

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin S (Homo sapiens (Human)) | BDBM19622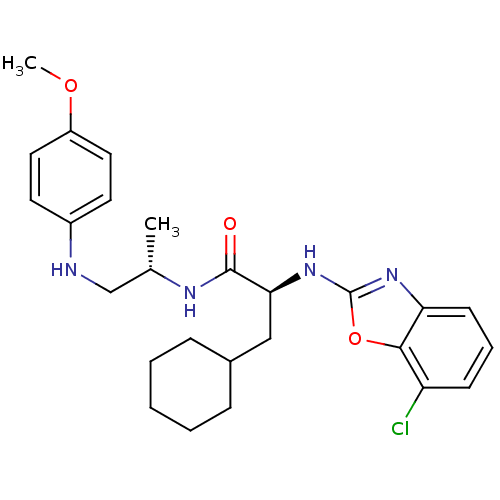 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19627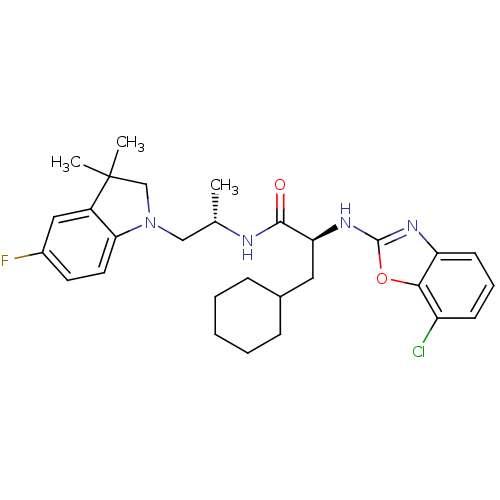 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -50.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19716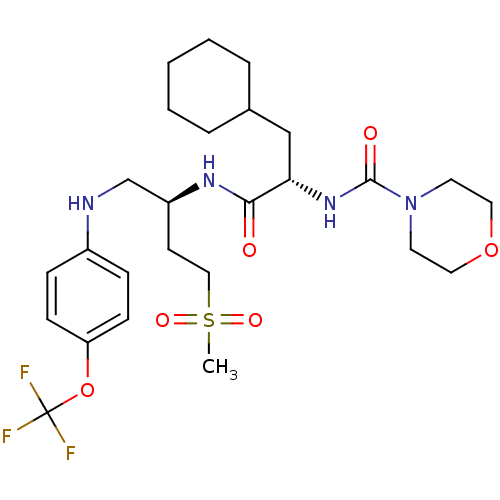 ((2S)-3-cyclohexyl-N-[(2S)-4-methanesulfonyl-1-{[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19631 ((2S)-2-(1,3-benzoxazol-2-ylamino)-N-[(2R)-1-(benzy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19716 ((2S)-3-cyclohexyl-N-[(2S)-4-methanesulfonyl-1-{[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5 | -49.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19704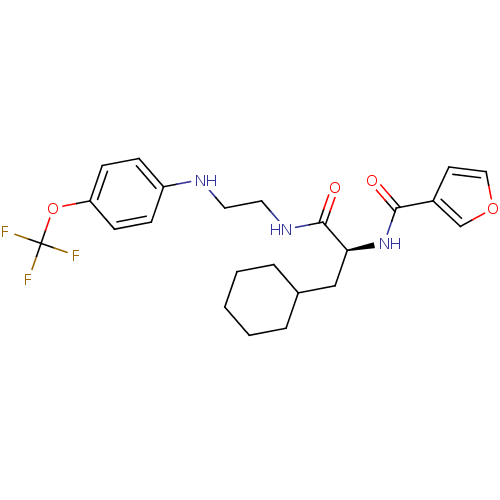 ((2S)-3-cyclohexyl-2-(furan-3-ylformamido)-N-(2-{[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6 | -48.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19715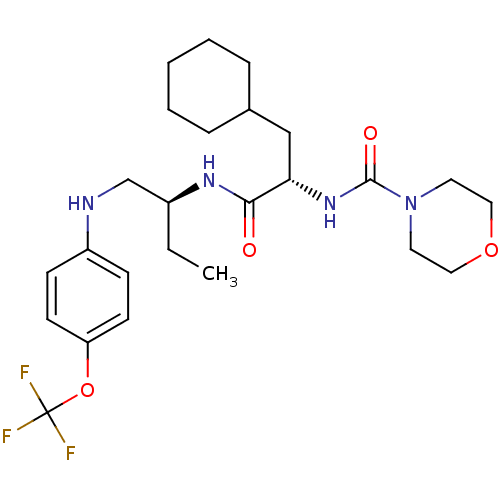 ((2S)-3-cyclohexyl-2-(morpholin-4-ylcarbonylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19488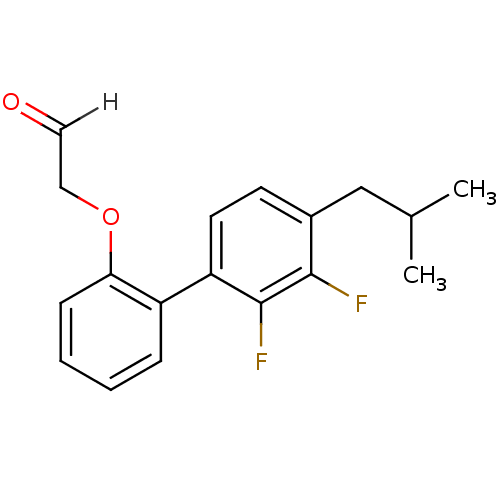 (2-{2-[2,3-difluoro-4-(2-methylpropyl)phenyl]phenox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.60 | -47.6 | n/a | n/a | n/a | n/a | n/a | 6.1 | 37 |
University of California at Berkeley | Assay Description The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read... | J Med Chem 50: 2693-9 (2007) Article DOI: 10.1021/jm070111+ BindingDB Entry DOI: 10.7270/Q2BP012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19726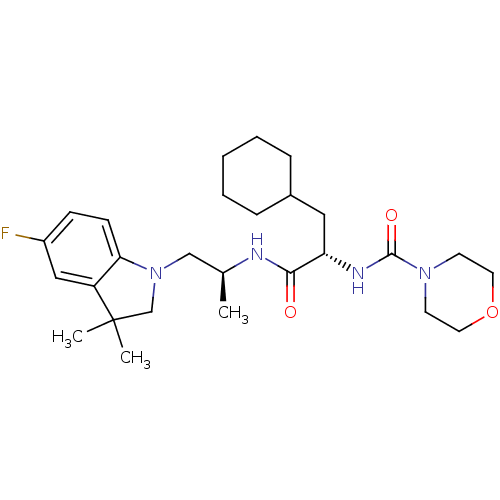 ((2S)-3-cyclohexyl-N-[(2S)-1-(5-fluoro-3,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19714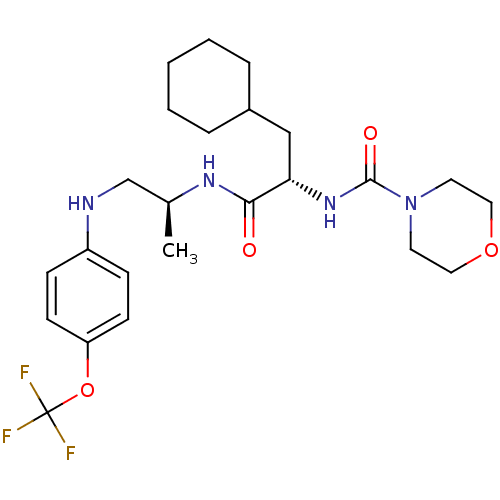 ((2S)-3-cyclohexyl-2-(morpholin-4-ylcarbonylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19715 ((2S)-3-cyclohexyl-2-(morpholin-4-ylcarbonylamino)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19623 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19700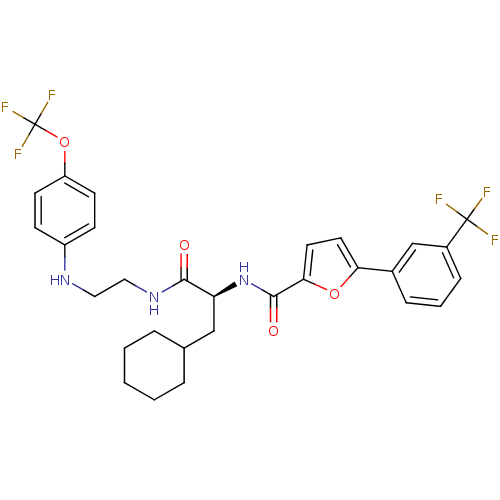 ((2S)-3-cyclohexyl-N-(2-{[4-(trifluoromethoxy)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -47.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19722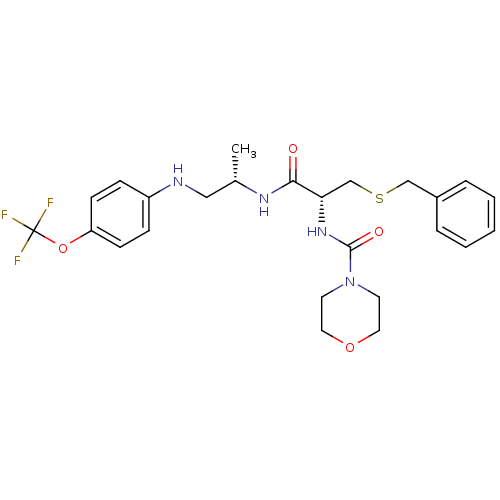 ((2R)-3-(benzylsulfanyl)-2-(morpholin-4-ylcarbonyla...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | -47.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19634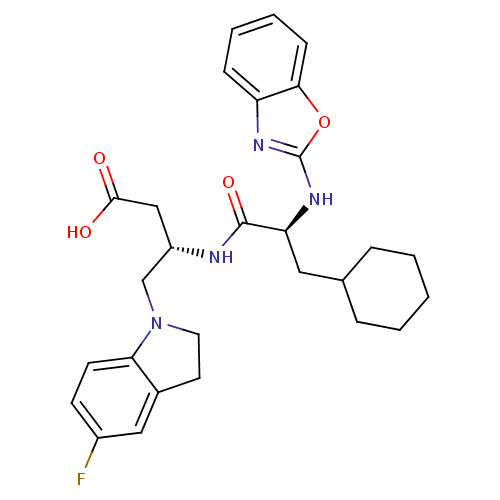 ((3S)-3-[(2S)-2-(1,3-benzoxazol-2-ylamino)-3-cycloh...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -47.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19713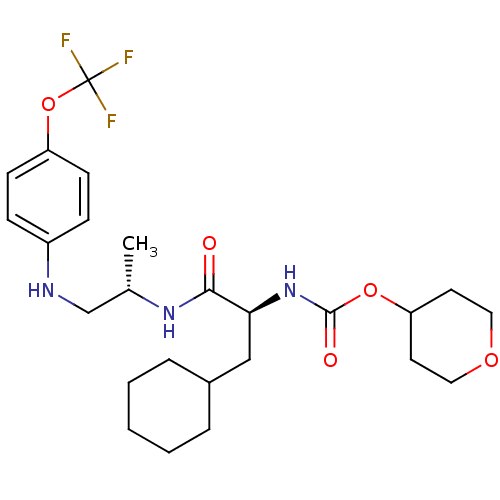 (arylaminoethyl amide deriv. 27 | oxan-4-yl N-[(1S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -47.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246998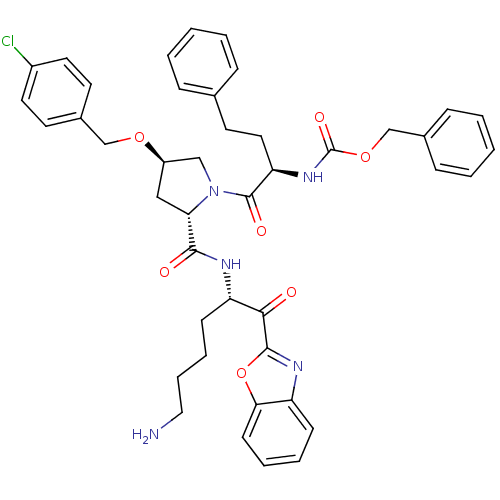 (CHEMBL505558 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19712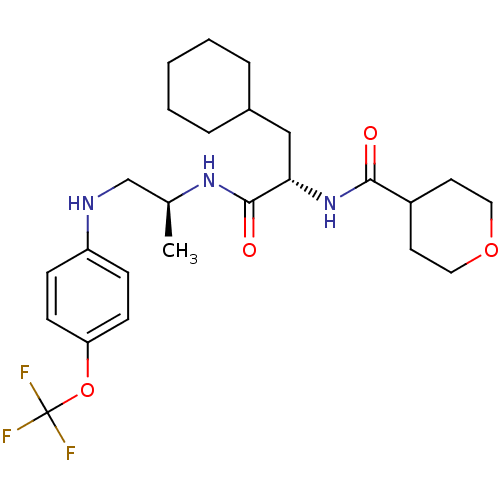 ((2S)-3-cyclohexyl-2-(oxan-4-ylformamido)-N-[(2S)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 12 | -47.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19625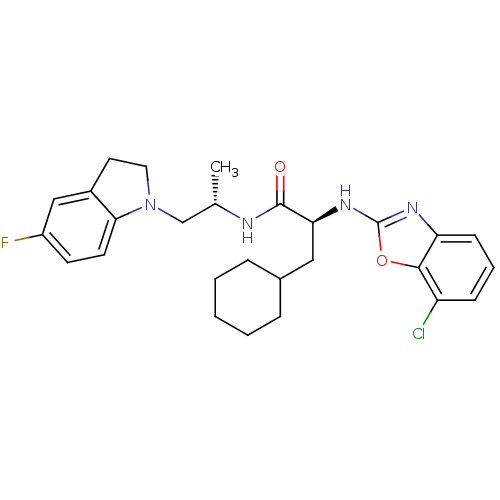 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19626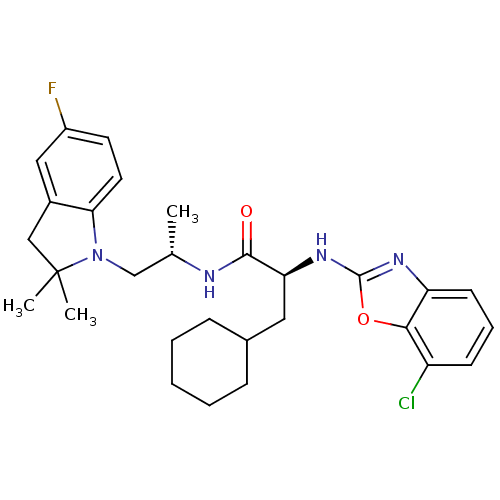 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19484 (1, 2, 3 -Triazole Nitrile Inhibitor, 11e | N-[(1S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -46.5 | n/a | n/a | n/a | n/a | n/a | 6.1 | 37 |
University of California at Berkeley | Assay Description The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read... | J Med Chem 49: 6298-307 (2006) Article DOI: 10.1021/jm060701s BindingDB Entry DOI: 10.7270/Q2GF0RSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19702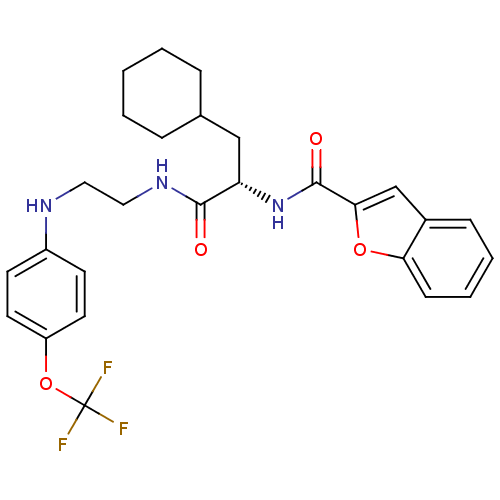 ((2S)-2-(1-benzofuran-2-ylformamido)-3-cyclohexyl-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 17 | -46.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19483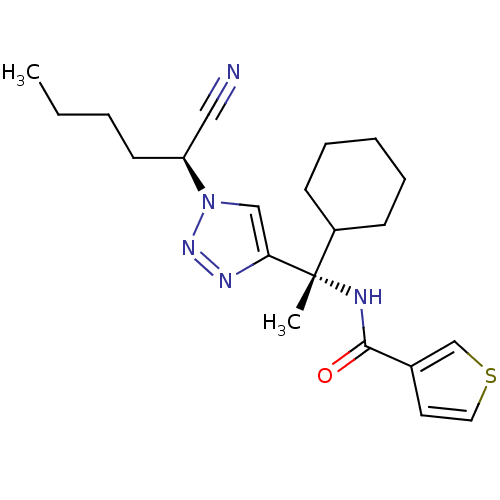 (1, 2, 3 -Triazole Nitrile Inhibitor, 11d | N-[(1S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -46.0 | n/a | n/a | n/a | n/a | n/a | 6.1 | 37 |
University of California at Berkeley | Assay Description The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read... | J Med Chem 49: 6298-307 (2006) Article DOI: 10.1021/jm060701s BindingDB Entry DOI: 10.7270/Q2GF0RSV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19707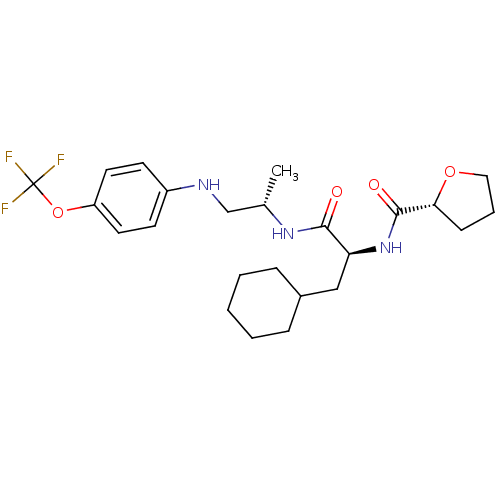 ((2S)-3-cyclohexyl-2-[(2R)-oxolan-2-ylformamido]-N-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 18 | -46.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246999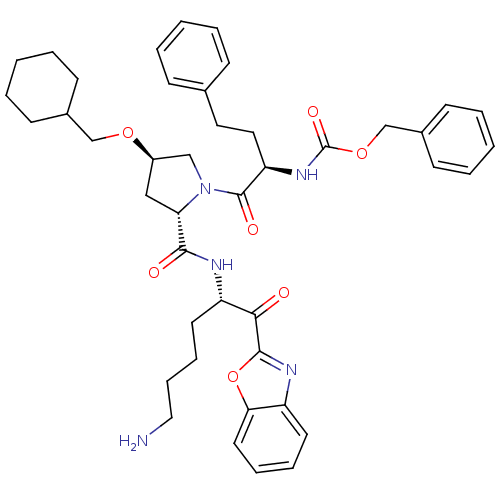 (CHEMBL500474 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19620 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | -45.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19709 ((2S)-3-cyclohexyl-2-(oxolan-3-ylformamido)-N-[(2S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -45.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19633 ((2S)-2-[(6-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 21 | -45.6 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19622 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19630 ((2S)-2-(1,3-benzoxazol-2-ylamino)-3-cyclohexyl-N-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -45.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19624 ((2S)-2-[(7-chloro-1,3-benzoxazol-2-yl)amino]-3-cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 26 | -45.0 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246997 (CHEMBL505738 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19635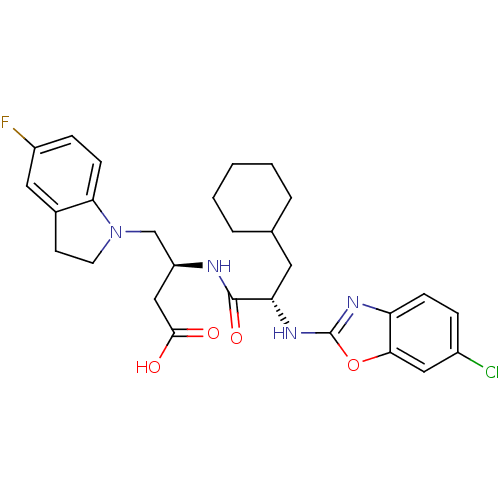 ((3S)-3-[(2S)-2-[(6-chloro-1,3-benzoxazol-2-yl)amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | -44.9 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246995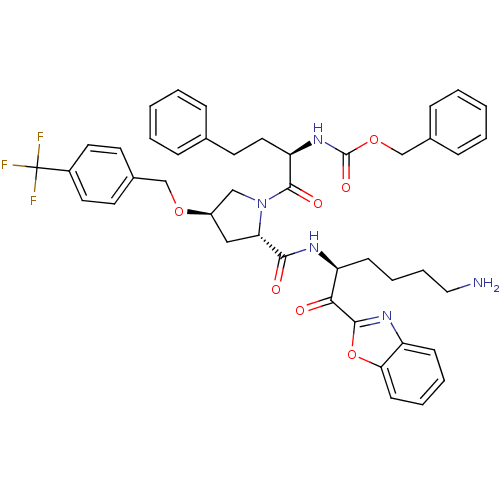 (CHEMBL505048 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19614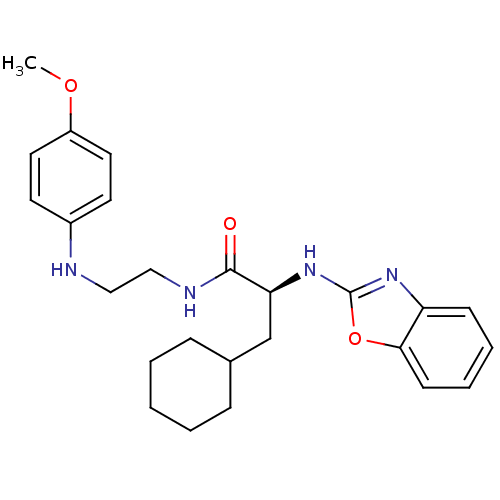 ((2S)-2-(1,3-benzoxazol-2-ylamino)-3-cyclohexyl-N-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | DrugBank MMDB PDB Article PubMed | 29 | -44.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50247004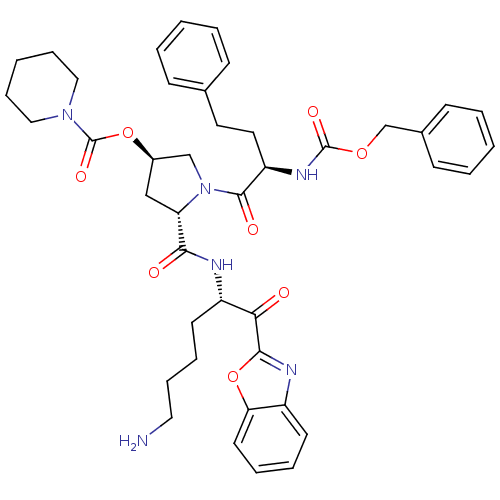 ((3R,5S)-5-(((S)-6-amino-1-(benzo[d]oxazol-2-yl)-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19723 ((2R)-2-(morpholin-4-ylcarbonylamino)-3-(phenylmeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 32 | -44.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19701 ((2S)-3-cyclohexyl-2-{[5-(3-fluorophenyl)furan-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 34 | -44.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246992 (CHEMBL498914 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246994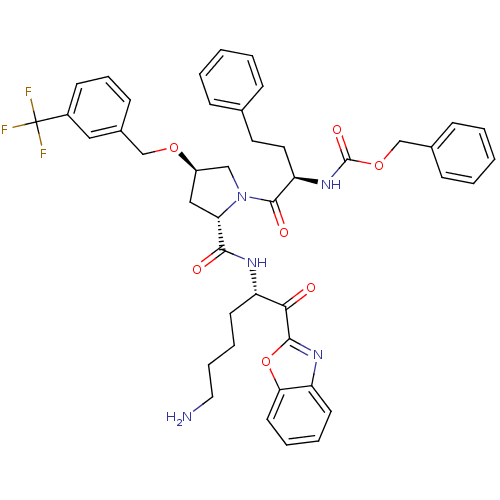 (CHEMBL443101 | benzyl (R)-1-((2S,4R)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19711 ((3R)-oxolan-3-yl N-[(1S)-2-cyclohexyl-1-{[(2S)-1-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 42 | -43.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19621 (Heterocyclic arylaminoethyl amide, 7g | methyl 2-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | -43.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19617 ((2S)-3-cyclohexyl-2-[(6-fluoro-1,3-benzoxazol-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 43 | -43.7 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 1975-80 (2006) Article DOI: 10.1016/j.bmcl.2005.12.095 BindingDB Entry DOI: 10.7270/Q2PZ573M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM19702 ((2S)-2-(1-benzofuran-2-ylformamido)-3-cyclohexyl-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246996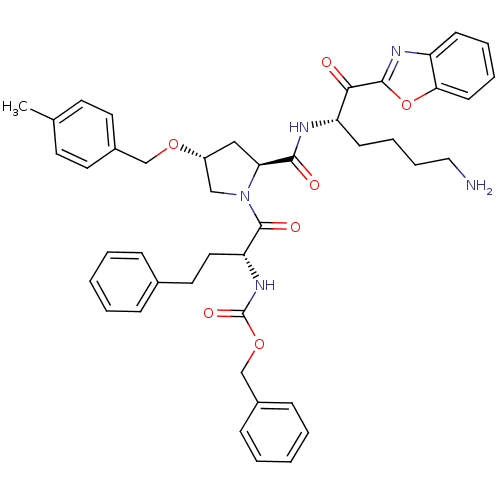 (CHEMBL506226 | {(R)-1-[(2S,4R)-2-[(S)-5-Amino-1-(b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19703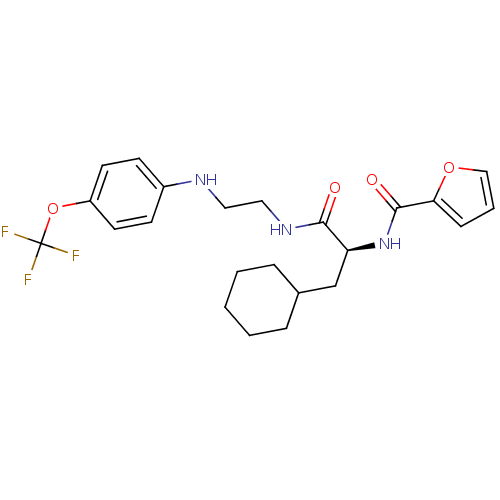 ((2S)-3-cyclohexyl-2-(furan-2-ylformamido)-N-(2-{[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 47 | -43.5 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19487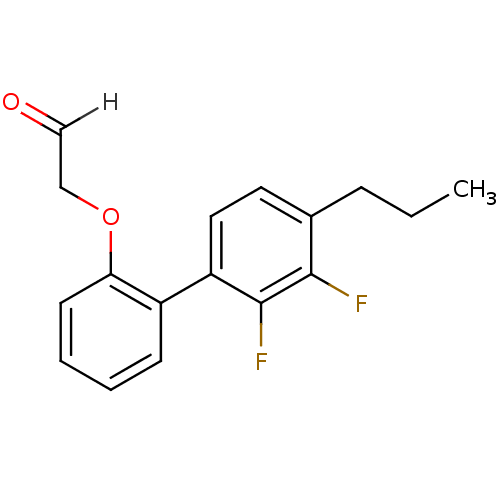 (2-[2-(2,3-difluoro-4-propylphenyl)phenoxy]acetalde...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 48.7 | -43.4 | n/a | n/a | n/a | n/a | n/a | 6.1 | 37 |
University of California at Berkeley | Assay Description The proteolytic cleavage of N-acyl aminocoumarins by cathepsins was conducted in Dynatech Microfluor fluorescence 96-well microtiter plates, and read... | J Med Chem 50: 2693-9 (2007) Article DOI: 10.1021/jm070111+ BindingDB Entry DOI: 10.7270/Q2BP012X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19710 ((3S)-oxolan-3-yl N-[(1S)-2-cyclohexyl-1-{[(2S)-1-{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -43.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostasin (Homo sapiens (Human)) | BDBM50246991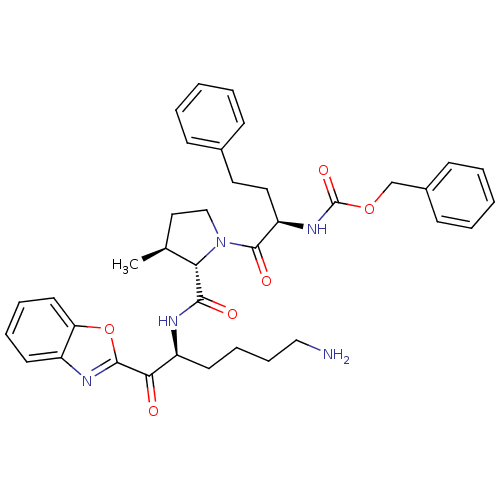 (CHEMBL509770 | benzyl (R)-1-((2S,3S)-2-(((S)-6-ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Binding affinity to human prostasin | Bioorg Med Chem Lett 18: 5895-9 (2008) Article DOI: 10.1016/j.bmcl.2008.08.029 BindingDB Entry DOI: 10.7270/Q2959HD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM19721 ((2S)-3-cyclopentyl-2-(morpholin-4-ylcarbonylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 49 | -43.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
GNF | Assay Description The recombinant human cathepsin enzyme was preincubated with inhibitor for 20 minutes prior to addition of substrate. The substrate hydrolysis was mo... | Bioorg Med Chem Lett 16: 5112-7 (2006) Article DOI: 10.1016/j.bmcl.2006.07.033 BindingDB Entry DOI: 10.7270/Q29P2ZXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 378 total ) | Next | Last >> |