

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Procathepsin L (Homo sapiens (Human)) | BDBM50121305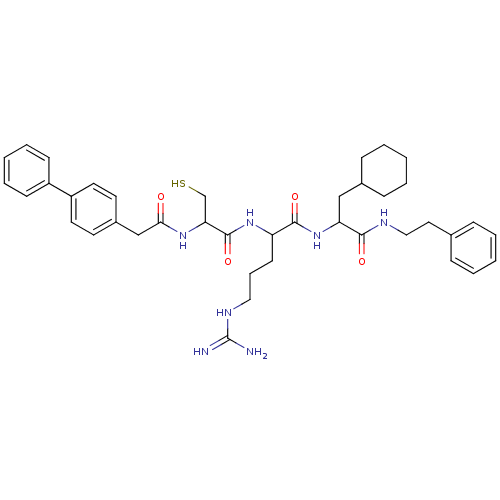 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121291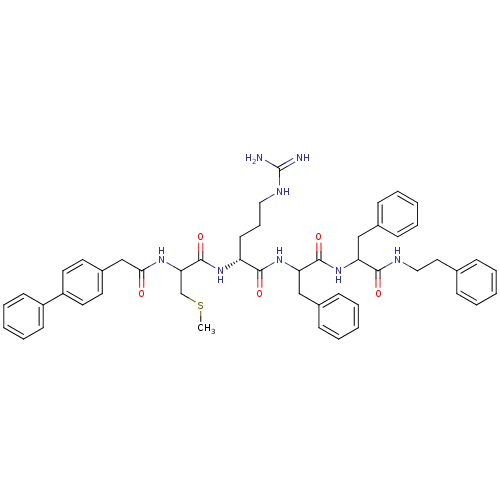 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulfany...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM21006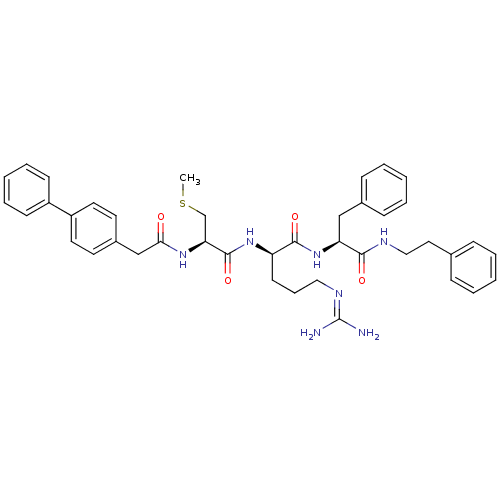 ((2R)-5-[(diaminomethylidene)amino]-2-[(2R)-3-(meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 19 | -44.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121299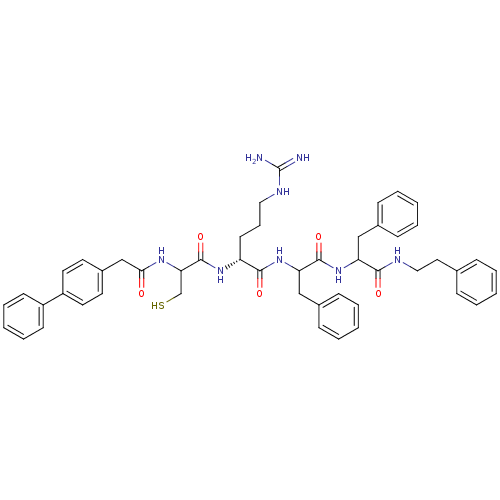 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM20998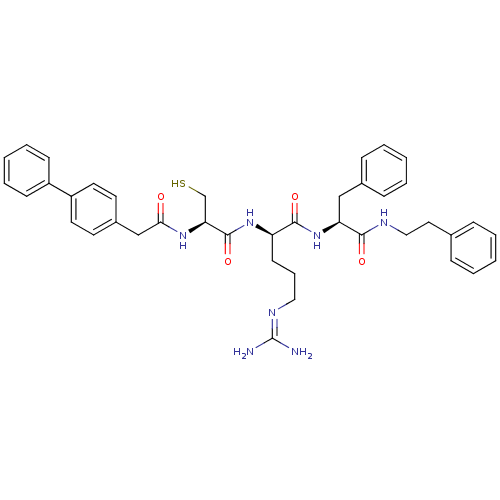 ((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 21 | -43.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM20997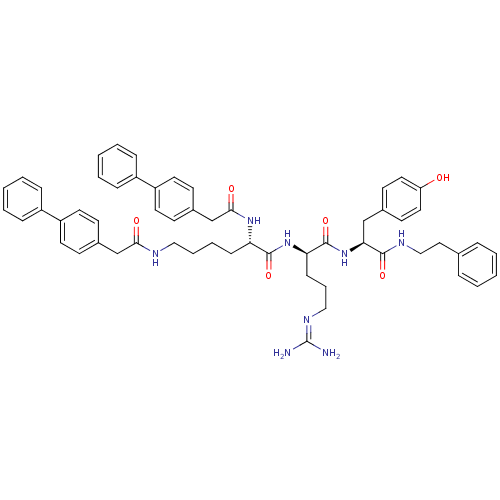 ((2S)-N-[(1R)-4-[(diaminomethylidene)amino]-1-{[(1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 23 | -43.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM20999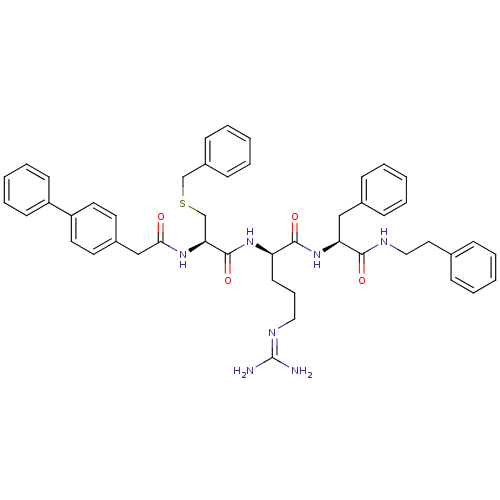 ((2R)-2-[(2R)-3-(benzylsulfanyl)-2-[1-(4-phenylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121290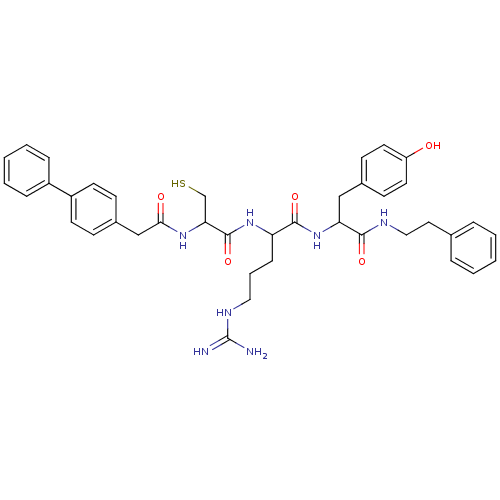 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM20993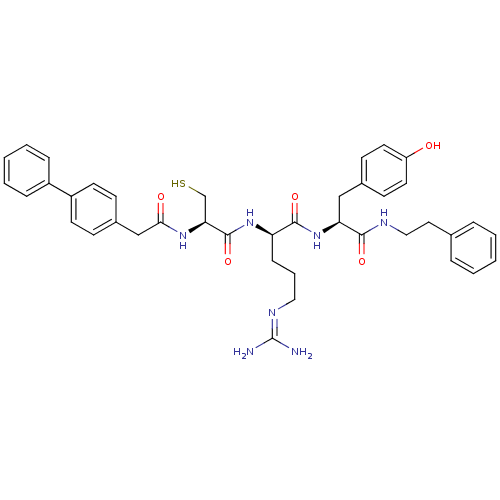 ((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG MMDB PC cid PC sid UniChem Similars | MMDB Article PubMed | 45 | -41.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121286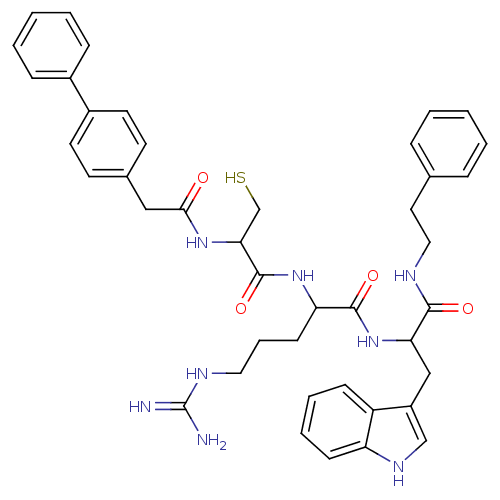 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM20996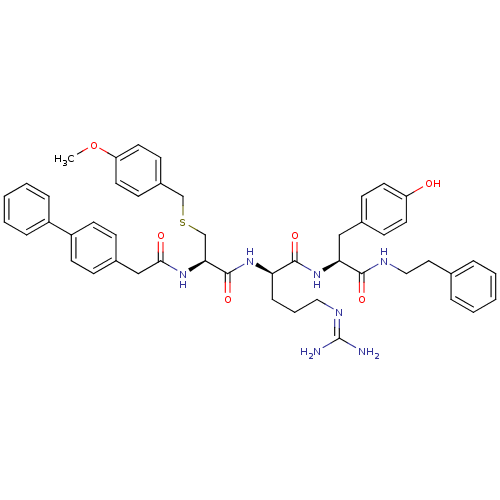 ((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 112 | -39.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM20995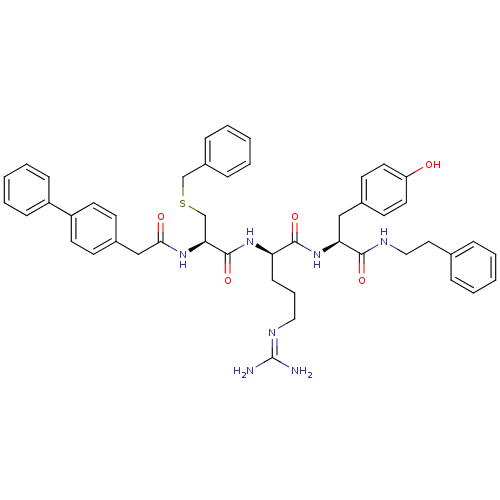 ((2R)-2-[(2R)-3-(benzylsulfanyl)-2-[1-(4-phenylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 155 | -38.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121298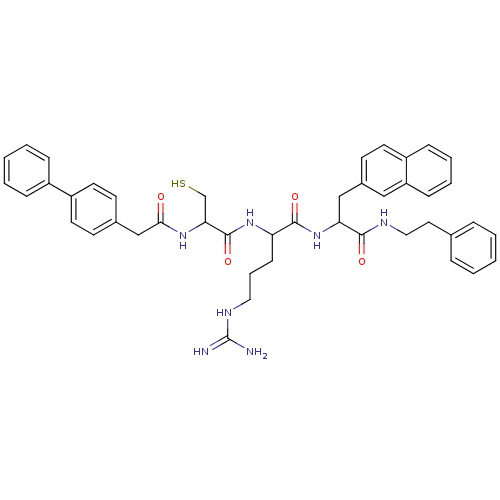 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121300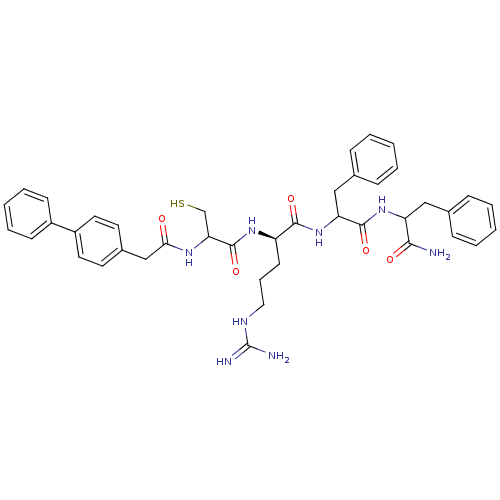 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121300 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121301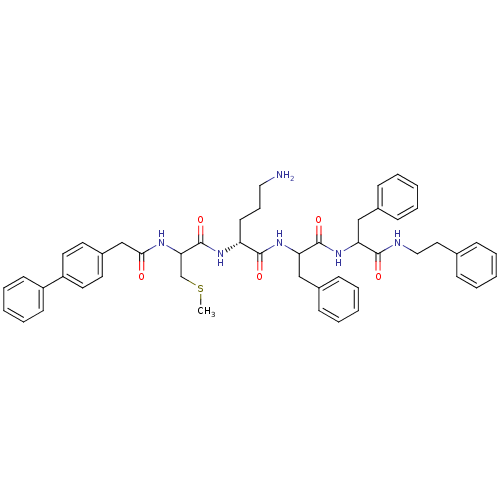 (5-Amino-2-[2-(2-biphenyl-4-yl-acetylamino)-3-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121292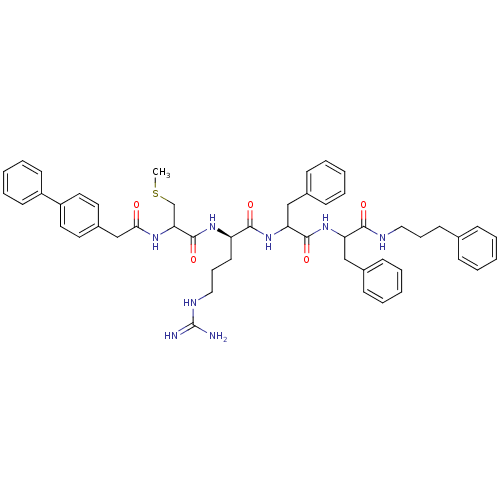 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulfany...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121288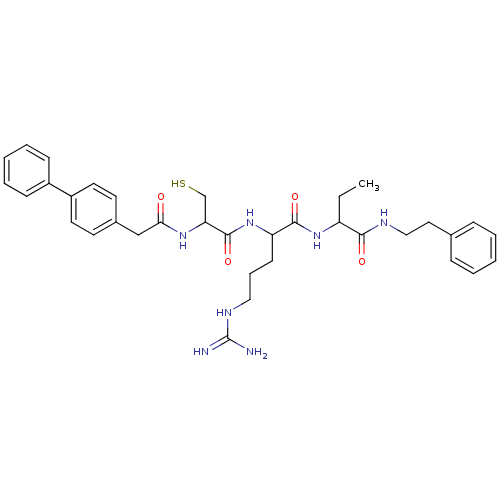 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121295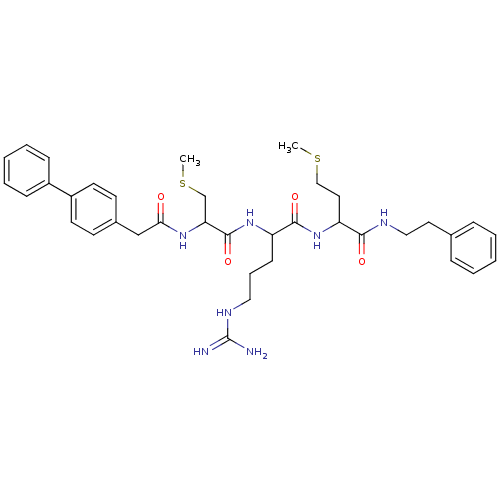 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulfany...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121293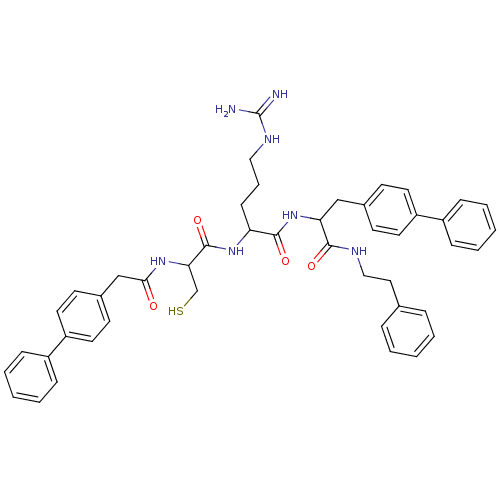 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121289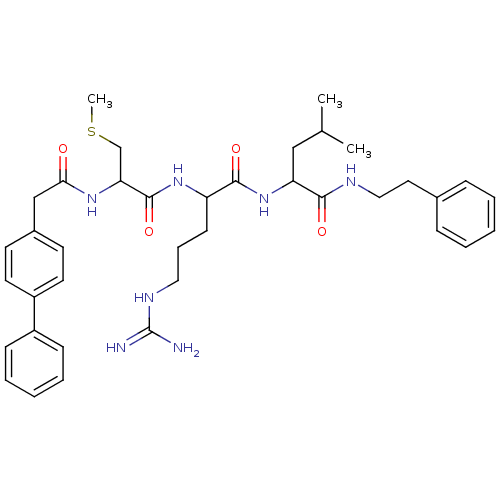 (2-{2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM21005 ((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 460 | -36.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM21000 ((2R)-5-[(diaminomethylidene)amino]-2-[(2R)-3-{[(4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 464 | -36.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121287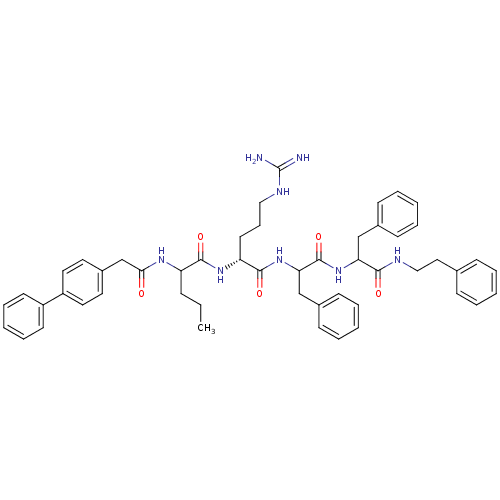 (2-[2-(2-Biphenyl-4-yl-acetylamino)-pentanoylamino]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM21001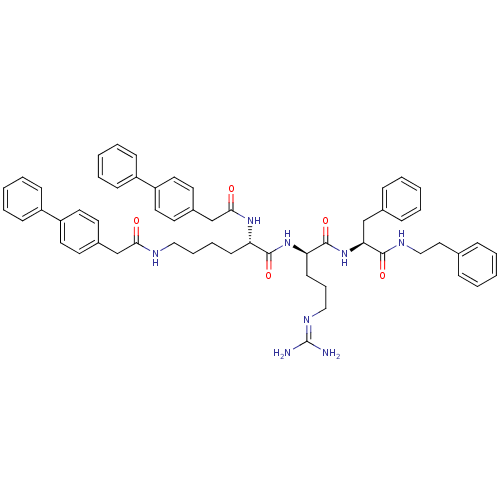 ((2S)-N-[(1R)-4-[(diaminomethylidene)amino]-1-{[(1S...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 511 | -35.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50121286 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Specificity of Cathepsin B inhibition by the compound | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121302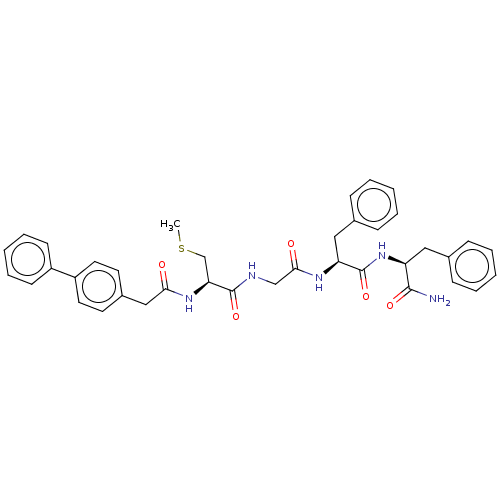 (2-(2-{2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methyls...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50121286 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Specificity of Cathepsin K inhibition by the compound | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50121290 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Specificity of Cathepsin K inhibition by the compound | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121297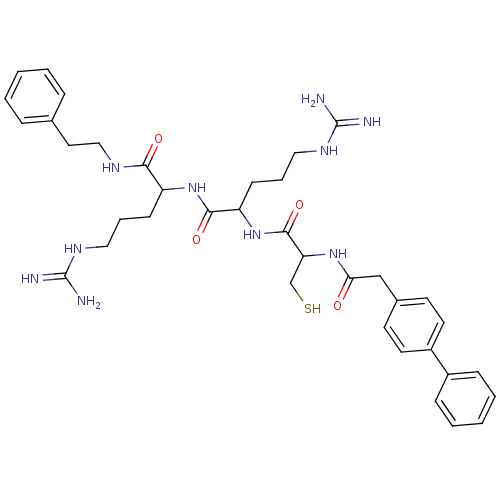 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50121291 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulfany...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Specificity of Cathepsin B inhibition by the compound | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50121291 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulfany...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Specificity of Cathepsin K inhibition by the compound | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM21002 ((2R)-5-[(diaminomethylidene)amino]-N-[(1S)-2-(4-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121303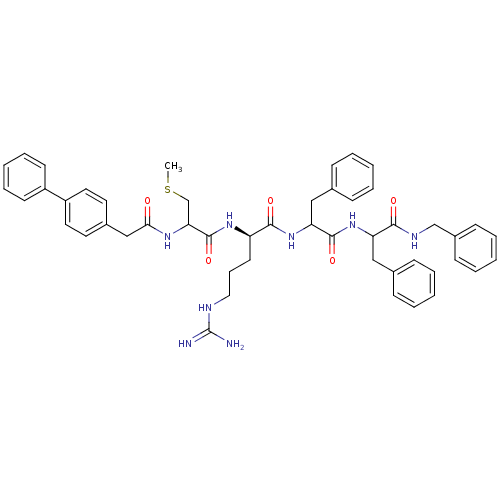 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-methylsulfany...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM21003 ((2R)-5-[(diaminomethylidene)amino]-2-[(2S)-2-forma...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.07E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50121290 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-mercapto-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Specificity of Cathepsin B inhibition by the compound | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121294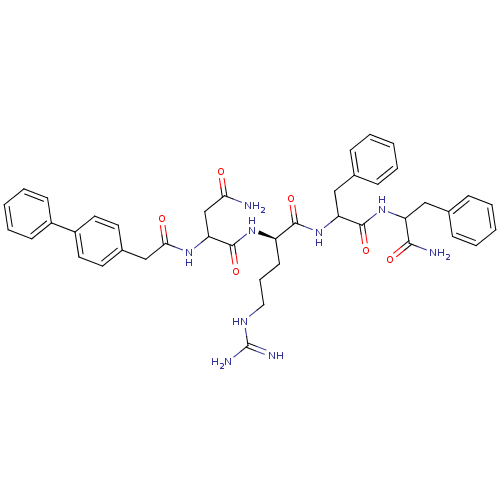 (2-(2-Biphenyl-4-yl-acetylamino)-N*1*-{1-[1-(1-carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM21004 ((3S)-3-{[(1R)-4-[(diaminomethylidene)amino]-1-{[(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.75E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
National Research Council Canada | Assay Description Fluorescence was monitored on a SPEX Fluorolog-2 spectrofluorometer with the excitation and emission wavelengths set at 380 and 440 nm, respectively.... | J Med Chem 51: 1361-8 (2008) Article DOI: 10.1021/jm701190v BindingDB Entry DOI: 10.7270/Q21Z42QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121304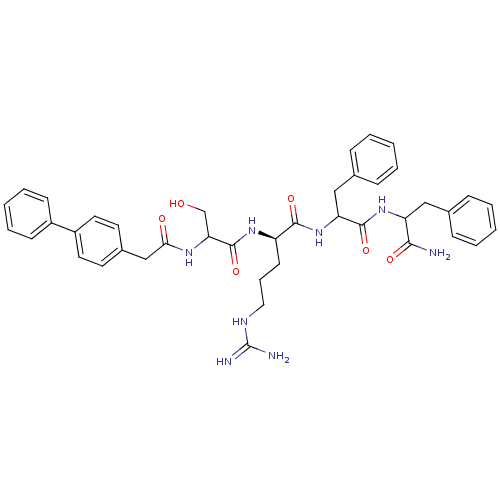 (2-[2-(2-Biphenyl-4-yl-acetylamino)-3-hydroxy-propi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50121296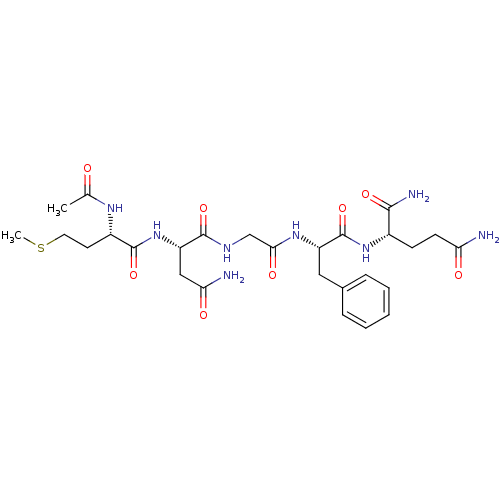 (2-(2-{2-[2-(2-Acetylamino-4-methylsulfanyl-butyryl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Council of Canada Curated by ChEMBL | Assay Description Inhibitory activity against human Cathepsin L | J Med Chem 45: 5321-9 (2002) BindingDB Entry DOI: 10.7270/Q23B5ZH6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||