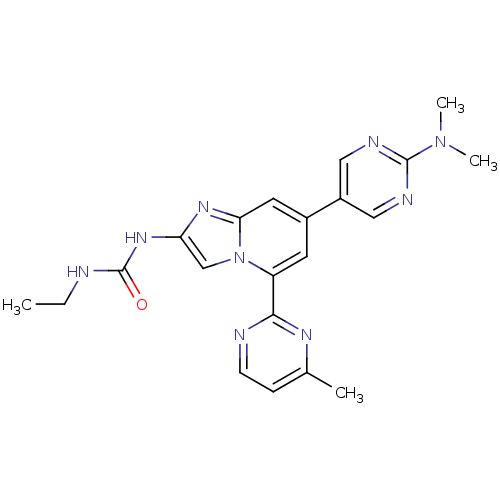
(CHEMBL577909)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cnc(nc1)N(C)C)-c1nccc(C)n1 Show InChI InChI=1S/C21H23N9O/c1-5-22-21(31)28-17-12-30-16(19-23-7-6-13(2)26-19)8-14(9-18(30)27-17)15-10-24-20(25-11-15)29(3)4/h6-12H,5H2,1-4H3,(H2,22,28,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378445
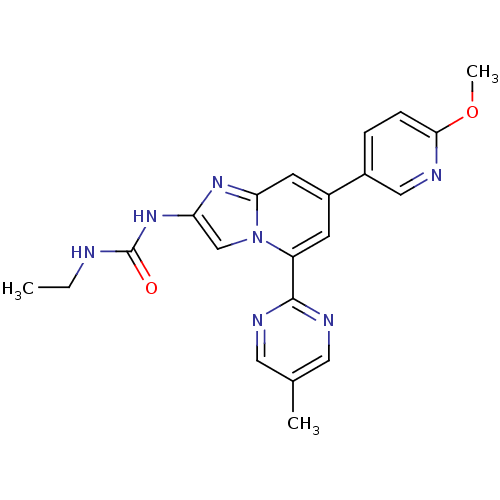
(CHEMBL565776)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1ccc(OC)nc1)-c1ncc(C)cn1 Show InChI InChI=1S/C21H21N7O2/c1-4-22-21(29)27-17-12-28-16(20-24-9-13(2)10-25-20)7-15(8-18(28)26-17)14-5-6-19(30-3)23-11-14/h5-12H,4H2,1-3H3,(H2,22,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 117 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase (ATP-hydrolyzing)
(Streptococcus pneumoniae) | BDBM50378443

(CHEMBL568020)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1ccc(C)nc1)-c1ncccn1 Show InChI InChI=1S/C20H19N7O/c1-3-21-20(28)26-17-12-27-16(19-22-7-4-8-23-19)9-15(10-18(27)25-17)14-6-5-13(2)24-11-14/h4-12H,3H2,1-2H3,(H2,21,26,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 119 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 parE after 30 mins |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378452
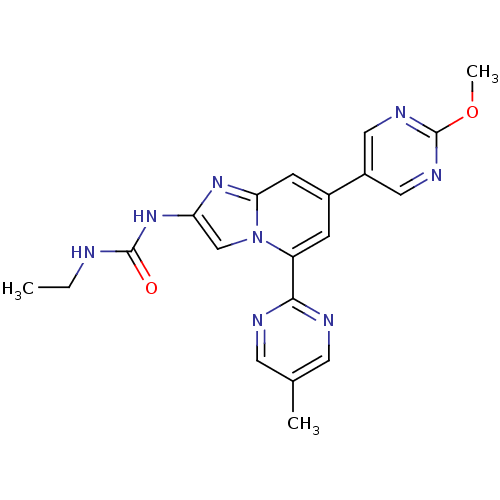
(CHEMBL583870)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cnc(OC)nc1)-c1ncc(C)cn1 Show InChI InChI=1S/C20H20N8O2/c1-4-21-19(29)27-16-11-28-15(18-22-7-12(2)8-23-18)5-13(6-17(28)26-16)14-9-24-20(30-3)25-10-14/h5-11H,4H2,1-3H3,(H2,21,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 132 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378448

(CHEMBL577041)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cnc(nc1)N(C)C)-c1ncccn1 Show InChI InChI=1S/C20H21N9O/c1-4-21-20(30)27-16-12-29-15(18-22-6-5-7-23-18)8-13(9-17(29)26-16)14-10-24-19(25-11-14)28(2)3/h5-12H,4H2,1-3H3,(H2,21,27,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 137 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase (ATP-hydrolyzing)
(Streptococcus pneumoniae) | BDBM50378445

(CHEMBL565776)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1ccc(OC)nc1)-c1ncc(C)cn1 Show InChI InChI=1S/C21H21N7O2/c1-4-22-21(29)27-17-12-28-16(20-24-9-13(2)10-25-20)7-15(8-18(28)26-17)14-5-6-19(30-3)23-11-14/h5-12H,4H2,1-3H3,(H2,22,27,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 147 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 parE after 30 mins |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378430

(CHEMBL568019)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(Cl)cn1 Show InChI InChI=1S/C19H16ClN7O/c1-2-22-19(28)26-16-11-27-15(18-23-9-14(20)10-24-18)6-13(7-17(27)25-16)12-4-3-5-21-8-12/h3-11H,2H2,1H3,(H2,22,26,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
Botulinum neurotoxin type A
(Clostridium botulinum) | BDBM50447108

(CHEMBL3112881)Show InChI InChI=1S/C11H7ClFNO2S/c12-11-7-2-1-6(13)5-9(7)17-8(11)3-4-10(15)14-16/h1-5,16H,(H,14,15)/b4-3+ | PDB
MMDB
NCI pathway
Reactome pathway
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Belgrade
Curated by ChEMBL
| Assay Description
Inhibition of Clostridium botulinum recombinant BoNT/A light chain using N-terminal acetylated, C-terminal aminated SNAP-25 (187-203) as substrate by... |
J Med Chem 57: 4134-53 (2014)
Article DOI: 10.1021/jm500033r
BindingDB Entry DOI: 10.7270/Q20Z74TB |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378427
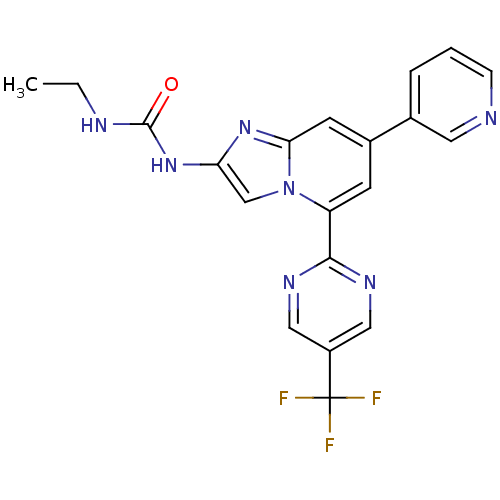
(CHEMBL565572)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(cn1)C(F)(F)F Show InChI InChI=1S/C20H16F3N7O/c1-2-25-19(31)29-16-11-30-15(18-26-9-14(10-27-18)20(21,22)23)6-13(7-17(30)28-16)12-4-3-5-24-8-12/h3-11H,2H2,1H3,(H2,25,29,31) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 163 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378435
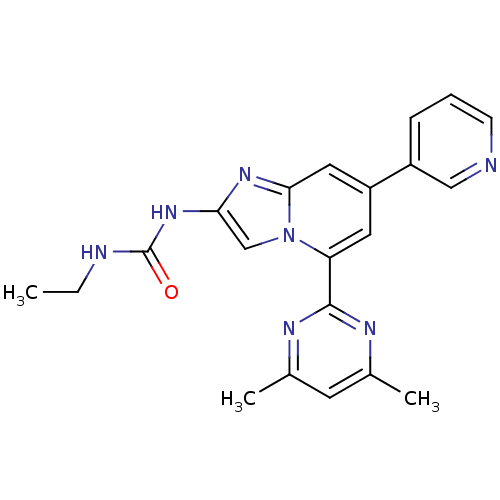
(CHEMBL578336)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1nc(C)cc(C)n1 Show InChI InChI=1S/C21H21N7O/c1-4-23-21(29)27-18-12-28-17(20-24-13(2)8-14(3)25-20)9-16(10-19(28)26-18)15-6-5-7-22-11-15/h5-12H,4H2,1-3H3,(H2,23,27,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378436
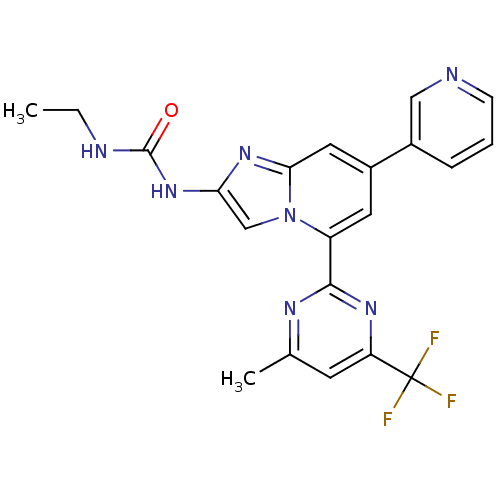
(CHEMBL578991)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1nc(C)cc(n1)C(F)(F)F Show InChI InChI=1S/C21H18F3N7O/c1-3-26-20(32)30-17-11-31-15(19-27-12(2)7-16(28-19)21(22,23)24)8-14(9-18(31)29-17)13-5-4-6-25-10-13/h4-11H,3H2,1-2H3,(H2,26,30,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378437
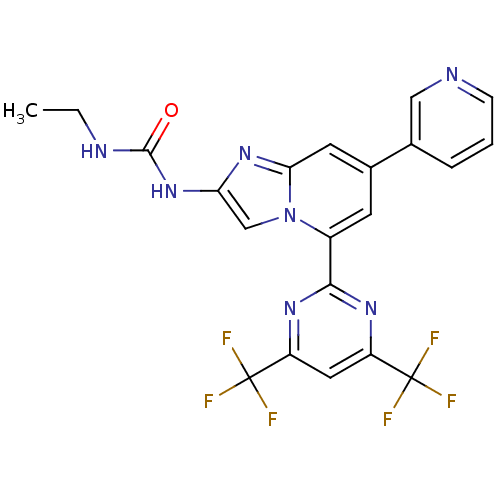
(CHEMBL578992)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1nc(cc(n1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C21H15F6N7O/c1-2-29-19(35)33-16-10-34-13(6-12(7-17(34)32-16)11-4-3-5-28-9-11)18-30-14(20(22,23)24)8-15(31-18)21(25,26)27/h3-10H,2H2,1H3,(H2,29,33,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 242 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378453

(CHEMBL567804)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cnc(nc1)N1CCCC1)-c1ncc(C)cn1 Show InChI InChI=1S/C23H25N9O/c1-3-24-23(33)30-19-14-32-18(21-25-10-15(2)11-26-21)8-16(9-20(32)29-19)17-12-27-22(28-13-17)31-6-4-5-7-31/h8-14H,3-7H2,1-2H3,(H2,24,30,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 244 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase (ATP-hydrolyzing)
(Streptococcus pneumoniae) | BDBM50378426

(CHEMBL577478)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(C)cn1 Show InChI InChI=1S/C20H19N7O/c1-3-22-20(28)26-17-12-27-16(19-23-9-13(2)10-24-19)7-15(8-18(27)25-17)14-5-4-6-21-11-14/h4-12H,3H2,1-2H3,(H2,22,26,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 parE after 30 mins |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378439

(CHEMBL565741)Show SMILES CCC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(C)cn1 Show InChI InChI=1S/C20H18N6O/c1-3-19(27)25-17-12-26-16(20-22-9-13(2)10-23-20)7-15(8-18(26)24-17)14-5-4-6-21-11-14/h4-12H,3H2,1-2H3,(H,25,27) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 255 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase (ATP-hydrolyzing)
(Streptococcus pneumoniae) | BDBM50378451

(CHEMBL578362)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cncnc1)-c1ncc(C)cn1 Show InChI InChI=1S/C19H18N8O/c1-3-22-19(28)26-16-10-27-15(18-23-6-12(2)7-24-18)4-13(5-17(27)25-16)14-8-20-11-21-9-14/h4-11H,3H2,1-2H3,(H2,22,26,28) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 258 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 parE after 30 mins |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA gyrase subunit B
(Streptococcus pneumoniae) | BDBM50378441
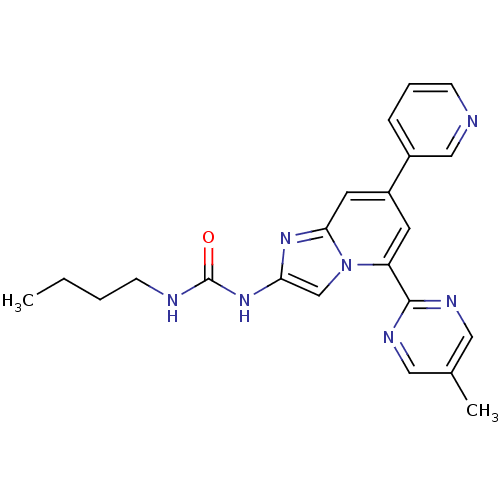
(CHEMBL578138)Show SMILES CCCCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(C)cn1 Show InChI InChI=1S/C22H23N7O/c1-3-4-8-24-22(30)28-19-14-29-18(21-25-11-15(2)12-26-21)9-17(10-20(29)27-19)16-6-5-7-23-13-16/h5-7,9-14H,3-4,8H2,1-2H3,(H2,24,28,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 261 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 gyrB by enzyme coupled phosphate assay |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |
DNA topoisomerase (ATP-hydrolyzing)
(Streptococcus pneumoniae) | BDBM50378428

(CHEMBL578120)Show SMILES CCNC(=O)Nc1cn2c(cc(cc2n1)-c1cccnc1)-c1ncc(CC)cn1 Show InChI InChI=1S/C21H21N7O/c1-3-14-10-24-20(25-11-14)17-8-16(15-6-5-7-22-12-15)9-19-26-18(13-28(17)19)27-21(29)23-4-2/h5-13H,3-4H2,1-2H3,(H2,23,27,29) | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 265 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Streptococcus pneumoniae SP-3 parE after 30 mins |
Bioorg Med Chem Lett 19: 5302-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.141
BindingDB Entry DOI: 10.7270/Q23X87KD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data