 Found 400 hits with Last Name = 'skinner' and Initial = 's'
Found 400 hits with Last Name = 'skinner' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM8610

(1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...)Show SMILES [H][C@]1(COc2ccc(cc2)N2CCN(CC2)C(C)=O)CO[C@@](Cn2ccnc2)(O1)c1ccc(Cl)cc1Cl |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293820

(2-(2-(2-amino-6-oxo-1,6,7,8-tetrahydropurin-9-yl)e...)Show InChI InChI=1S/C9H16N5O5P/c10-9-12-7-6(8(15)13-9)11-5-14(7)1-2-19-3-4-20(16,17)18/h11H,1-5H2,(H2,16,17,18)(H3,10,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293821

(2-(2-(6-oxo-1,6,7,8-tetrahydropurin-9-yl)ethoxy)et...)Show InChI InChI=1S/C9H15N4O5P/c14-9-7-8(10-5-11-9)13(6-12-7)1-2-18-3-4-19(15,16)17/h5,12H,1-4,6H2,(H,10,11,14)(H2,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293826
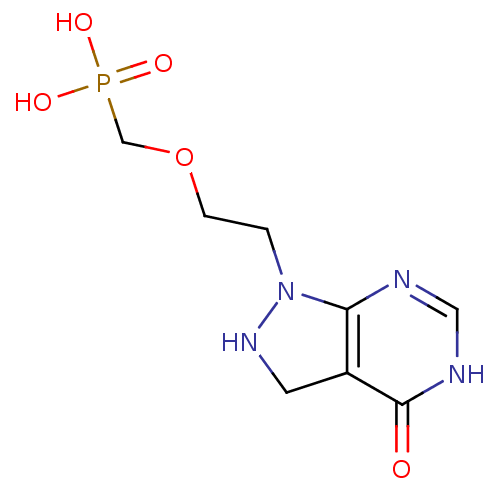
((2-(4-oxo-2,3,4,5-tetrahydropyrazolo[3,4-d]pyrimid...)Show InChI InChI=1S/C8H13N4O5P/c13-8-6-3-11-12(7(6)9-4-10-8)1-2-17-5-18(14,15)16/h4,11H,1-3,5H2,(H,9,10,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293819
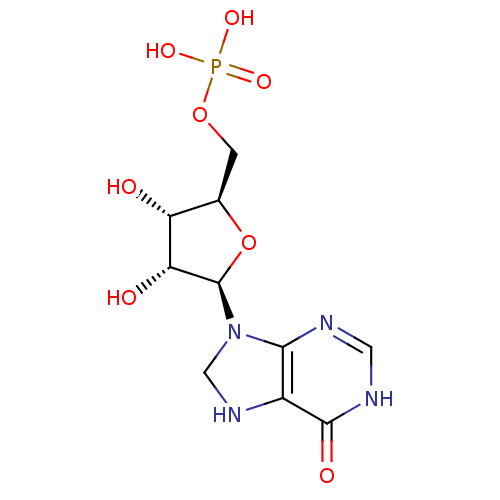
(((2R,3S,4R,5R)-3,4-dihydroxy-5-(6-oxo-1,6,7,8-tetr...)Show SMILES O[C@@H]1[C@@H](COP(O)(O)=O)O[C@H]([C@@H]1O)N1CNc2c1nc[nH]c2=O |r| Show InChI InChI=1S/C10H15N4O8P/c15-6-4(1-21-23(18,19)20)22-10(7(6)16)14-3-13-5-8(14)11-2-12-9(5)17/h2,4,6-7,10,13,15-16H,1,3H2,(H,11,12,17)(H2,18,19,20)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293818
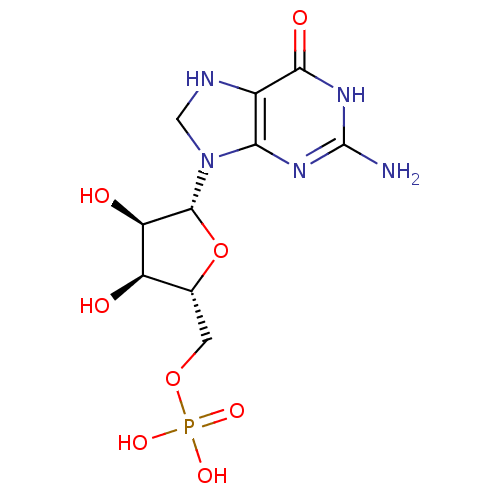
(((2R,3S,4R,5R)-5-(2-amino-6-oxo-1,6,7,8-tetrahydro...)Show SMILES Nc1nc2N(CNc2c(=O)[nH]1)[C@@H]1O[C@H](COP(O)(O)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H16N5O8P/c11-10-13-7-4(8(18)14-10)12-2-15(7)9-6(17)5(16)3(23-9)1-22-24(19,20)21/h3,5-6,9,12,16-17H,1-2H2,(H2,19,20,21)(H3,11,13,14,18)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293830
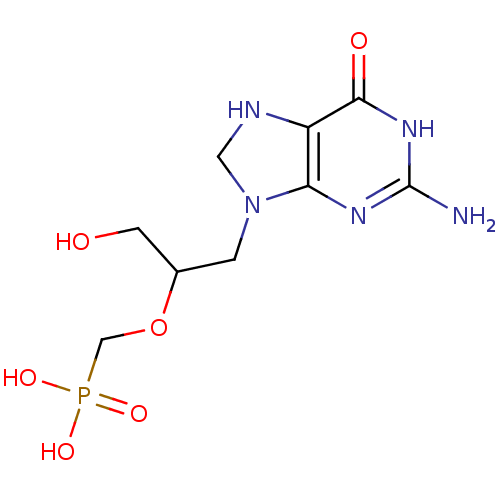
(CHEMBL561493 | RS-(3-(2-amino-6-oxo-1,6,7,8-tetrah...)Show InChI InChI=1S/C9H16N5O6P/c10-9-12-7-6(8(16)13-9)11-3-14(7)1-5(2-15)20-4-21(17,18)19/h5,11,15H,1-4H2,(H2,17,18,19)(H3,10,12,13,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293835
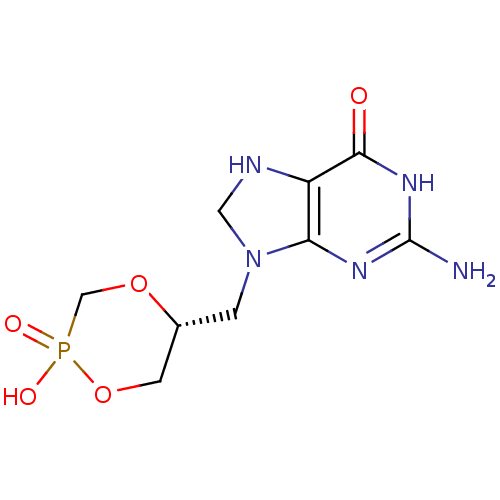
((R)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293831

((S)-(1-(5-amino-7-oxo-1,2,6,7-tetrahydro-[1,2,3]tr...)Show SMILES C[C@@H](CN1NNc2c1nc(N)[nH]c2=O)OCP(O)(O)=O |r| Show InChI InChI=1S/C8H15N6O5P/c1-4(19-3-20(16,17)18)2-14-6-5(12-13-14)7(15)11-8(9)10-6/h4,12-13H,2-3H2,1H3,(H2,16,17,18)(H3,9,10,11,15)/t4-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293835

((R)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293836
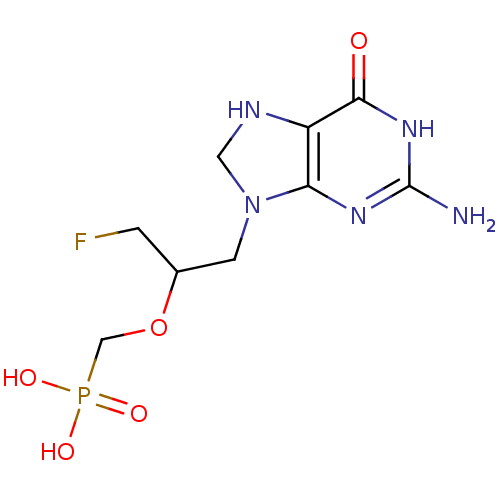
(CHEMBL541006 | RS-(1-(2-amino-6-oxo-7,8-dihydro-1H...)Show InChI InChI=1S/C9H15FN5O5P/c10-1-5(20-4-21(17,18)19)2-15-3-12-6-7(15)13-9(11)14-8(6)16/h5,12H,1-4H2,(H2,17,18,19)(H3,11,13,14,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50225188
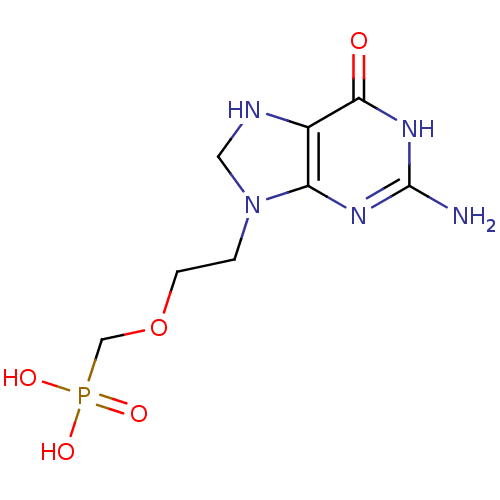
((2-(2-amino-6-oxo-1,6,7,8-tetrahydropurin-9-yl)eth...)Show InChI InChI=1S/C8H14N5O5P/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-18-4-19(15,16)17/h10H,1-4H2,(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293833
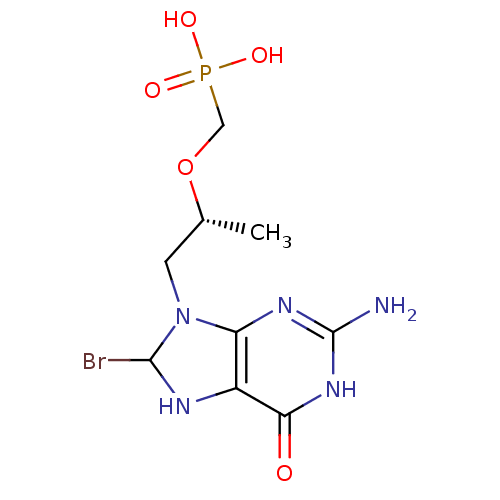
(((R)-1-(2-amino-8-bromo-6-oxo-1,6,7,8-tetrahydropu...)Show SMILES C[C@H](CN1C(Br)Nc2c1nc(N)[nH]c2=O)OCP(O)(O)=O |r| Show InChI InChI=1S/C9H15BrN5O5P/c1-4(20-3-21(17,18)19)2-15-6-5(12-8(15)10)7(16)14-9(11)13-6/h4,8,12H,2-3H2,1H3,(H2,17,18,19)(H3,11,13,14,16)/t4-,8?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50225188

((2-(2-amino-6-oxo-1,6,7,8-tetrahydropurin-9-yl)eth...)Show InChI InChI=1S/C8H14N5O5P/c9-8-11-6-5(7(14)12-8)10-3-13(6)1-2-18-4-19(15,16)17/h10H,1-4H2,(H2,15,16,17)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088527
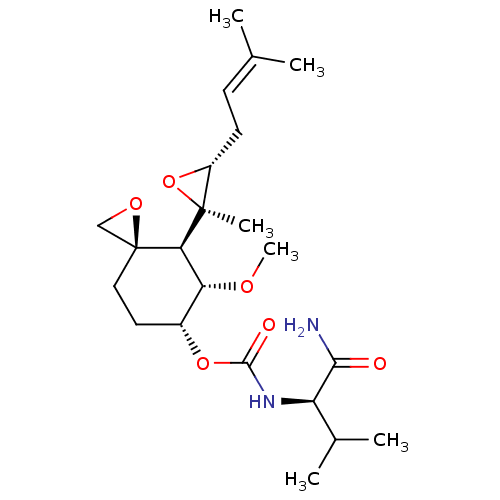
(CHEMBL3527358)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6@H](-[#6](-[#6])-[#6])-[#6](-[#7])=O)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C22H36N2O6/c1-12(2)7-8-15-21(5,30-15)18-17(27-6)14(9-10-22(18)11-28-22)29-20(26)24-16(13(3)4)19(23)25/h7,13-18H,8-11H2,1-6H3,(H2,23,25)(H,24,26)/t14-,15-,16-,17-,18-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293824

((2-(2-amino-8-hydroxy-6-oxo-1,6,7,8-tetrahydropuri...)Show InChI InChI=1S/C8H14N5O6P/c9-7-11-5-4(6(14)12-7)10-8(15)13(5)1-2-19-3-20(16,17)18/h8,10,15H,1-3H2,(H2,16,17,18)(H3,9,11,12,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293834
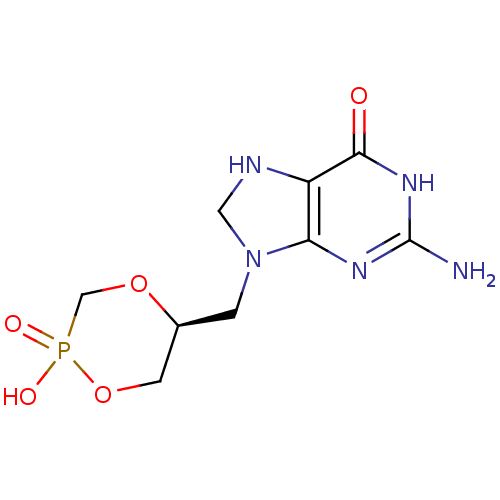
((S)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293834

((S)-5-(2-Amino-6-oxo-1,6,7,8-tetrahydro-purin-9-yl...)Show SMILES Nc1nc2N(C[C@H]3COP(O)(=O)CO3)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H14N5O5P/c10-9-12-7-6(8(15)13-9)11-3-14(7)1-5-2-19-20(16,17)4-18-5/h5,11H,1-4H2,(H,16,17)(H3,10,12,13,15)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293828
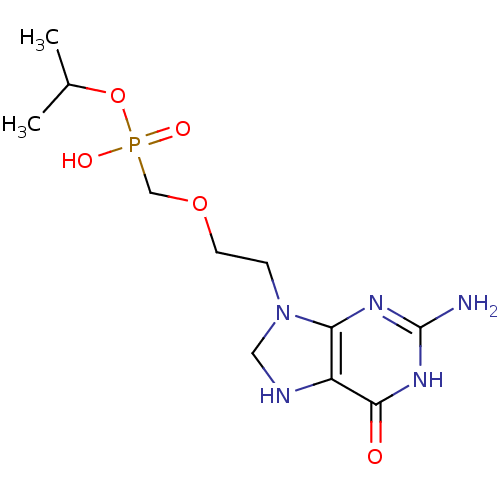
(CHEMBL560759 | isopropyl(2-(2-amino-6-oxo-1,6,7,8-...)Show InChI InChI=1S/C11H20N5O5P/c1-7(2)21-22(18,19)6-20-4-3-16-5-13-8-9(16)14-11(12)15-10(8)17/h7,13H,3-6H2,1-2H3,(H,18,19)(H3,12,14,15,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293823

((2-(5-amino-7-oxo-1,2,6,7-tetrahydro-[1,2,3]triazo...)Show InChI InChI=1S/C7H13N6O5P/c8-7-9-5-4(6(14)10-7)11-12-13(5)1-2-18-3-19(15,16)17/h11-12H,1-3H2,(H2,15,16,17)(H3,8,9,10,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293837
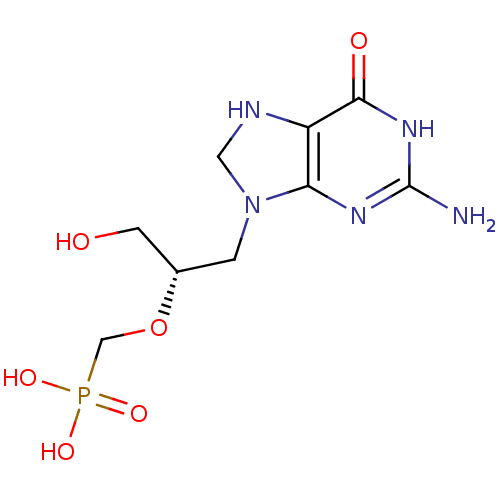
((S)-9-[3-hydroxy-2-(phosphonomethoxy)propyl]guanin...)Show SMILES Nc1nc2N(C[C@@H](CO)OCP(O)(O)=O)CNc2c(=O)[nH]1 |r| Show InChI InChI=1S/C9H16N5O6P/c10-9-12-7-6(8(16)13-9)11-3-14(7)1-5(2-15)20-4-21(17,18)19/h5,11,15H,1-4H2,(H2,17,18,19)(H3,10,12,13,16)/t5-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.77E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293838

((2-(6-oxo-1,6,7,8-tetrahydropurin-9-yl)ethoxy)meth...)Show InChI InChI=1S/C8H13N4O5P/c13-8-6-7(9-3-10-8)12(4-11-6)1-2-17-5-18(14,15)16/h3,11H,1-2,4-5H2,(H,9,10,13)(H2,14,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.5 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 8.5 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088527

(CHEMBL3527358)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6@H](-[#6](-[#6])-[#6])-[#6](-[#7])=O)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C22H36N2O6/c1-12(2)7-8-15-21(5,30-15)18-17(27-6)14(9-10-22(18)11-28-22)29-20(26)24-16(13(3)4)19(23)25/h7,13-18H,8-11H2,1-6H3,(H2,23,25)(H,24,26)/t14-,15-,16-,17-,18-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293832
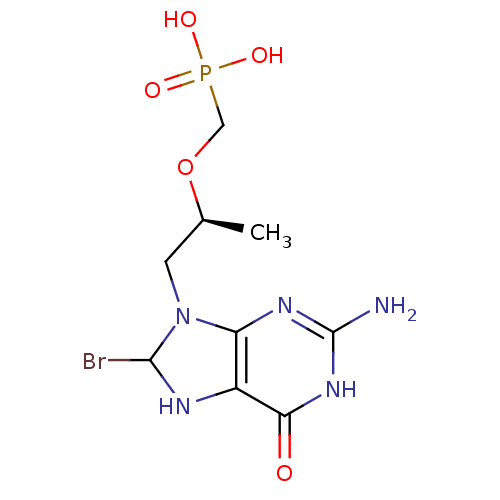
(((S)-1-(2-amino-8-bromo-6-oxo-1,6,7,8-tetrahydropu...)Show SMILES C[C@@H](CN1C(Br)Nc2c1nc(N)[nH]c2=O)OCP(O)(O)=O |r| Show InChI InChI=1S/C9H15BrN5O5P/c1-4(20-3-21(17,18)19)2-15-6-5(12-8(15)10)7(16)14-9(11)13-6/h4,8,12H,2-3H2,1H3,(H2,17,18,19)(H3,11,13,14,16)/t4-,8?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293825
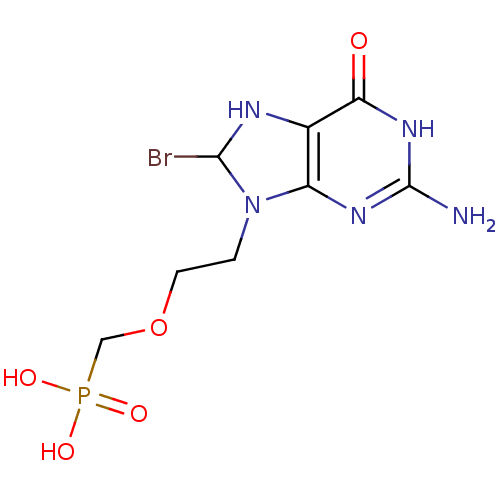
((2-(2-amino-8-bromo-6-oxo-1,6,7,8-tetrahydropurin-...)Show InChI InChI=1S/C8H13BrN5O5P/c9-7-11-4-5(12-8(10)13-6(4)15)14(7)1-2-19-3-20(16,17)18/h7,11H,1-3H2,(H2,16,17,18)(H3,10,12,13,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50088527

(CHEMBL3527358)Show SMILES [H][C@@]1([#6@H](-[#8]-[#6])-[#6@@H](-[#6]-[#6][C@]11[#6]-[#8]1)-[#8]-[#6](=O)-[#7]-[#6@H](-[#6](-[#6])-[#6])-[#6](-[#7])=O)[C@@]1([#6])[#8]-[#6@@H]1-[#6]\[#6]=[#6](\[#6])-[#6] |r| Show InChI InChI=1S/C22H36N2O6/c1-12(2)7-8-15-21(5,30-15)18-17(27-6)14(9-10-22(18)11-28-22)29-20(26)24-16(13(3)4)19(23)25/h7,13-18H,8-11H2,1-6H3,(H2,23,25)(H,24,26)/t14-,15-,16-,17-,18-,21+,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis |
Drug Metab Dispos 41: 814-26 (2013)
Article DOI: 10.1124/dmd.112.048355
BindingDB Entry DOI: 10.7270/Q2NC62XH |
More data for this
Ligand-Target Pair | |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293829
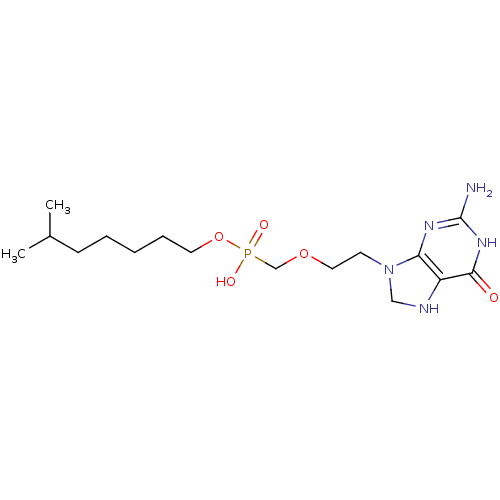
(6-methylheptyl(2-(2-amino-6-oxo-1,6,7,8-tetrahydro...)Show SMILES CC(C)CCCCCOP(O)(=O)COCCN1CNc2c1nc(N)[nH]c2=O Show InChI InChI=1S/C16H30N5O5P/c1-12(2)6-4-3-5-8-26-27(23,24)11-25-9-7-21-10-18-13-14(21)19-16(17)20-15(13)22/h12,18H,3-11H2,1-2H3,(H,23,24)(H3,17,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Hypoxanthine-guanine phosphoribosyltransferase
(Homo sapiens (Human)) | BDBM50293827
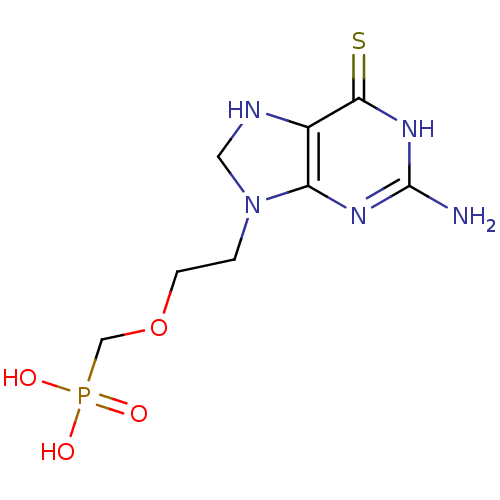
((2-(2-amino-6-thioxo-1,6,7,8-tetrahydropurin-9-yl)...)Show InChI InChI=1S/C8H14N5O4PS/c9-8-11-6-5(7(19)12-8)10-3-13(6)1-2-17-4-18(14,15)16/h10H,1-4H2,(H2,14,15,16)(H3,9,11,12,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HGPRT at pH 7.4 by spectrophotometric assay |
J Med Chem 52: 4391-9 (2009)
Article DOI: 10.1021/jm900267n
BindingDB Entry DOI: 10.7270/Q27M0809 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566635
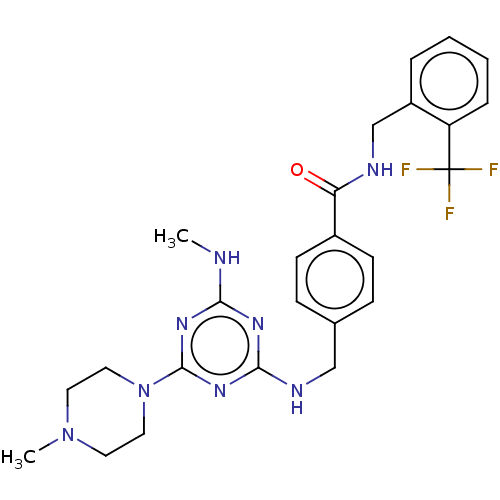
(CHEMBL4875337)Show SMILES CNc1nc(NCc2ccc(cc2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of MMP-13 using 5-FAM-TPGPLGL[Dap- (DNP)]ARRK(5-TAMRA)-amide as substrate after 45 mins |
J Med Chem 55: 7061-79 (2012)
Article DOI: 10.1021/jm300449x
BindingDB Entry DOI: 10.7270/Q2RX9D6T |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566638
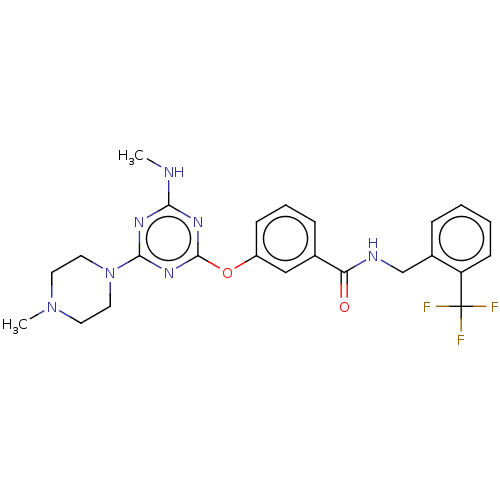
(CHEMBL4862566)Show SMILES CNc1nc(Oc2cccc(c2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of TACE using Mca-PLAQAV-Dpa-RSSSR-NH2 as substrate preincubated 15 mins measured every 30 sec for 30 mins |
J Med Chem 55: 7061-79 (2012)
Article DOI: 10.1021/jm300449x
BindingDB Entry DOI: 10.7270/Q2RX9D6T |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Lille
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using RHK-K(Ac) as substrate by homogeneous fluorescence release assay |
Eur J Med Chem 161: 277-291 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.041
BindingDB Entry DOI: 10.7270/Q21V5J76 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM36462
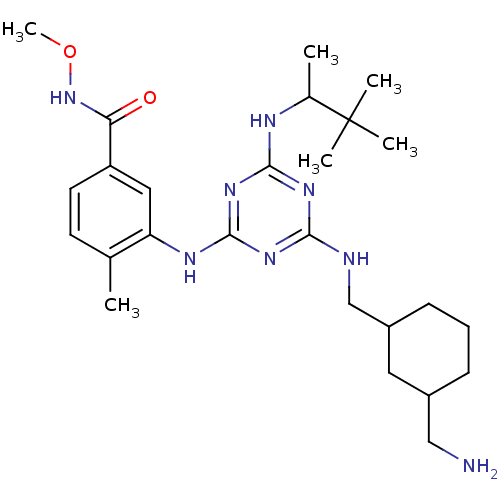
(3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(NCC3CCCC(CN)C3)nc(NC(C)C(C)(C)C)n2)c1 Show InChI InChI=1S/C26H42N8O2/c1-16-10-11-20(22(35)34-36-6)13-21(16)30-25-32-23(28-15-19-9-7-8-18(12-19)14-27)31-24(33-25)29-17(2)26(3,4)5/h10-11,13,17-19H,7-9,12,14-15,27H2,1-6H3,(H,34,35)(H3,28,29,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals
| Assay Description
Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... |
Nat Chem Biol 5: 647-54 (2009)
Article DOI: 10.1038/nchembio.211
BindingDB Entry DOI: 10.7270/Q2MP51NX |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM36463
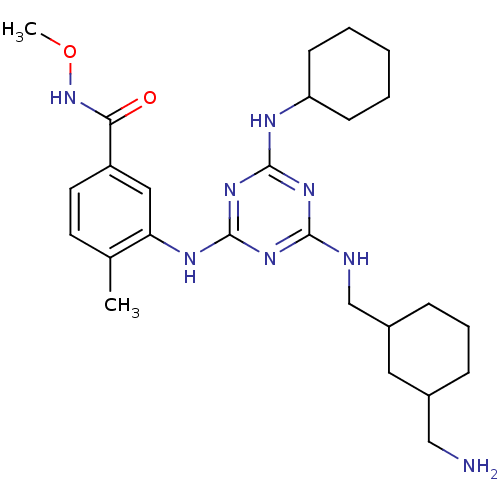
(3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...)Show SMILES CONC(=O)c1ccc(C)c(Nc2nc(NCC3CCCC(CN)C3)nc(NC3CCCCC3)n2)c1 Show InChI InChI=1S/C26H40N8O2/c1-17-11-12-20(23(35)34-36-2)14-22(17)30-26-32-24(28-16-19-8-6-7-18(13-19)15-27)31-25(33-26)29-21-9-4-3-5-10-21/h11-12,14,18-19,21H,3-10,13,15-16,27H2,1-2H3,(H,34,35)(H3,28,29,30,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals
| Assay Description
Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... |
Nat Chem Biol 5: 647-54 (2009)
Article DOI: 10.1038/nchembio.211
BindingDB Entry DOI: 10.7270/Q2MP51NX |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091691
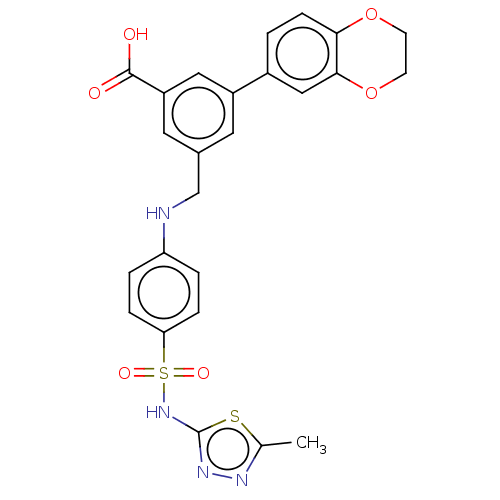
(CHEMBL3582356)Show SMILES Cc1nnc(NS(=O)(=O)c2ccc(NCc3cc(cc(c3)-c3ccc4OCCOc4c3)C(O)=O)cc2)s1 Show InChI InChI=1S/C25H22N4O6S2/c1-15-27-28-25(36-15)29-37(32,33)21-5-3-20(4-6-21)26-14-16-10-18(12-19(11-16)24(30)31)17-2-7-22-23(13-17)35-9-8-34-22/h2-7,10-13,26H,8-9,14H2,1H3,(H,28,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091689
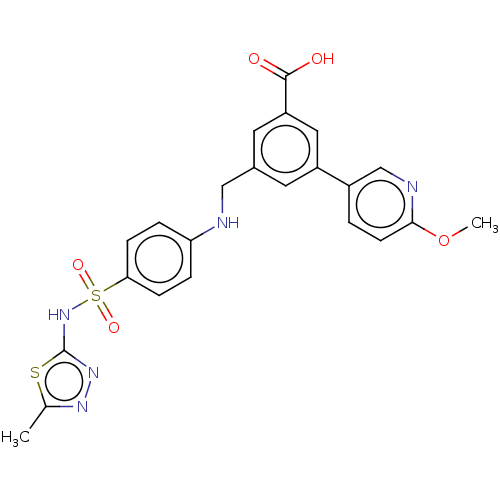
(CHEMBL3582354)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(O)=O Show InChI InChI=1S/C23H21N5O5S2/c1-14-26-27-23(34-14)28-35(31,32)20-6-4-19(5-7-20)24-12-15-9-17(11-18(10-15)22(29)30)16-3-8-21(33-2)25-13-16/h3-11,13,24H,12H2,1-2H3,(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 using WAAG-3R as substrate preincubated for 15 mins measured after 1 hr |
J Med Chem 55: 7061-79 (2012)
Article DOI: 10.1021/jm300449x
BindingDB Entry DOI: 10.7270/Q2RX9D6T |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566636

(CHEMBL4870025)Show SMILES CNc1nc(NCc2cc(oc2C)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50539700
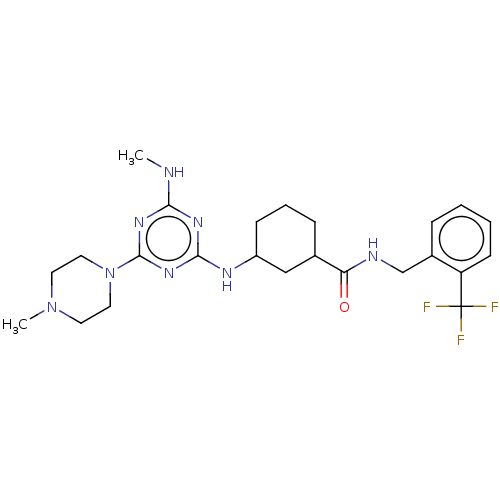
(CHEMBL4637413)Show SMILES CNc1nc(NC2CCCC(C2)C(=O)NCc2ccccc2C(F)(F)F)nc(n1)N1CCN(C)CC1 Show InChI InChI=1S/C24H33F3N8O/c1-28-21-31-22(33-23(32-21)35-12-10-34(2)11-13-35)30-18-8-5-7-16(14-18)20(36)29-15-17-6-3-4-9-19(17)24(25,26)27/h3-4,6,9,16,18H,5,7-8,10-15H2,1-2H3,(H,29,36)(H2,28,30,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM8798
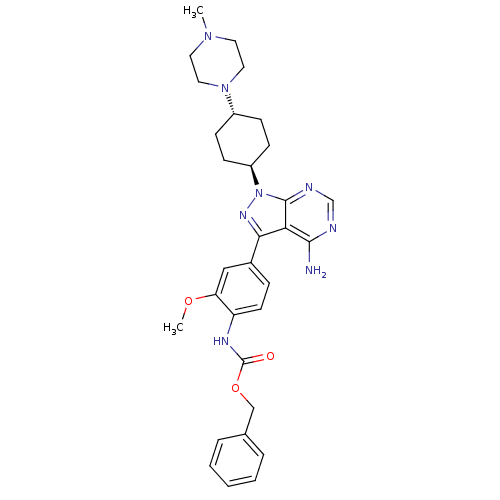
(CHEMBL47787 | Pyrazolo[3,4-d]pyrimidine 6 | benzyl...)Show SMILES COc1cc(ccc1NC(=O)OCc1ccccc1)-c1nn([C@H]2CC[C@@H](CC2)N2CCN(C)CC2)c2ncnc(N)c12 |r,wU:22.23,wD:25.30,(.85,5.16,;-.29,6.2,;-1.75,5.73,;-2.08,4.23,;-3.55,3.76,;-4.69,4.8,;-4.36,6.3,;-2.89,6.77,;-2.56,8.27,;-1.1,8.74,;.04,7.7,;-.77,10.25,;.7,10.71,;1.03,12.22,;-.05,13.31,;.36,14.8,;1.85,15.19,;2.93,14.09,;2.52,12.61,;-4.03,2.3,;-3.12,1.05,;-4.03,-.2,;-3.55,-1.66,;-2.06,-2.04,;-1.64,-3.52,;-2.72,-4.63,;-4.21,-4.25,;-4.63,-2.76,;-2.3,-6.11,;-3.34,-7.24,;-2.87,-8.71,;-1.37,-9.04,;-.91,-10.51,;-.33,-7.91,;-.8,-6.44,;-5.49,.28,;-6.82,-.49,;-8.16,.28,;-8.16,1.82,;-6.82,2.59,;-6.82,4.13,;-5.49,1.82,)| Show InChI InChI=1S/C31H38N8O3/c1-37-14-16-38(17-15-37)23-9-11-24(12-10-23)39-30-27(29(32)33-20-34-30)28(36-39)22-8-13-25(26(18-22)41-2)35-31(40)42-19-21-6-4-3-5-7-21/h3-8,13,18,20,23-24H,9-12,14-17,19H2,1-2H3,(H,35,40)(H2,32,33,34)/t23-,24- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Abbott Bioresearch Center
| Assay Description
The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... |
Bioorg Med Chem Lett 12: 1687-90 (2002)
Article DOI: 10.1016/s0960-894x(02)00196-8
BindingDB Entry DOI: 10.7270/Q2154F84 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091692

(CHEMBL3582357)Show SMILES Cc1nnc(NS(=O)(=O)c2ccc(NCc3cc(cc(c3)-c3ccc4OCOc4c3)C(O)=O)cc2)s1 Show InChI InChI=1S/C24H20N4O6S2/c1-14-26-27-24(35-14)28-36(31,32)20-5-3-19(4-6-20)25-12-15-8-17(10-18(9-15)23(29)30)16-2-7-21-22(11-16)34-13-33-21/h2-11,25H,12-13H2,1H3,(H,27,28)(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50091696
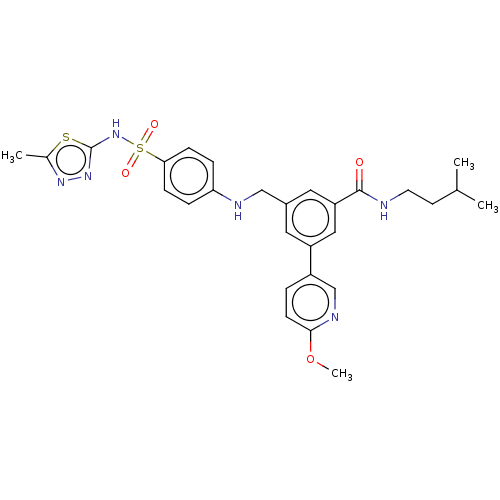
(CHEMBL3582351)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(=O)NCCC(C)C Show InChI InChI=1S/C28H32N6O4S2/c1-18(2)11-12-29-27(35)23-14-20(13-22(15-23)21-5-10-26(38-4)31-17-21)16-30-24-6-8-25(9-7-24)40(36,37)34-28-33-32-19(3)39-28/h5-10,13-15,17-18,30H,11-12,16H2,1-4H3,(H,29,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50091695
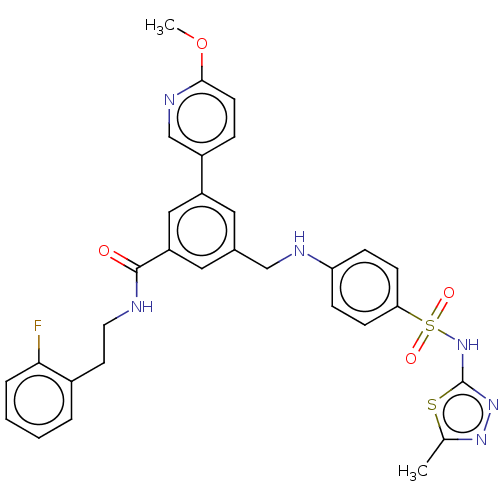
(CHEMBL3582350)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(=O)NCCc1ccccc1F Show InChI InChI=1S/C31H29FN6O4S2/c1-20-36-37-31(43-20)38-44(40,41)27-10-8-26(9-11-27)34-18-21-15-24(23-7-12-29(42-2)35-19-23)17-25(16-21)30(39)33-14-13-22-5-3-4-6-28(22)32/h3-12,15-17,19,34H,13-14,18H2,1-2H3,(H,33,39)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50566637
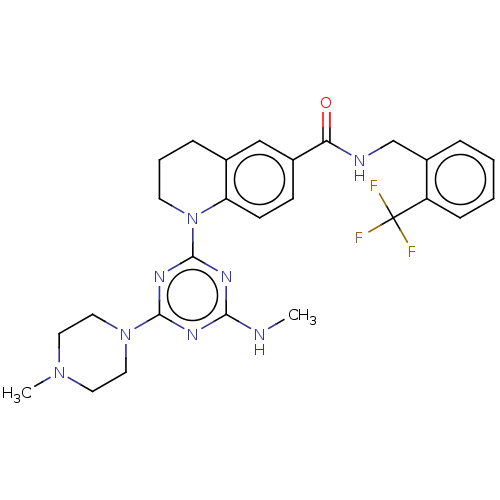
(CHEMBL4871885)Show SMILES CNc1nc(nc(n1)N1CCCc2cc(ccc12)C(=O)NCc1ccccc1C(F)(F)F)N1CCN(C)CC1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... |
Citation and Details
Article DOI: 10.1016/j.bmc.2021.116216
BindingDB Entry DOI: 10.7270/Q22J6GNB |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50122453
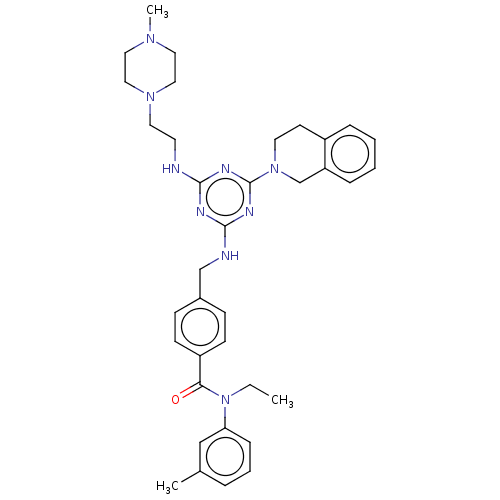
(CHEMBL3622491)Show SMILES CCN(C(=O)c1ccc(CNc2nc(NCCN3CCN(C)CC3)nc(n2)N2CCc3ccccc3C2)cc1)c1cccc(C)c1 Show InChI InChI=1S/C36H45N9O/c1-4-45(32-11-7-8-27(2)24-32)33(46)30-14-12-28(13-15-30)25-38-35-39-34(37-17-19-43-22-20-42(3)21-23-43)40-36(41-35)44-18-16-29-9-5-6-10-31(29)26-44/h5-15,24H,4,16-23,25-26H2,1-3H3,(H2,37,38,39,40,41) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human ADAMTS4 (213 to 575 amino acid residues) using WAAG-3R as substrate preincubated for 15 mins followed by substrate ad... |
ACS Med Chem Lett 6: 888-93 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00138
BindingDB Entry DOI: 10.7270/Q2416ZWQ |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 5
(Homo sapiens (Human)) | BDBM50168737

((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...)Show SMILES C[C@@]1(O)CCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(OCc2ccc(F)cc2Cl)cc1 Show InChI InChI=1S/C20H22ClFN2O6S/c1-20(26)9-2-10-24(18(20)19(25)23-27)31(28,29)16-7-5-15(6-8-16)30-12-13-3-4-14(22)11-17(13)21/h3-8,11,18,26-27H,2,9-10,12H2,1H3,(H,23,25)/t18-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human ADAMTS-5 expressed in CHO cells using WAAG-3R as substrate preincubated for 15 mins measured after 1 hr by FRET assay |
J Med Chem 55: 7061-79 (2012)
Article DOI: 10.1021/jm300449x
BindingDB Entry DOI: 10.7270/Q2RX9D6T |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50091695

(CHEMBL3582350)Show SMILES COc1ccc(cn1)-c1cc(CNc2ccc(cc2)S(=O)(=O)Nc2nnc(C)s2)cc(c1)C(=O)NCCc1ccccc1F Show InChI InChI=1S/C31H29FN6O4S2/c1-20-36-37-31(43-20)38-44(40,41)27-10-8-26(9-11-27)34-18-21-15-24(23-7-12-29(42-2)35-19-23)17-25(16-21)30(39)33-14-13-22-5-3-4-6-28(22)32/h3-12,15-17,19,34H,13-14,18H2,1-2H3,(H,33,39)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type his-tagged PI3Kalpha (unknown origin) |
ACS Med Chem Lett 6: 531-6 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00025
BindingDB Entry DOI: 10.7270/Q2BP04JF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data