

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neprilysin (Homo sapiens (Human)) | BDBM50354041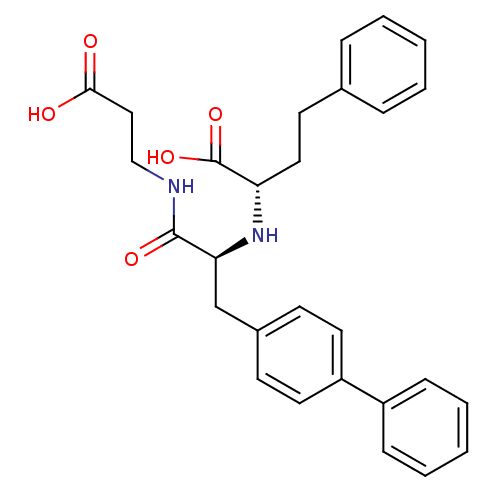 (CHEMBL1829584) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354042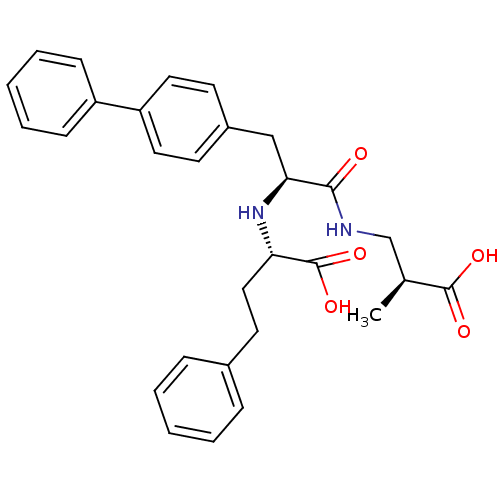 (CHEMBL1829585) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50251742 ((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182852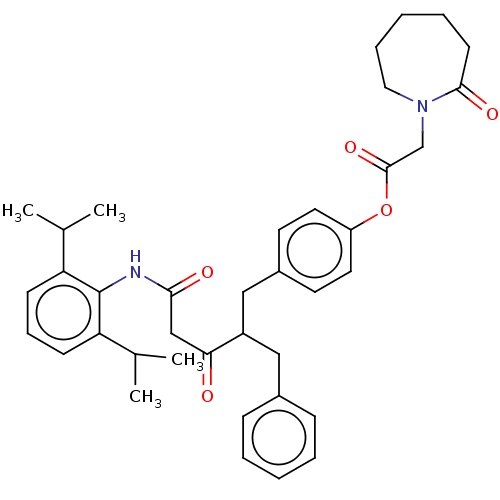 (US9149492, Compound 1D) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182851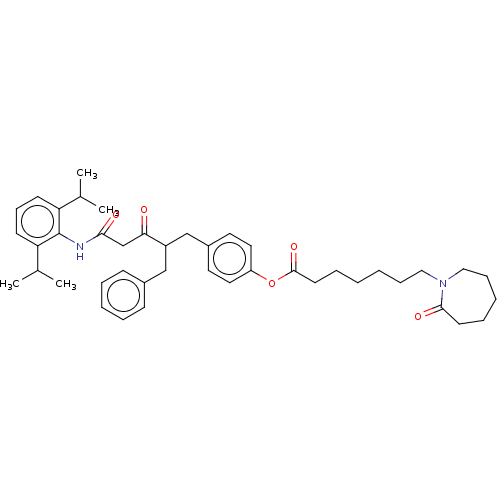 (US9149492, Compound 1C) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182849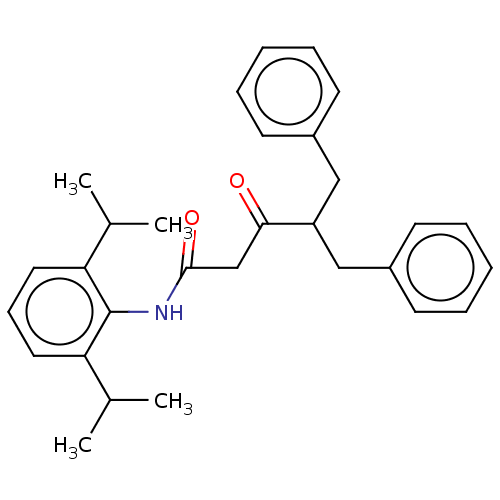 (US9149492, Compound 1A) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354040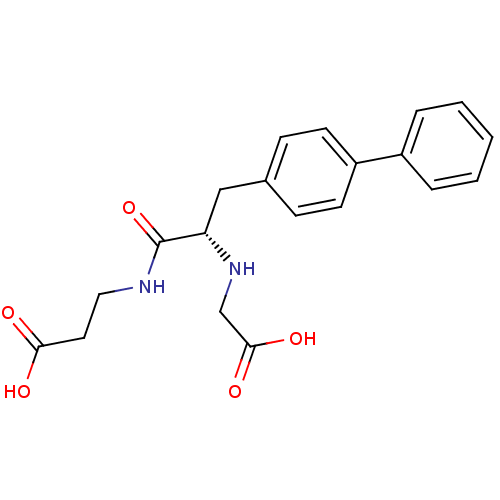 (CHEMBL1829583) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182850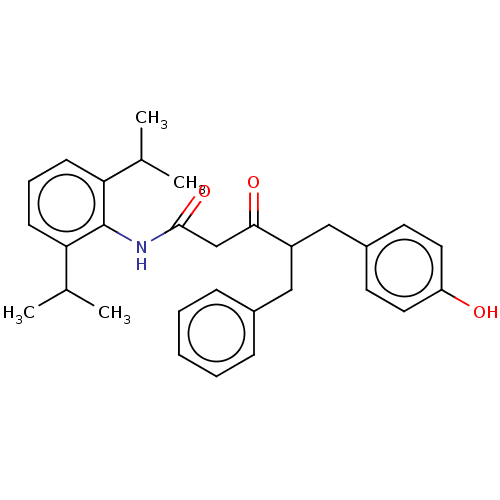 (US9149492, Compound 1B) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182848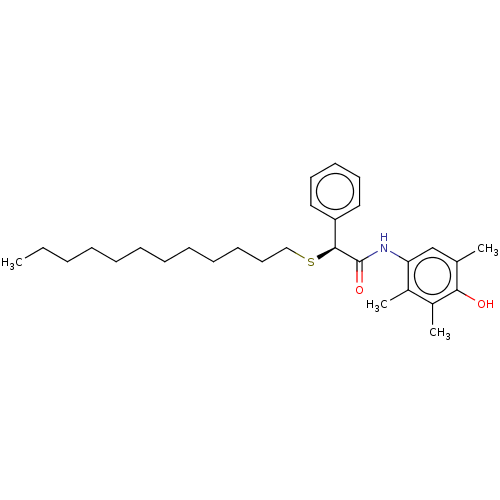 (US9149492, Eflucimibe (F12511)) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182848 (US9149492, Eflucimibe (F12511)) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182849 (US9149492, Compound 1A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354044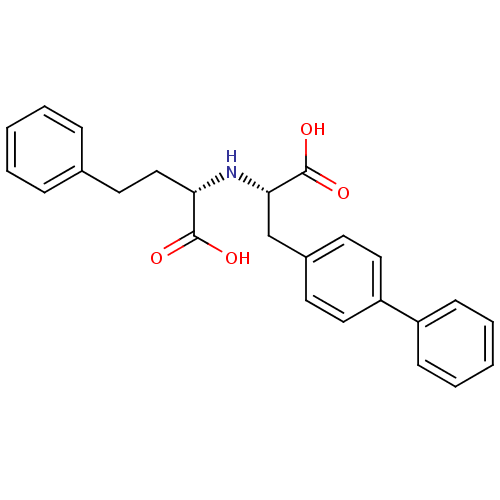 (CHEMBL1829587) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354043 (CHEMBL1829586) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182847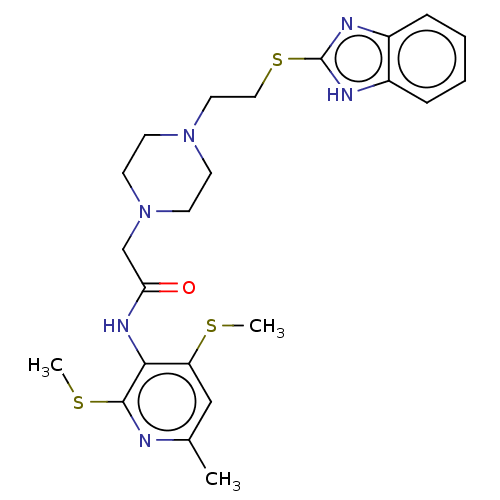 (US9149492, K-604) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182851 (US9149492, Compound 1C) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50360304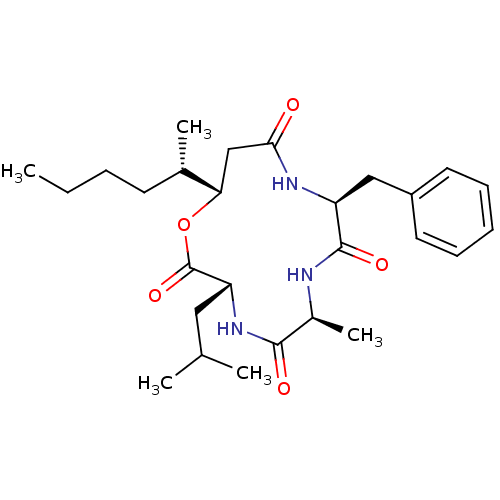 (CHEMBL516093 | US9149492, Beauveriolide I) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182852 (US9149492, Compound 1D) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50360305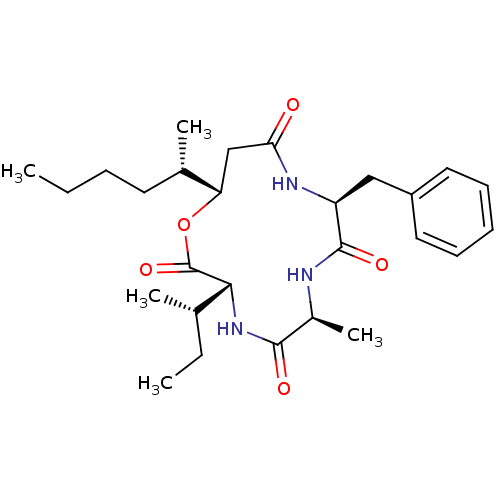 (CHEMBL409855 | US9149492, Beauveriolide III) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182850 (US9149492, Compound 1B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM50360304 (CHEMBL516093 | US9149492, Beauveriolide I) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM50360305 (CHEMBL409855 | US9149492, Beauveriolide III) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182854 (US9149492, 14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182853 (US9149492, 13) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM182855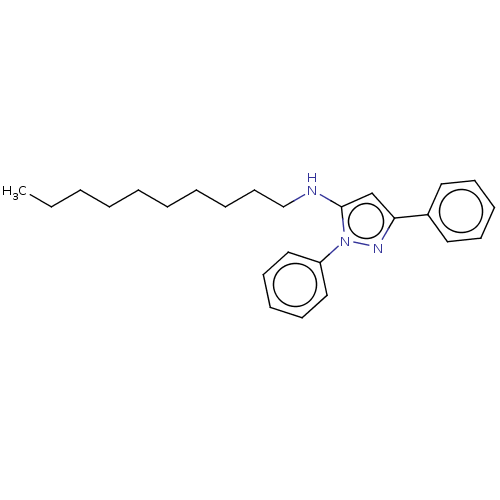 (US9149492, 16) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50354039 (CHEMBL1829582) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Kao Corporation Curated by ChEMBL | Assay Description Inhibition of neutral endopeptidase in human fibroblasts homogenates using glutaryl-Ala-Ala-Phe-4-methoxy-2-naphtylamide as substrate after 1 hrs by ... | Bioorg Med Chem 19: 5935-47 (2011) Article DOI: 10.1016/j.bmc.2011.08.064 BindingDB Entry DOI: 10.7270/Q2154HF6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182853 (US9149492, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50371698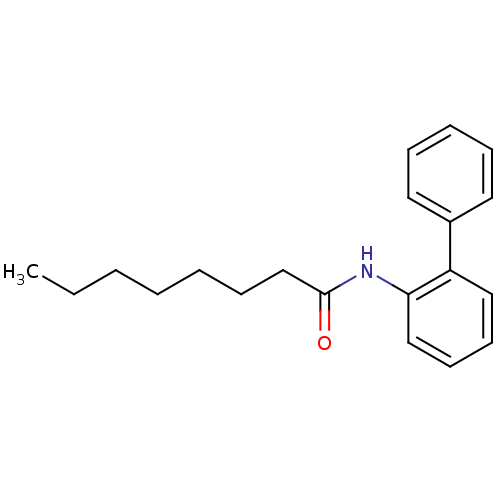 (CHEMBL408322 | US9149492, 1) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 1 (Homo sapiens (Human)) | BDBM50371699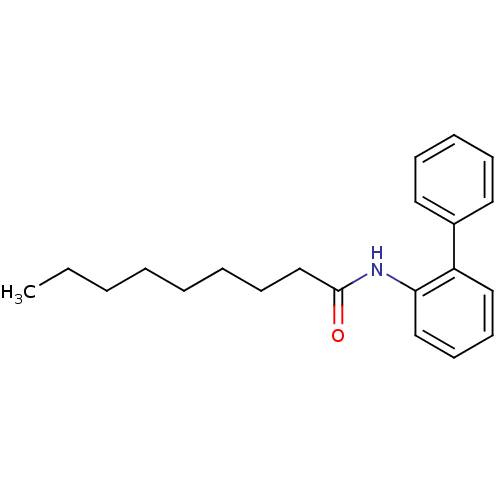 (CHEMBL270041 | US9149492, 2) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182854 (US9149492, 14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182855 (US9149492, 16) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM182847 (US9149492, K-604) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM50371698 (CHEMBL408322 | US9149492, 1) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sterol O-acyltransferase 2 (Homo sapiens (Human)) | BDBM50371699 (CHEMBL270041 | US9149492, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.14E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Trustees of Dartmouth College US Patent | Assay Description Methods for assessing the selectively of ACAT1 inhibitors are known in the art and can be based upon any conventional assay including, but not limite... | US Patent US9149492 (2015) BindingDB Entry DOI: 10.7270/Q2N015BF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269673 (CHEMBL1909927) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269674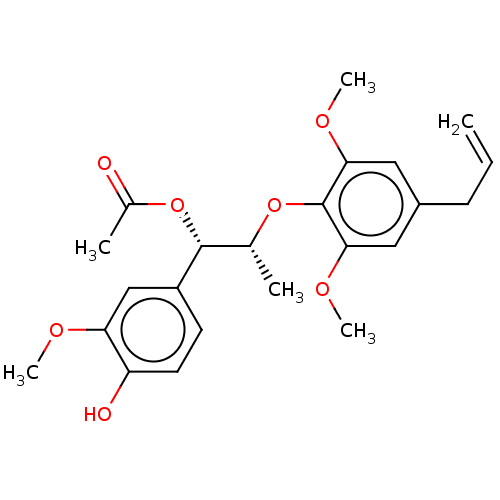 (CHEMBL4065676) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269675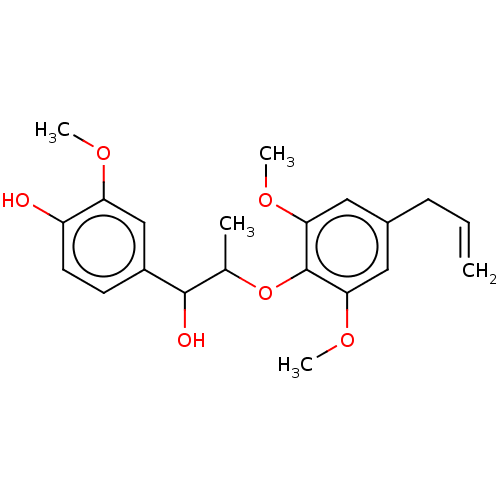 (CHEMBL4087839) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269676 (CHEMBL4074380) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 87 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269678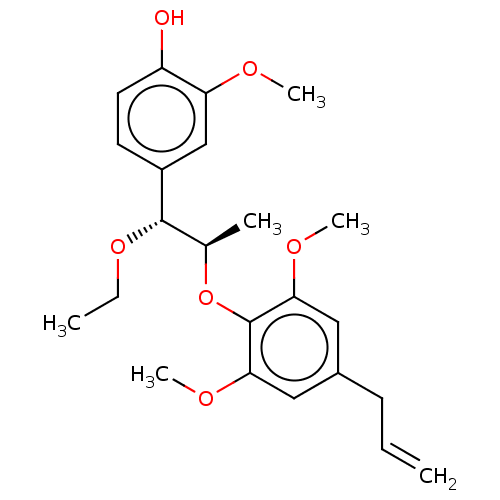 (CHEMBL4092140) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 583 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269680 (CHEMBL4073694) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269681 (CHEMBL4077565) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 66 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269682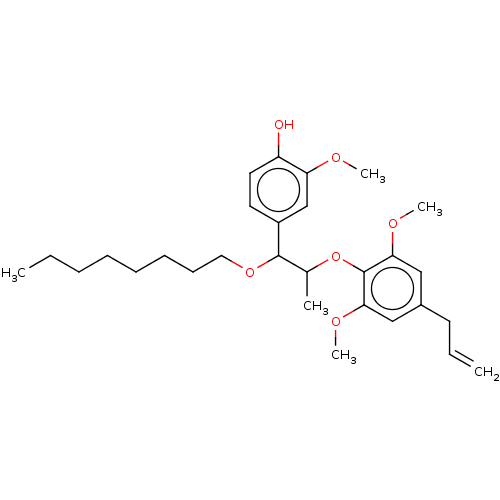 (CHEMBL4076994) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 86 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269683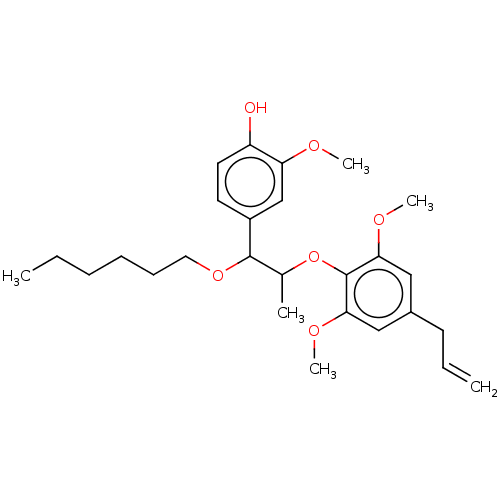 (CHEMBL4084074) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 329 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269672 (CHEMBL463097) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269659 (CHEMBL4069984) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 332 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50318482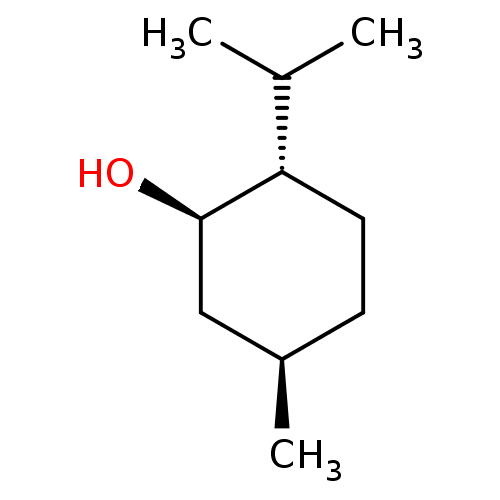 ((-)-menthol | CHEMBL470670 | MENTHOL | US11406649,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 1.01E+4 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269671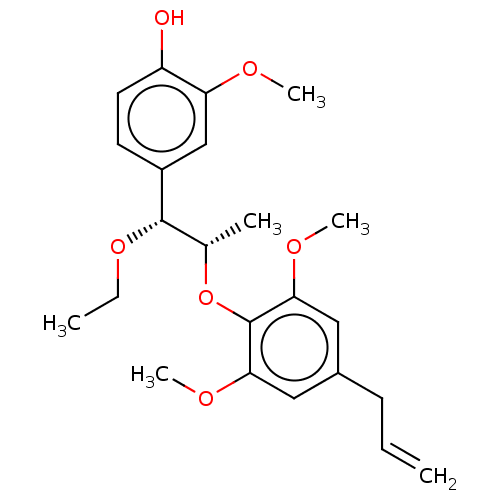 (CHEMBL4084423) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily A member 1 (Homo sapiens (Human)) | BDBM50318482 ((-)-menthol | CHEMBL470670 | MENTHOL | US11406649,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at recombinant human TRPA1 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for 5 mins by Fl... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269670 (CHEMBL4072058) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 153 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269669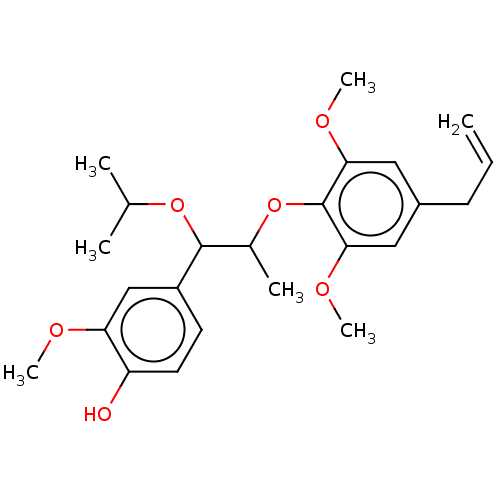 (CHEMBL4065256) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 82 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transient receptor potential cation channel subfamily M member 8 (Homo sapiens (Human)) | BDBM50269664 (CHEMBL4076657) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 54 | n/a | n/a | n/a | n/a |
Kansei Science Research and Biological Science Research, Kao Corporation, 2606 Akabane, Ichikai-Machi, Haga-Gun, Tochigi 321-3497, Japan. Curated by ChEMBL | Assay Description Agonist activity at full length recombinant human TRPM8 expressed in HEK293 cells assessed as increase in intracellular Ca2+ level pre-incubated for ... | ACS Med Chem Lett 8: 715-719 (2017) Article DOI: 10.1021/acsmedchemlett.7b00104 BindingDB Entry DOI: 10.7270/Q2CZ39N2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 55 total ) | Next | Last >> |