 Found 2096 hits with Last Name = 'soldermann' and Initial = 'n'
Found 2096 hits with Last Name = 'soldermann' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324315

(CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc2cnccc2c(n1)N1CCNCC1 Show InChI InChI=1S/C23H28N6/c1-2-4-19(5-3-1)27-22-15-17(6-9-26-22)21-14-18-16-25-8-7-20(18)23(28-21)29-12-10-24-11-13-29/h6-9,14-16,19,24H,1-5,10-13H2,(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324324

(2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2)c1)N1CCNCC1 Show InChI InChI=1S/C21H22N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h1-7,12-14,23H,8-11H2,(H2,22,28)(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324346

(CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C23H29N7/c1-2-4-20(5-3-1)28-22-13-17(6-7-25-22)21-12-18(19-15-26-27-16-19)14-23(29-21)30-10-8-24-9-11-30/h6-7,12-16,20,24H,1-5,8-11H2,(H,25,28)(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324325
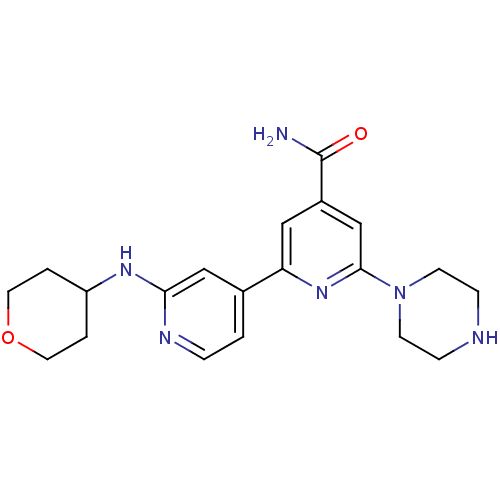
(6-Piperazin-1-yl-2'-(tetrahydropyran-4-ylamino)[2,...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(NC2CCOCC2)c1)N1CCNCC1 Show InChI InChI=1S/C20H26N6O2/c21-20(27)15-11-17(25-19(13-15)26-7-5-22-6-8-26)14-1-4-23-18(12-14)24-16-2-9-28-10-3-16/h1,4,11-13,16,22H,2-3,5-10H2,(H2,21,27)(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324326
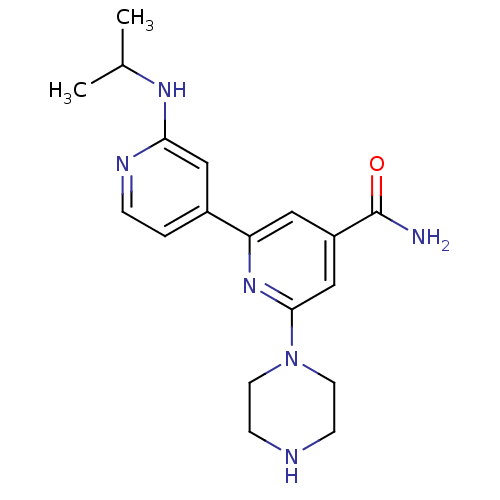
(4'-tert-Butylcarbamoyl-2''-isopropylamino-3,4,5,6-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)C(N)=O Show InChI InChI=1S/C18H24N6O/c1-12(2)22-16-10-13(3-4-21-16)15-9-14(18(19)25)11-17(23-15)24-7-5-20-6-8-24/h3-4,9-12,20H,5-8H2,1-2H3,(H2,19,25)(H,21,22) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324324

(2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2)c1)N1CCNCC1 Show InChI InChI=1S/C21H22N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h1-7,12-14,23H,8-11H2,(H2,22,28)(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324322
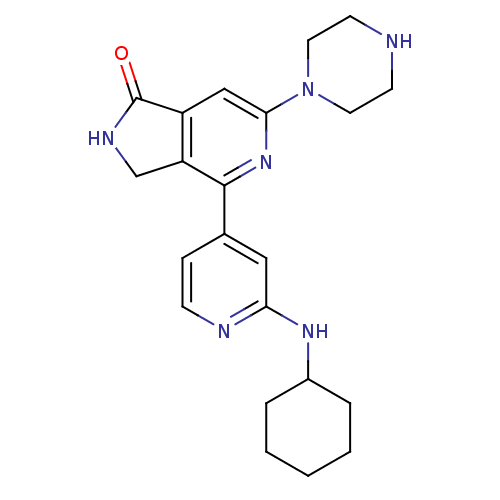
(4-(2-cyclohexylaminopyridin-4-yl)-6-(piperazin-1-y...)Show SMILES O=C1NCc2c1cc(nc2-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C22H28N6O/c29-22-17-13-20(28-10-8-23-9-11-28)27-21(18(17)14-25-22)15-6-7-24-19(12-15)26-16-4-2-1-3-5-16/h6-7,12-13,16,23H,1-5,8-11,14H2,(H,24,26)(H,25,29) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324328
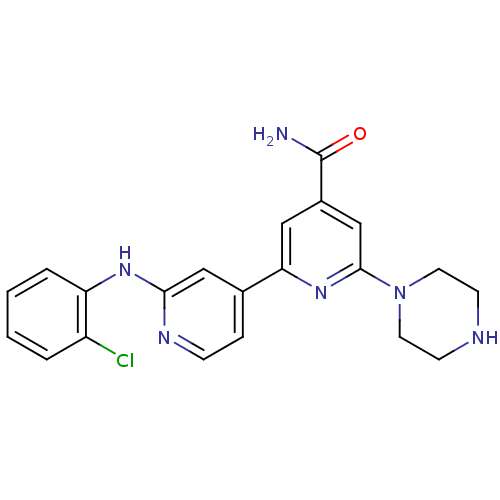
(2'-(2-Chlorophenylamino)-6-piperazin-1-yl[2,4']bip...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(Nc2ccccc2Cl)c1)N1CCNCC1 Show InChI InChI=1S/C21H21ClN6O/c22-16-3-1-2-4-17(16)26-19-12-14(5-6-25-19)18-11-15(21(23)29)13-20(27-18)28-9-7-24-8-10-28/h1-6,11-13,24H,7-10H2,(H2,23,29)(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324323
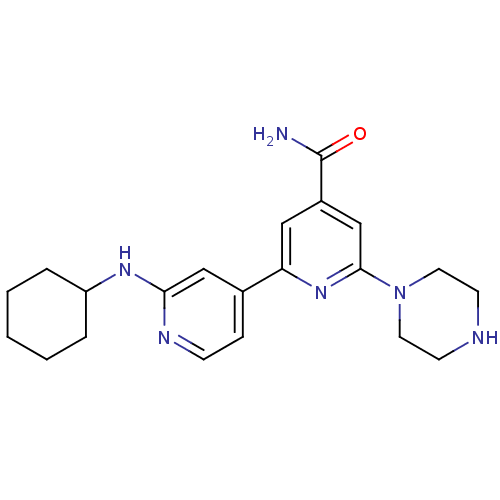
(2'-Cyclohexylamino-6-piperazin-1-yl[2,4']bipyridin...)Show SMILES NC(=O)c1cc(nc(c1)-c1ccnc(NC2CCCCC2)c1)N1CCNCC1 Show InChI InChI=1S/C21H28N6O/c22-21(28)16-12-18(26-20(14-16)27-10-8-23-9-11-27)15-6-7-24-19(13-15)25-17-4-2-1-3-5-17/h6-7,12-14,17,23H,1-5,8-11H2,(H2,22,28)(H,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324348
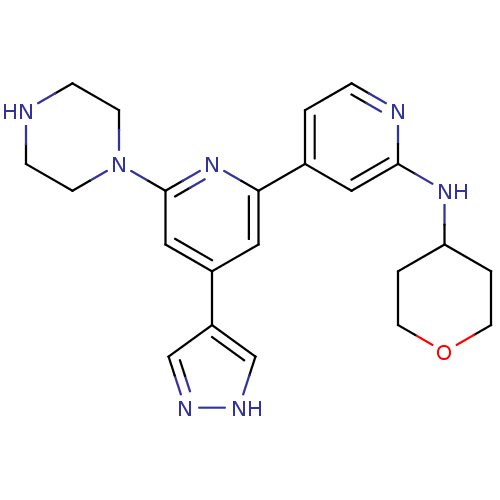
(6-(piperazin-1-yl)-4-(4H-pyrazol-4-yl)-N-(tetrahyd...)Show SMILES C1CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCOCC2)c1)-c1cn[nH]c1 Show InChI InChI=1S/C22H27N7O/c1-4-24-21(27-19-2-9-30-10-3-19)12-16(1)20-11-17(18-14-25-26-15-18)13-22(28-20)29-7-5-23-6-8-29/h1,4,11-15,19,23H,2-3,5-10H2,(H,24,27)(H,25,26) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324347
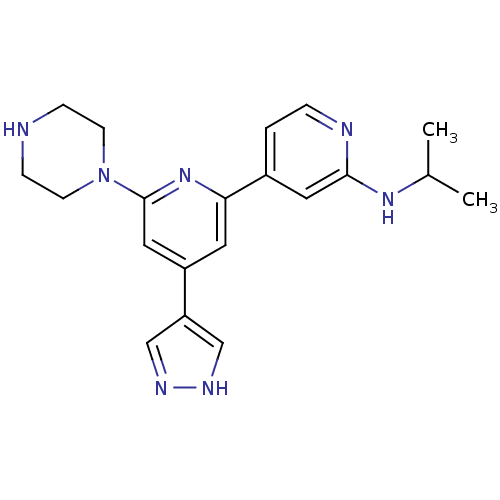
(CHEMBL1215153 | Isopropyl-[6-piperazin-1-yl-4-(1H-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C20H25N7/c1-14(2)25-19-10-15(3-4-22-19)18-9-16(17-12-23-24-13-17)11-20(26-18)27-7-5-21-6-8-27/h3-4,9-14,21H,5-8H2,1-2H3,(H,22,25)(H,23,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324346

(CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...)Show SMILES C1CCC(CC1)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C23H29N7/c1-2-4-20(5-3-1)28-22-13-17(6-7-25-22)21-12-18(19-15-26-27-16-19)14-23(29-21)30-10-8-24-9-11-30/h6-7,12-16,20,24H,1-5,8-11H2,(H,25,28)(H,26,27) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324327
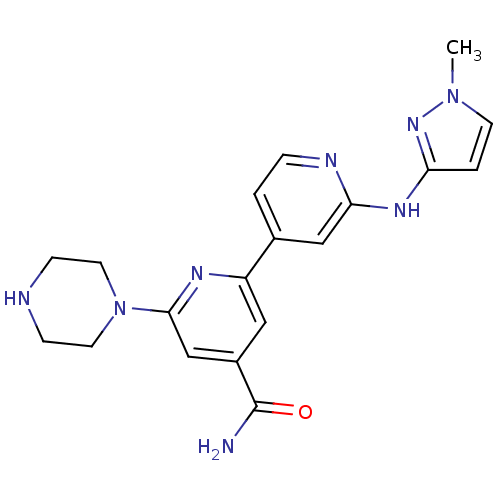
(2'-(1-Methyl-1H-pyrazol-3-ylamino)-6-piperazin-1-y...)Show SMILES Cn1ccc(Nc2cc(ccn2)-c2cc(cc(n2)N2CCNCC2)C(N)=O)n1 Show InChI InChI=1S/C19H22N8O/c1-26-7-3-16(25-26)24-17-11-13(2-4-22-17)15-10-14(19(20)28)12-18(23-15)27-8-5-21-6-9-27/h2-4,7,10-12,21H,5-6,8-9H2,1H3,(H2,20,28)(H,22,24,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324347

(CHEMBL1215153 | Isopropyl-[6-piperazin-1-yl-4-(1H-...)Show SMILES CC(C)Nc1cc(ccn1)-c1cc(cc(n1)N1CCNCC1)-c1cn[nH]c1 Show InChI InChI=1S/C20H25N7/c1-14(2)25-19-10-15(3-4-22-19)18-9-16(17-12-23-24-13-17)11-20(26-18)27-7-5-21-6-8-27/h3-4,9-14,21H,5-8H2,1-2H3,(H,22,25)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Protein kinase C eta type
(Homo sapiens (Human)) | BDBM50391897
(CHEMBL2147537)Show InChI InChI=1S/C16H17N5/c1-11(17)9-20-16-14-4-7-19-10-13(14)8-15(21-16)12-2-5-18-6-3-12/h2-8,10-11H,9,17H2,1H3,(H,20,21)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... |
Bioorg Med Chem Lett 21: 7367-72 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.025
BindingDB Entry DOI: 10.7270/Q22J6CZ4 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase D1
(Homo sapiens (Human)) | BDBM50324335
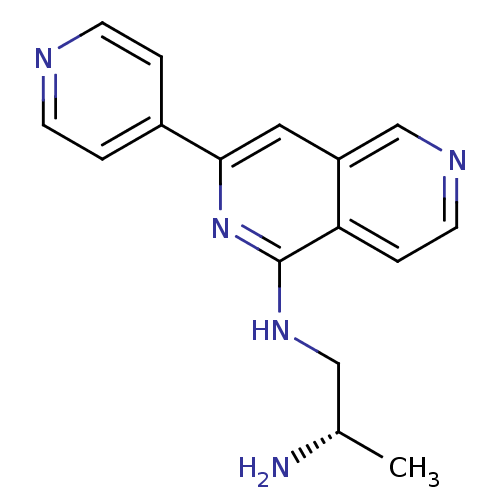
(2'-Cyclohexylamino-6-((R)-pyrrolidin-3-ylamino)[2,...)Show SMILES NC(=O)c1cc(N[C@@H]2CCNC2)nc(c1)-c1ccnc(NC2CCCCC2)c1 |r| Show InChI InChI=1S/C21H28N6O/c22-21(28)15-10-18(27-20(12-15)26-17-7-8-23-13-17)14-6-9-24-19(11-14)25-16-4-2-1-3-5-16/h6,9-12,16-17,23H,1-5,7-8,13H2,(H2,22,28)(H,24,25)(H,26,27)/t17-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD1 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Polycystin-2
(Homo sapiens (Human)) | BDBM50324348

(6-(piperazin-1-yl)-4-(4H-pyrazol-4-yl)-N-(tetrahyd...)Show SMILES C1CN(CCN1)c1cc(cc(n1)-c1ccnc(NC2CCOCC2)c1)-c1cn[nH]c1 Show InChI InChI=1S/C22H27N7O/c1-4-24-21(27-19-2-9-30-10-3-19)12-16(1)20-11-17(18-14-25-26-15-18)13-22(28-20)29-7-5-23-6-8-29/h1,4,11-15,19,23H,2-3,5-10H2,(H,24,27)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PKD2 by TR-FRET assay |
J Med Chem 53: 5422-38 (2010)
Article DOI: 10.1021/jm100076w
BindingDB Entry DOI: 10.7270/Q2P84C2J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50533774

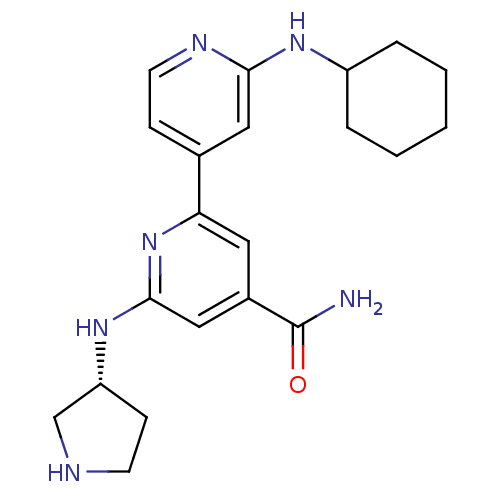
(CHEMBL4469006)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cccc(c1)-c1ncnc2ccc(cc12)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H25N5O2/c1-20(36)34-11-13-35(14-12-34)30(37)24-7-4-6-23(16-24)29-26-17-21(9-10-28(26)32-19-33-29)25-15-22-5-2-3-8-27(22)31-18-25/h2-10,15-19H,11-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) assessed as reduction in ADP formation using phosphatidyl inositol as substrate after 30 to 60 mins by TR-FR... |
ACS Med Chem Lett 7: 762-7 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00119
BindingDB Entry DOI: 10.7270/Q2V98CKP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM202490
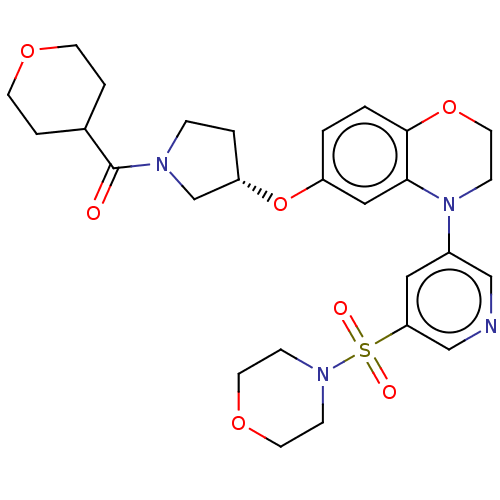
(US9539260, A3 | US9763952, Example A3)Show SMILES O=C(C1CCOCC1)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cncc(c3)S(=O)(=O)N3CCOCC3)c2c1 |r| Show InChI InChI=1S/C27H34N4O7S/c32-27(20-4-10-35-11-5-20)29-6-3-23(19-29)38-22-1-2-26-25(16-22)31(9-14-37-26)21-15-24(18-28-17-21)39(33,34)30-7-12-36-13-8-30/h1-2,15-18,20,23H,3-14,19H2/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM202513
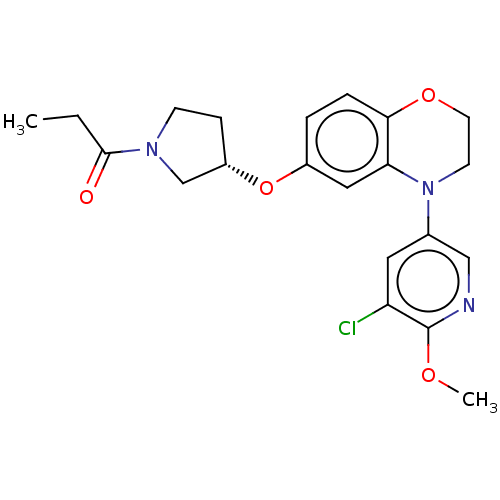
(US9539260, A12 | US9763952, Example A12)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(Cl)c3)c2c1 |r| Show InChI InChI=1S/C21H24ClN3O4/c1-3-20(26)24-7-6-16(13-24)29-15-4-5-19-18(11-15)25(8-9-28-19)14-10-17(22)21(27-2)23-12-14/h4-5,10-12,16H,3,6-9,13H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204120

(US9539260, K | US9763952, Example K)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)[C@@H]3CCN(C3)C(C)=O)cc12 |r| Show InChI InChI=1S/C26H29N5O5/c1-17(32)29-7-5-18(15-29)26(33)30-8-6-22(16-30)36-21-3-4-24-23(12-21)31(9-10-35-24)20-11-19(13-27)25(34-2)28-14-20/h3-4,11-12,14,18,22H,5-10,15-16H2,1-2H3/t18-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203908

(US9539260, C24 | US9539260, C25 | US9763952, Examp...)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3COCCO3)cc12 |r,w:24.27| Show InChI InChI=1S/C24H26N4O6/c1-30-23-16(12-25)10-17(13-26-23)28-6-7-32-21-3-2-18(11-20(21)28)34-19-4-5-27(14-19)24(29)22-15-31-8-9-33-22/h2-3,10-11,13,19,22H,4-9,14-15H2,1H3/t19-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204008
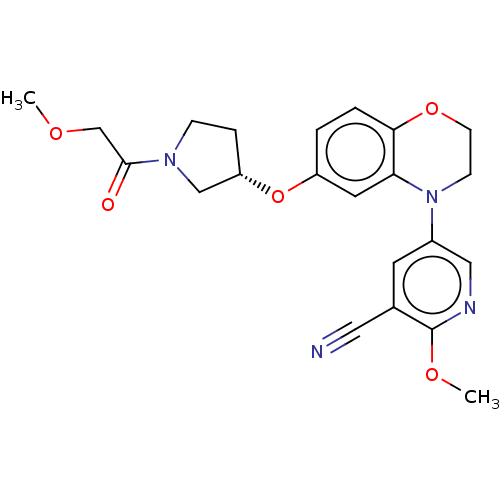
(US9539260, D12 | US9763952, Example D12)Show SMILES COCC(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r| Show InChI InChI=1S/C22H24N4O5/c1-28-14-21(27)25-6-5-18(13-25)31-17-3-4-20-19(10-17)26(7-8-30-20)16-9-15(11-23)22(29-2)24-12-16/h3-4,9-10,12,18H,5-8,13-14H2,1-2H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204062
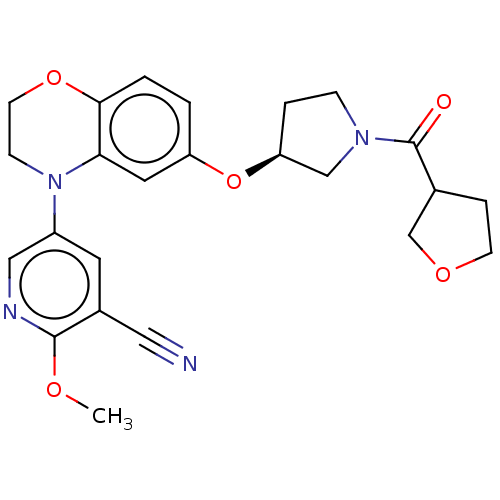
(US9539260, D28 | US9539260, D29 | US9763952, Examp...)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r| Show InChI InChI=1S/C24H26N4O5/c1-30-23-17(12-25)10-18(13-26-23)28-7-9-32-22-3-2-19(11-21(22)28)33-20-4-6-27(14-20)24(29)16-5-8-31-15-16/h2-3,10-11,13,16,20H,4-9,14-15H2,1H3/t16?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204070

(US9539260, D35 | US9539260, D36 | US9763952, Examp...)Show SMILES COC1CCC(CC1)C(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r,wD:13.16,(8.9,-5.23,;8.51,-3.74,;7.02,-3.35,;5.93,-4.43,;4.44,-4.04,;4.04,-2.55,;5.13,-1.46,;6.62,-1.86,;2.56,-2.15,;2.16,-.66,;1.47,-3.24,;.56,-4.48,;-.9,-4.01,;-.9,-2.47,;.56,-1.99,;-2.24,-1.7,;-3.57,-2.47,;-3.57,-4.01,;-4.9,-4.78,;-6.24,-4.01,;-7.57,-4.78,;-8.9,-4.01,;-8.9,-2.47,;-7.57,-1.7,;-7.57,-.16,;-8.9,.61,;-8.9,2.15,;-7.57,2.92,;-7.57,4.46,;-6.24,5.23,;-6.24,2.15,;-6.24,.61,;-4.9,2.92,;-3.57,3.69,;-6.24,-2.47,;-4.9,-1.7,)| Show InChI InChI=1S/C27H32N4O5/c1-33-21-5-3-18(4-6-21)27(32)30-10-9-23(17-30)36-22-7-8-25-24(14-22)31(11-12-35-25)20-13-19(15-28)26(34-2)29-16-20/h7-8,13-14,16,18,21,23H,3-6,9-12,17H2,1-2H3/t18?,21?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204077

(US9539260, E3 | US9763952, Example E3)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H30N4O5/c1-16-11-18(13-26-23(16)30-2)28-7-10-32-21-14-25-22(12-20(21)28)33-19-3-6-27(15-19)24(29)17-4-8-31-9-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204083

(US9539260, E11 | US9763952, Example E11)Show SMILES Cc1cc(cnc1OC(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CC3)cc12 |r| Show InChI InChI=1S/C22H24F2N4O4/c1-13-8-15(10-26-20(13)32-22(23)24)28-6-7-30-18-11-25-19(9-17(18)28)31-16-4-5-27(12-16)21(29)14-2-3-14/h8-11,14,16,22H,2-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM341005
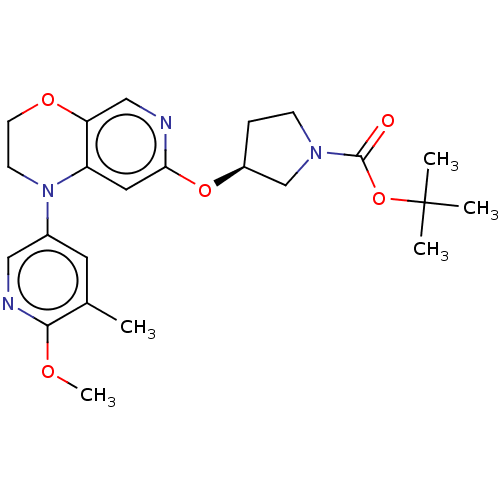
(US9763952, Example E1 | US9763952, Example F1 | {(...)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)OC(C)(C)C)cc12 |r| Show InChI InChI=1S/C23H30N4O5/c1-15-10-16(12-25-21(15)29-5)27-8-9-30-19-13-24-20(11-18(19)27)31-17-6-7-26(14-17)22(28)32-23(2,3)4/h10-13,17H,6-9,14H2,1-5H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204087

(US9539260, F4 | US9763952, Example F4)Show SMILES COc1ncc(cc1C#N)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H27N5O5/c1-31-23-17(12-25)10-18(13-27-23)29-6-9-33-21-14-26-22(11-20(21)29)34-19-2-5-28(15-19)24(30)16-3-7-32-8-4-16/h10-11,13-14,16,19H,2-9,15H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204089

(US9539260, F6 | US9763952, Example F6)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H30N4O6S/c1-16-11-18(13-26-23(16)35(2,30)31)28-7-10-33-21-14-25-22(12-20(21)28)34-19-3-6-27(15-19)24(29)17-4-8-32-9-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204090
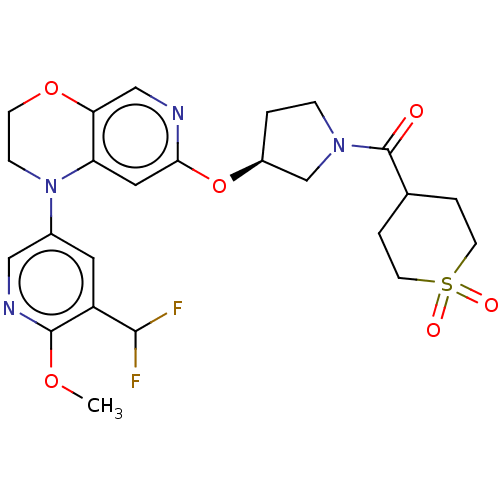
(US9539260, F7 | US9763952, Example F7)Show SMILES COc1ncc(cc1C(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCS(=O)(=O)CC3)cc12 |r| Show InChI InChI=1S/C24H28F2N4O6S/c1-34-23-18(22(25)26)10-16(12-28-23)30-6-7-35-20-13-27-21(11-19(20)30)36-17-2-5-29(14-17)24(31)15-3-8-37(32,33)9-4-15/h10-13,15,17,22H,2-9,14H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204092
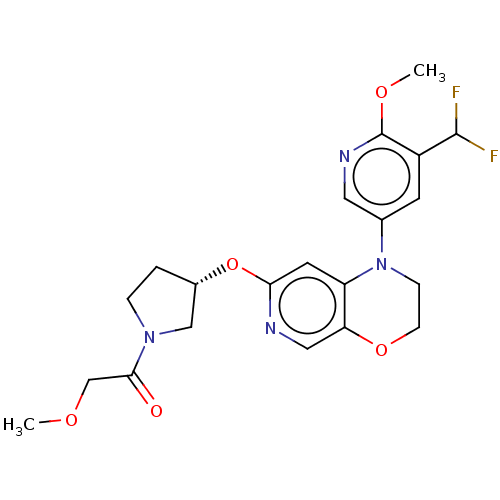
(US9539260, F9 | US9763952, Example F9)Show SMILES COCC(=O)N1CC[C@@H](C1)Oc1cc2N(CCOc2cn1)c1cnc(OC)c(c1)C(F)F |r| Show InChI InChI=1S/C21H24F2N4O5/c1-29-12-19(28)26-4-3-14(11-26)32-18-8-16-17(10-24-18)31-6-5-27(16)13-7-15(20(22)23)21(30-2)25-9-13/h7-10,14,20H,3-6,11-12H2,1-2H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204093
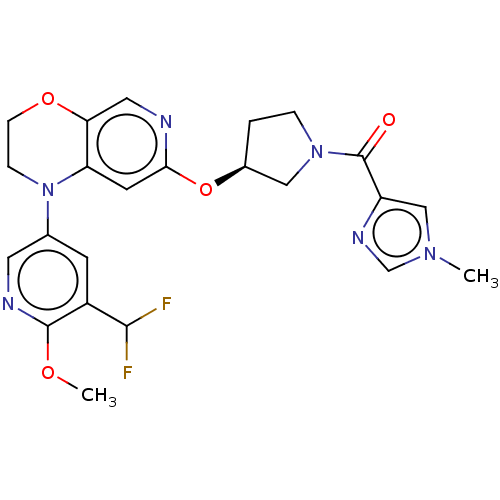
(US9539260, F10 | US9763952, Example F10)Show SMILES COc1ncc(cc1C(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)c3cn(C)cn3)cc12 |r| Show InChI InChI=1S/C23H24F2N6O4/c1-29-12-17(28-13-29)23(32)30-4-3-15(11-30)35-20-8-18-19(10-26-20)34-6-5-31(18)14-7-16(21(24)25)22(33-2)27-9-14/h7-10,12-13,15,21H,3-6,11H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204095

(US9539260, F12 | US9763952, Example F12)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(OC3CCN(C3)C(=O)C3CCS(=O)(=O)CC3)cc12 Show InChI InChI=1S/C24H30N4O6S/c1-16-11-18(13-26-23(16)32-2)28-7-8-33-21-14-25-22(12-20(21)28)34-19-3-6-27(15-19)24(29)17-4-9-35(30,31)10-5-17/h11-14,17,19H,3-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203425
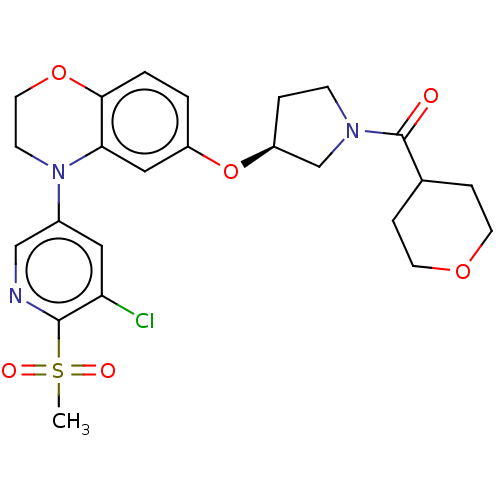
(US9539260, B79 | US9763952, Example B79)Show SMILES CS(=O)(=O)c1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H28ClN3O6S/c1-35(30,31)23-20(25)12-17(14-26-23)28-8-11-33-22-3-2-18(13-21(22)28)34-19-4-7-27(15-19)24(29)16-5-9-32-10-6-16/h2-3,12-14,16,19H,4-11,15H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203661
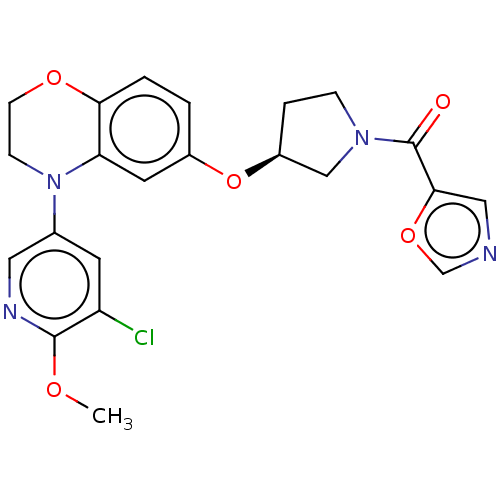
(US9539260, B108 | US9763952, Example B108)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)c3cnco3)cc12 |r| Show InChI InChI=1S/C22H21ClN4O5/c1-29-21-17(23)8-14(10-25-21)27-6-7-30-19-3-2-15(9-18(19)27)32-16-4-5-26(12-16)22(28)20-11-24-13-31-20/h2-3,8-11,13,16H,4-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203662
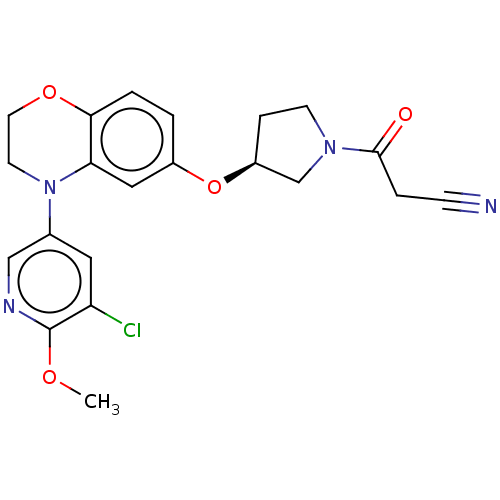
(US9539260, B109 | US9763952, Example B109)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)CC#N)cc12 |r| Show InChI InChI=1S/C21H21ClN4O4/c1-28-21-17(22)10-14(12-24-21)26-8-9-29-19-3-2-15(11-18(19)26)30-16-5-7-25(13-16)20(27)4-6-23/h2-3,10-12,16H,4-5,7-9,13H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203663
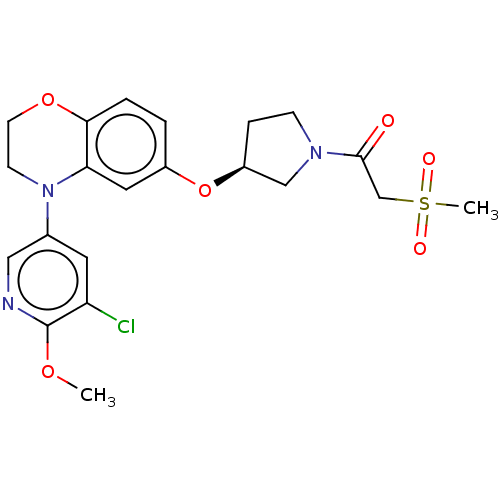
(US9539260, B110 | US9763952, Example B110)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)CS(C)(=O)=O)cc12 |r| Show InChI InChI=1S/C21H24ClN3O6S/c1-29-21-17(22)9-14(11-23-21)25-7-8-30-19-4-3-15(10-18(19)25)31-16-5-6-24(12-16)20(26)13-32(2,27)28/h3-4,9-11,16H,5-8,12-13H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203396
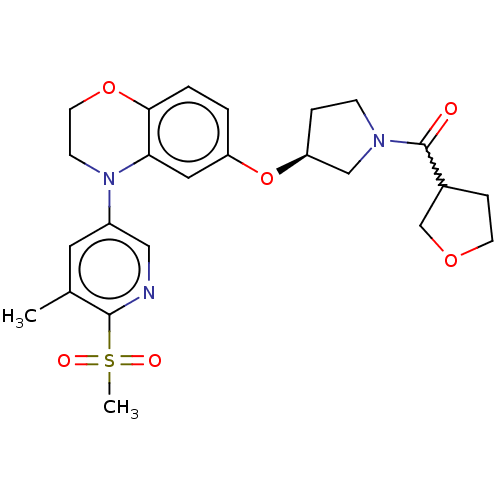
(US9539260, B52 | US9539260, B53 | US9763952, Examp...)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r,w:25.28| Show InChI InChI=1S/C24H29N3O6S/c1-16-11-18(13-25-23(16)34(2,29)30)27-8-10-32-22-4-3-19(12-21(22)27)33-20-5-7-26(14-20)24(28)17-6-9-31-15-17/h3-4,11-13,17,20H,5-10,14-15H2,1-2H3/t17?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204102
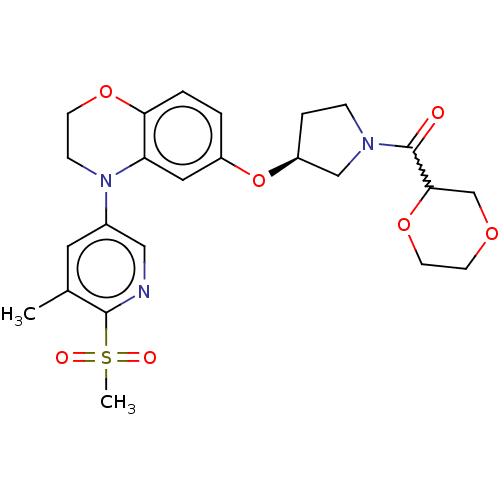
(US9539260, H3 | US9539260, H4 | US9763952, Example...)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3COCCO3)cc12 |r,w:25.28| Show InChI InChI=1S/C24H29N3O7S/c1-16-11-17(13-25-23(16)35(2,29)30)27-7-8-32-21-4-3-18(12-20(21)27)34-19-5-6-26(14-19)24(28)22-15-31-9-10-33-22/h3-4,11-13,19,22H,5-10,14-15H2,1-2H3/t19-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203381
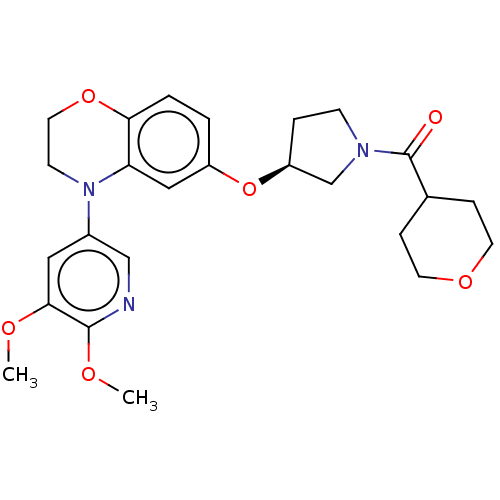
(US9539260, B38 | US9763952, Example B38)Show SMILES COc1cc(cnc1OC)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C25H31N3O6/c1-30-23-13-18(15-26-24(23)31-2)28-9-12-33-22-4-3-19(14-21(22)28)34-20-5-8-27(16-20)25(29)17-6-10-32-11-7-17/h3-4,13-15,17,20H,5-12,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203396

(US9539260, B52 | US9539260, B53 | US9763952, Examp...)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r,w:25.28| Show InChI InChI=1S/C24H29N3O6S/c1-16-11-18(13-25-23(16)34(2,29)30)27-8-10-32-22-4-3-19(12-21(22)27)33-20-5-7-26(14-20)24(28)17-6-9-31-15-17/h3-4,11-13,17,20H,5-10,14-15H2,1-2H3/t17?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203414

(US9539260, B70 | US9763952, Example B70)Show SMILES CS(=O)(=O)c1ncc(cc1C(F)F)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C25H29F2N3O6S/c1-37(32,33)24-20(23(26)27)12-17(14-28-24)30-8-11-35-22-3-2-18(13-21(22)30)36-19-4-7-29(15-19)25(31)16-5-9-34-10-6-16/h2-3,12-14,16,19,23H,4-11,15H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204120

(US9539260, K | US9763952, Example K)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)[C@@H]3CCN(C3)C(C)=O)cc12 |r| Show InChI InChI=1S/C26H29N5O5/c1-17(32)29-7-5-18(15-29)26(33)30-8-6-22(16-30)36-21-3-4-24-23(12-21)31(9-10-35-24)20-11-19(13-27)25(34-2)28-14-20/h3-4,11-12,14,18,22H,5-10,15-16H2,1-2H3/t18-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203908

(US9539260, C24 | US9539260, C25 | US9763952, Examp...)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3COCCO3)cc12 |r,w:24.27| Show InChI InChI=1S/C24H26N4O6/c1-30-23-16(12-25)10-17(13-26-23)28-6-7-32-21-3-2-18(11-20(21)28)34-19-4-5-27(14-19)24(29)22-15-31-8-9-33-22/h2-3,10-11,13,19,22H,4-9,14-15H2,1H3/t19-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204008

(US9539260, D12 | US9763952, Example D12)Show SMILES COCC(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r| Show InChI InChI=1S/C22H24N4O5/c1-28-14-21(27)25-6-5-18(13-25)31-17-3-4-20-19(10-17)26(7-8-30-20)16-9-15(11-23)22(29-2)24-12-16/h3-4,9-10,12,18H,5-8,13-14H2,1-2H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204062

(US9539260, D28 | US9539260, D29 | US9763952, Examp...)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r| Show InChI InChI=1S/C24H26N4O5/c1-30-23-17(12-25)10-18(13-26-23)28-7-9-32-22-3-2-19(11-21(22)28)33-20-4-6-27(14-20)24(29)16-5-8-31-15-16/h2-3,10-11,13,16,20H,4-9,14-15H2,1H3/t16?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204070

(US9539260, D35 | US9539260, D36 | US9763952, Examp...)Show SMILES COC1CCC(CC1)C(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r,wD:13.16,(8.9,-5.23,;8.51,-3.74,;7.02,-3.35,;5.93,-4.43,;4.44,-4.04,;4.04,-2.55,;5.13,-1.46,;6.62,-1.86,;2.56,-2.15,;2.16,-.66,;1.47,-3.24,;.56,-4.48,;-.9,-4.01,;-.9,-2.47,;.56,-1.99,;-2.24,-1.7,;-3.57,-2.47,;-3.57,-4.01,;-4.9,-4.78,;-6.24,-4.01,;-7.57,-4.78,;-8.9,-4.01,;-8.9,-2.47,;-7.57,-1.7,;-7.57,-.16,;-8.9,.61,;-8.9,2.15,;-7.57,2.92,;-7.57,4.46,;-6.24,5.23,;-6.24,2.15,;-6.24,.61,;-4.9,2.92,;-3.57,3.69,;-6.24,-2.47,;-4.9,-1.7,)| Show InChI InChI=1S/C27H32N4O5/c1-33-21-5-3-18(4-6-21)27(32)30-10-9-23(17-30)36-22-7-8-25-24(14-22)31(11-12-35-25)20-13-19(15-28)26(34-2)29-16-20/h7-8,13-14,16,18,21,23H,3-6,9-12,17H2,1-2H3/t18?,21?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204077

(US9539260, E3 | US9763952, Example E3)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H30N4O5/c1-16-11-18(13-26-23(16)30-2)28-7-10-32-21-14-25-22(12-20(21)28)33-19-3-6-27(15-19)24(29)17-4-8-31-9-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204083

(US9539260, E11 | US9763952, Example E11)Show SMILES Cc1cc(cnc1OC(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CC3)cc12 |r| Show InChI InChI=1S/C22H24F2N4O4/c1-13-8-15(10-26-20(13)32-22(23)24)28-6-7-30-18-11-25-19(9-17(18)28)31-16-4-5-27(12-16)21(29)14-2-3-14/h8-11,14,16,22H,2-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data