

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamine--fructose-6-phosphate aminotransferase [isomerizing] 1 (Homo sapiens (Human)) | BDBM50356009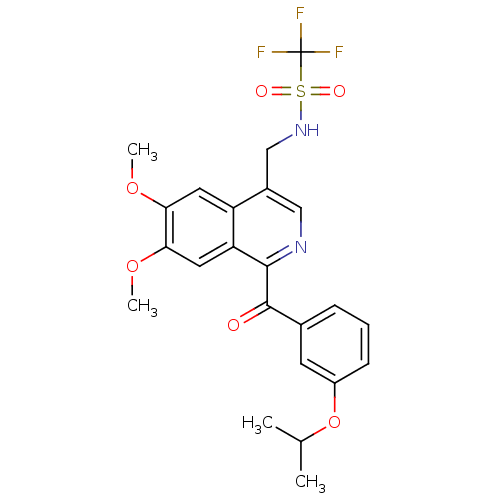 (CHEMBL1911368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Competitive inhibition of GFAT in presence of glutamine | Bioorg Med Chem Lett 21: 6264-9 (2011) Article DOI: 10.1016/j.bmcl.2011.09.009 BindingDB Entry DOI: 10.7270/Q21J9B6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine--fructose-6-phosphate aminotransferase [isomerizing] 1 (Homo sapiens (Human)) | BDBM50356009 (CHEMBL1911368) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Noncompetitive inhibition of GFAT in presence of fructose-6-phosphate | Bioorg Med Chem Lett 21: 6264-9 (2011) Article DOI: 10.1016/j.bmcl.2011.09.009 BindingDB Entry DOI: 10.7270/Q21J9B6P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317628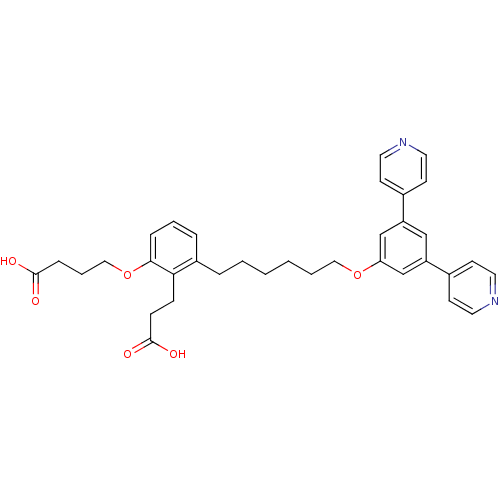 (4-{2-(2-Carboxy-ethyl)-3-[6-(3,5-di-pyridin-4-yl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317631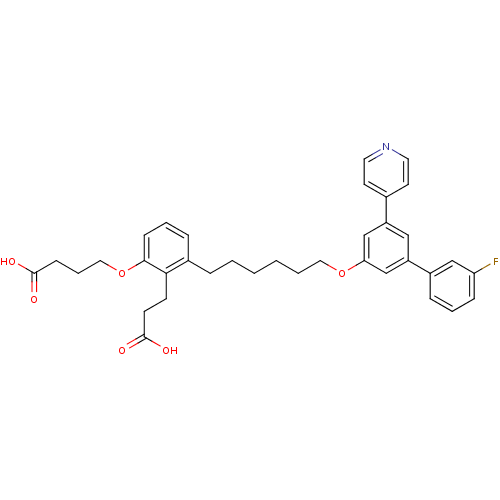 (4-{2-(2-Carboxy-ethyl)-3-[6-(3'-fluoro-5-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317632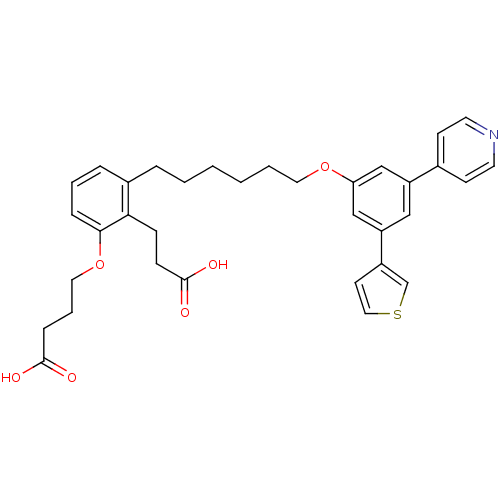 (4-{2-(2-Carboxy-ethyl)-3-[6-(3-pyridin-4-yl-5-thio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317625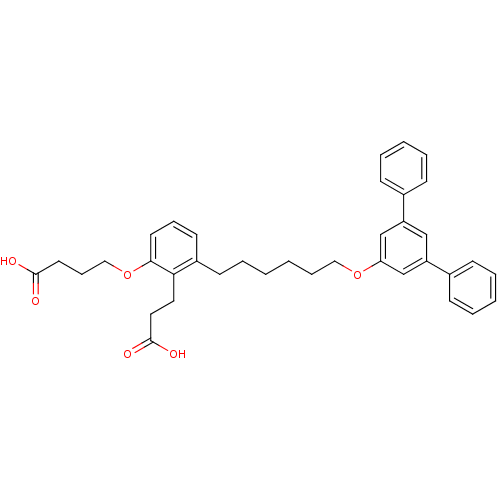 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317633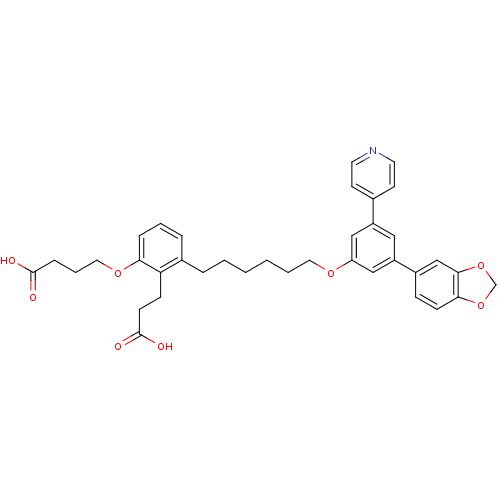 (4-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-pyridin-4-yl-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317634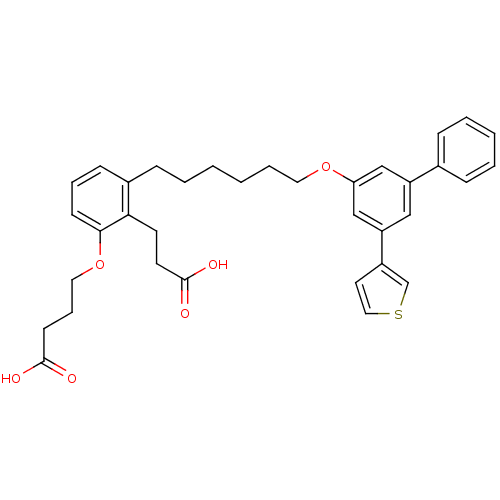 (4-{2-(2-Carboxyethyl)-3-[6-(5-thiophen-3-ylbipheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317635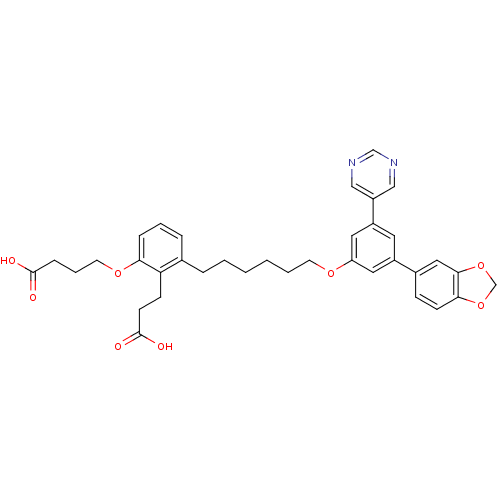 (4-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-pyrimidin-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317636 (4-{2-(2-Carboxy-ethyl)-3-[6-(3-pyrimidin-5-yl-5-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317626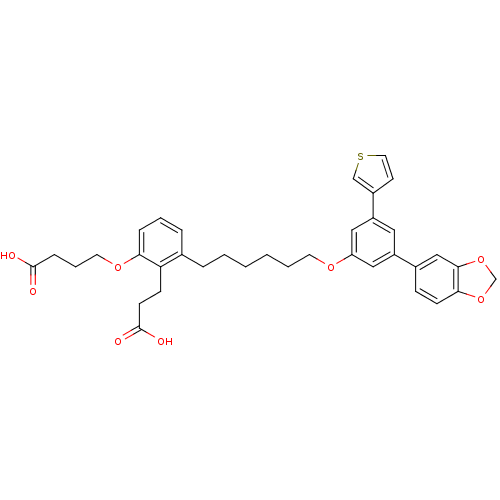 (4-{3-[6-(3-5-Benzo[1,3]dioxolyl-5-thiophen-3-ylphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317624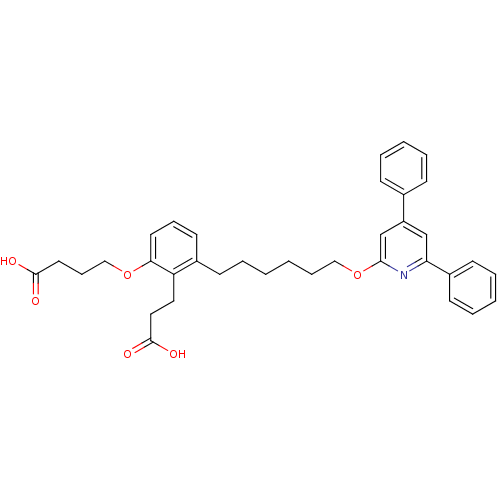 (4-(2-(2-carboxyethyl)-3-(6-(4,6-diphenylpyridin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317637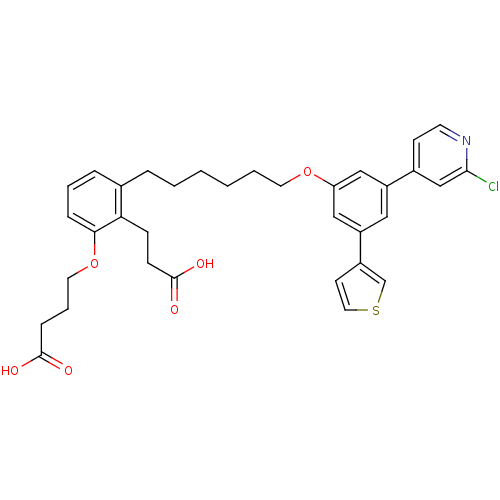 (4-(2-(2-Carboxy-ethyl)-3-{6-[3-(2-chloro-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317638 (4-{2-(2-Carboxy-ethyl)-3-[6-(3,5-di-thiophen-3-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317639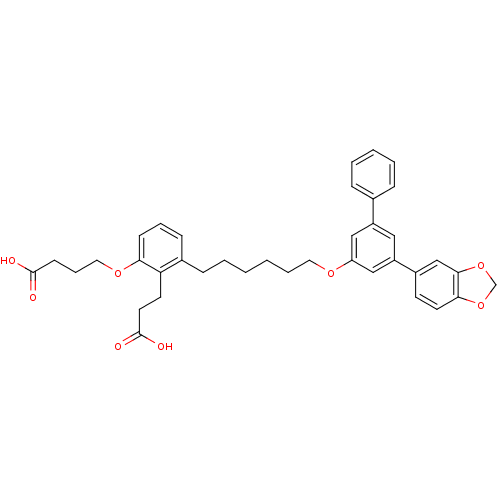 (4-[3-[6-(5-Benzo[1,3]dioxol-5-yl-biphenyl-3-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317630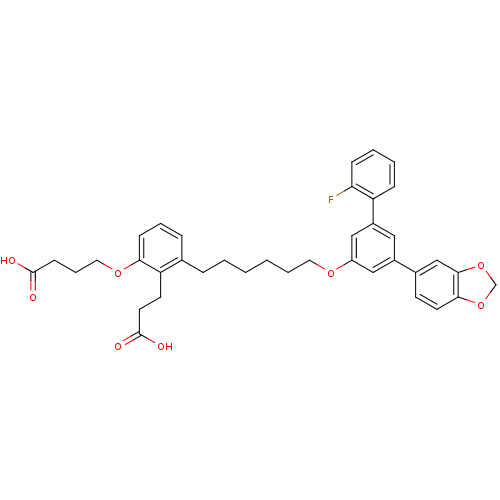 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-2'-fluorobip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317623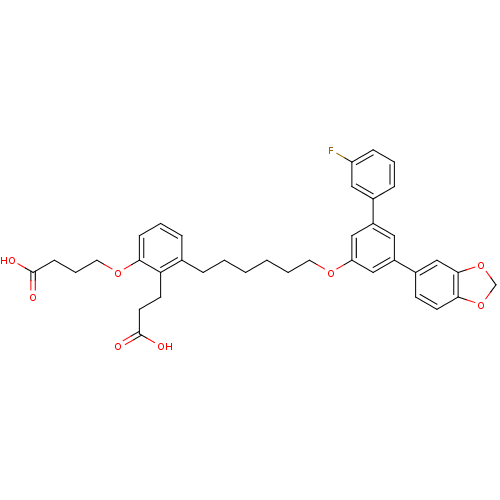 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-3'-fluorobip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317641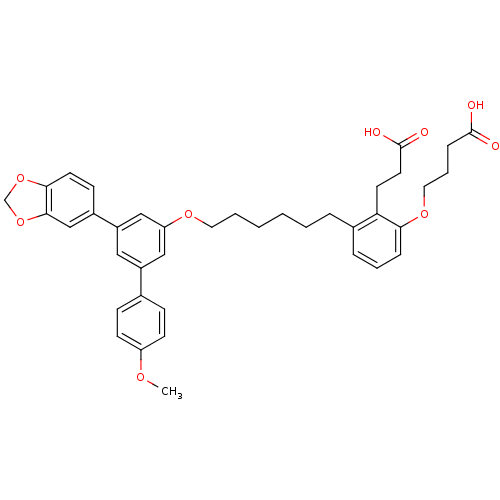 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-4'-methoxybi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441819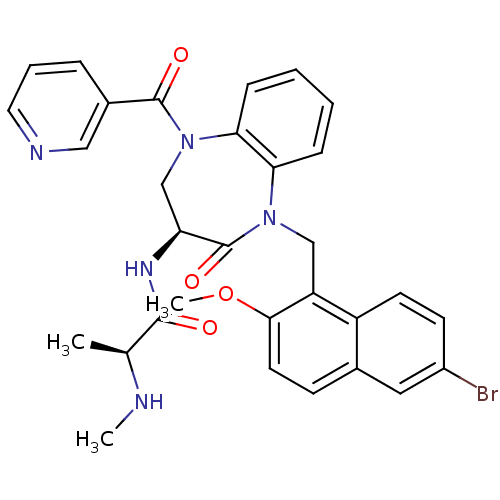 (CHEMBL2436209 | US10053431, 89b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317642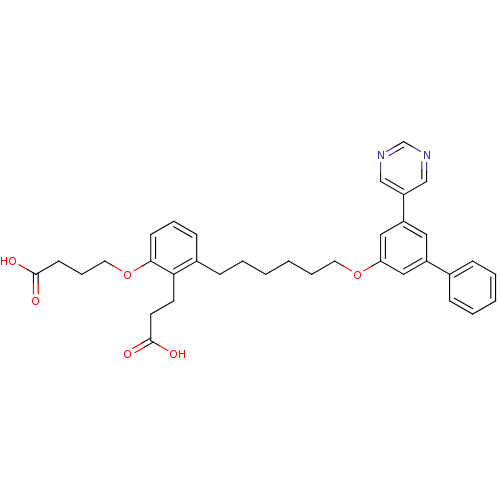 (4-{2-(2-Carboxyethyl)-3-[6-(3,5-dipyrimidin-5-ylph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317643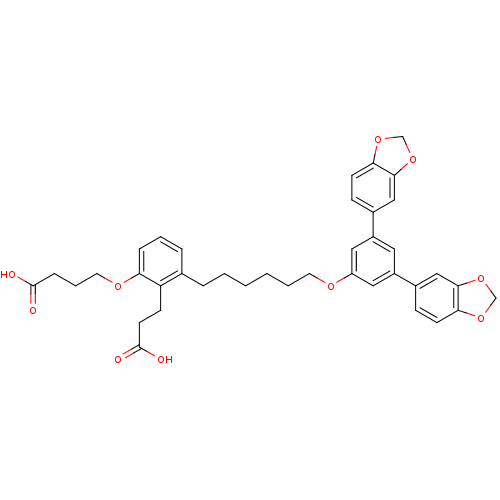 (4-[3-[6-(3,5-Bis-benzo[1,3]dioxol-5-yl-phenoxy)-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.39 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441832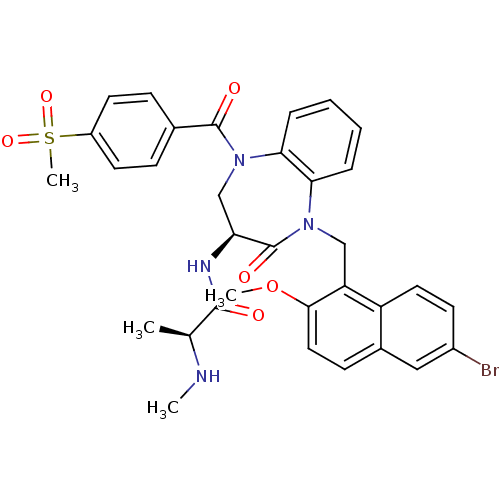 (CHEMBL2436205 | US10053431, 90d) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441816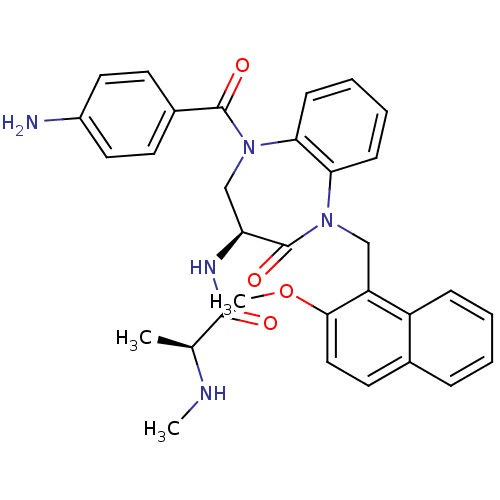 (CHEMBL2436208 | US10053431, 41) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441831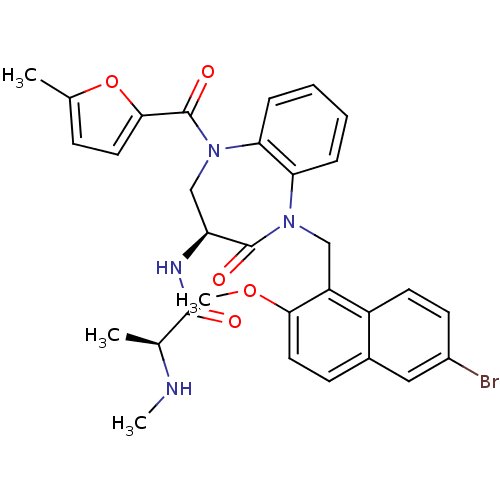 (CHEMBL2436212 | US10053431, 91c) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441834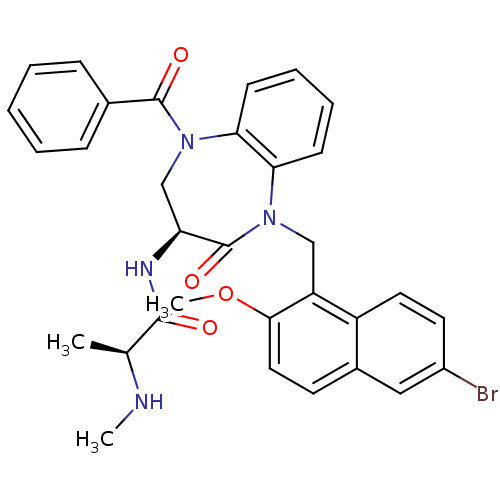 (CHEMBL2436330 | US10053431, 91a) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441833 (CHEMBL2436334 | US10053431, 75b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441818 (CHEMBL2436213 | US10053431, 75d) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441830 (CHEMBL2436210 | US10053431, 85) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441829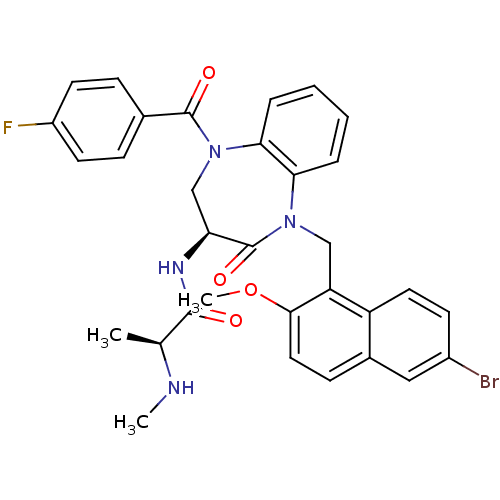 (CHEMBL2436331 | US10053431, 75a) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317644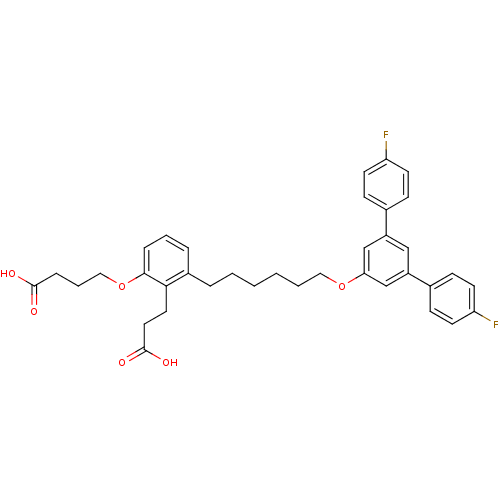 (4-{2-(2-Carboxy-ethyl)-3-[6-(4,4''-difluoro-[1.1':...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441810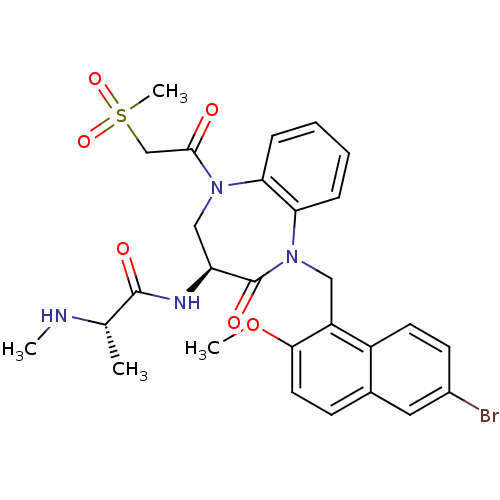 (CHEMBL2436215 | US10053431, 76b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441827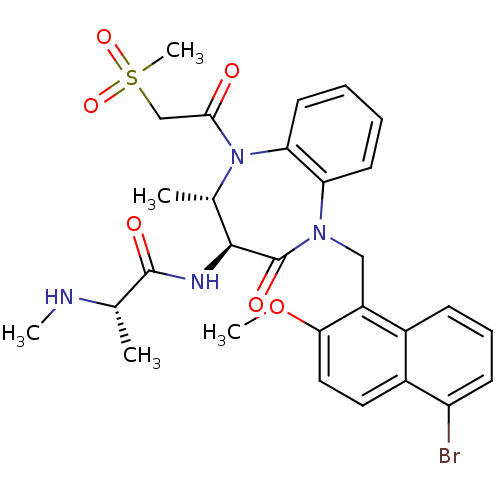 (CHEMBL2436217 | US9422331, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441828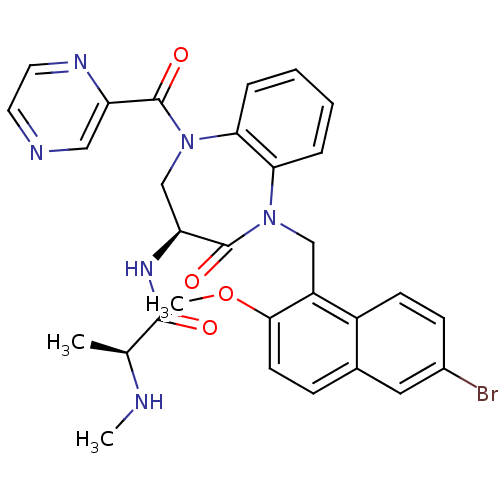 (CHEMBL2436211 | US10053431, 76c) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441826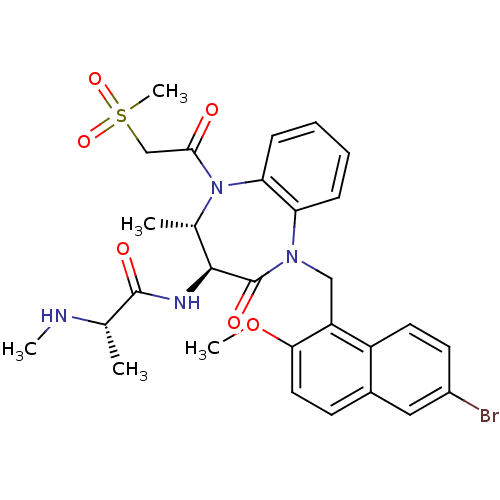 (CHEMBL2436216 | US9422331, 10) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441821 (CHEMBL2436223 | US9422331, 27) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441825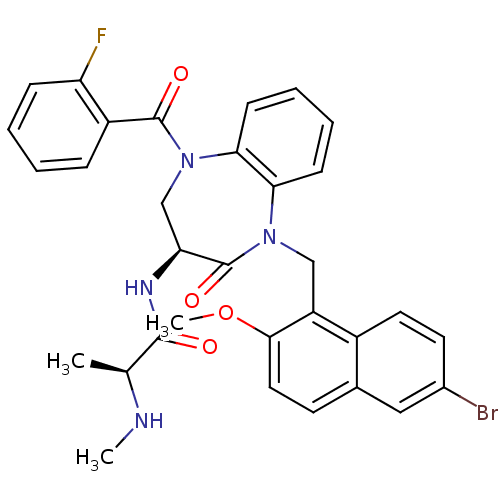 (CHEMBL2436333 | US10053431, 90a) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441817 (CHEMBL2436332 | US10053431, 90b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317645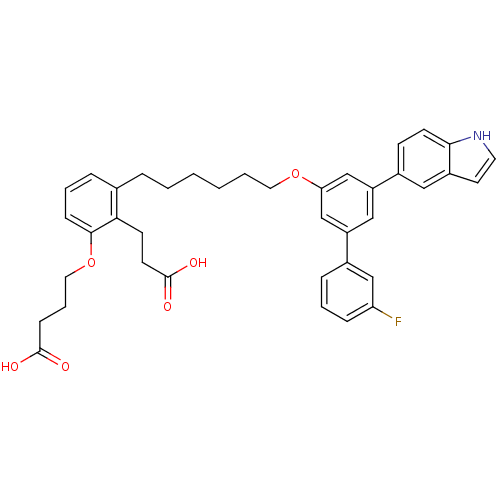 (4-(2-(2-Carboxy-ethyl)-3-{6-[3'-fluoro-5-(1H-indol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441811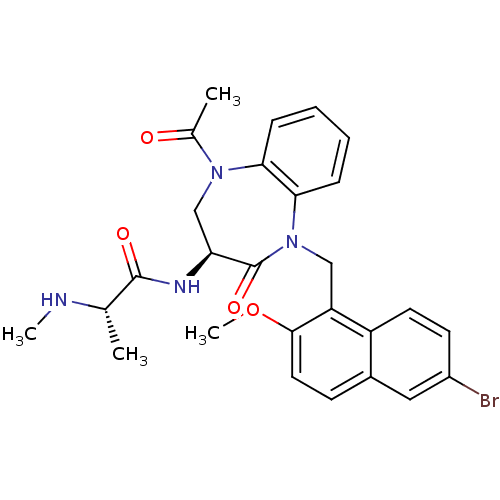 (CHEMBL2436214 | US10053431, 91b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441809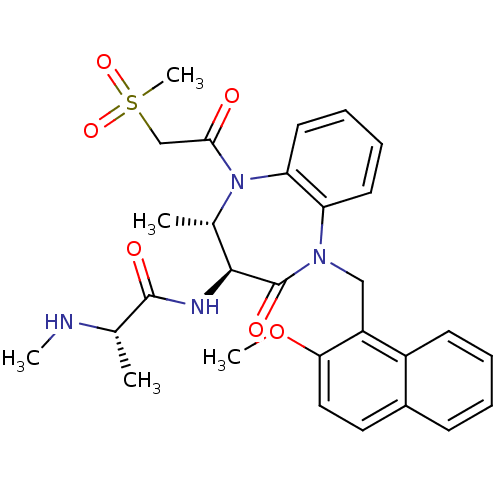 (CHEMBL2436218 | US9422331, 13) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441807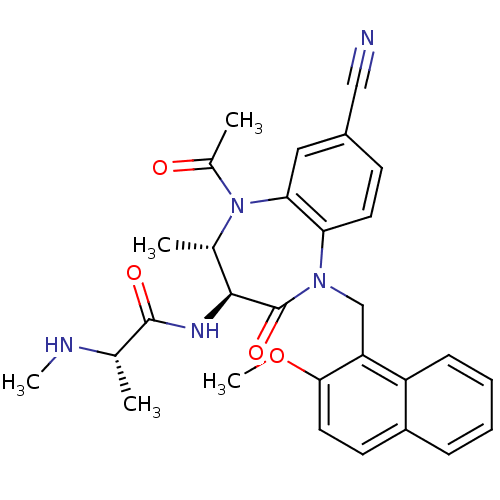 (CHEMBL2436225 | US9422331, 31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441820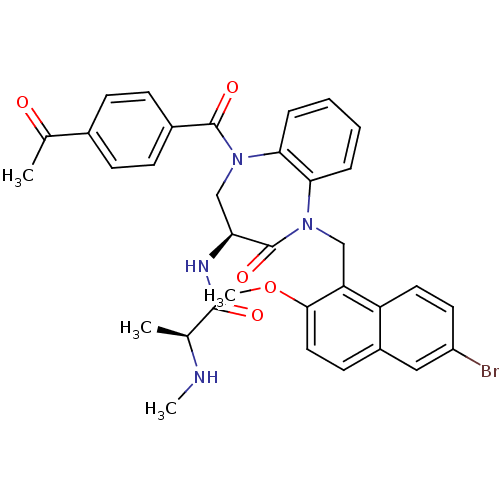 (CHEMBL2436206 | US10053431, 69l) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441356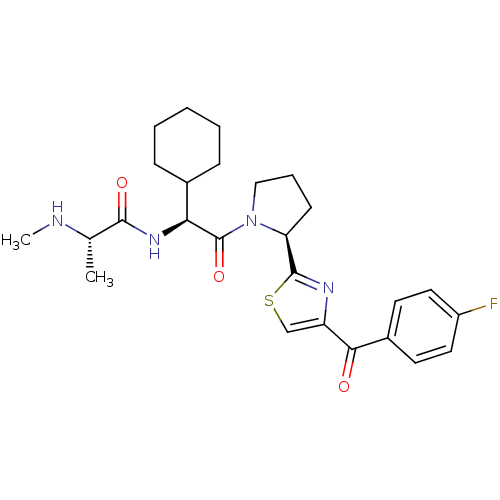 (CHEMBL2431768) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of XIAP BIR3 domain (unknown origin) | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317640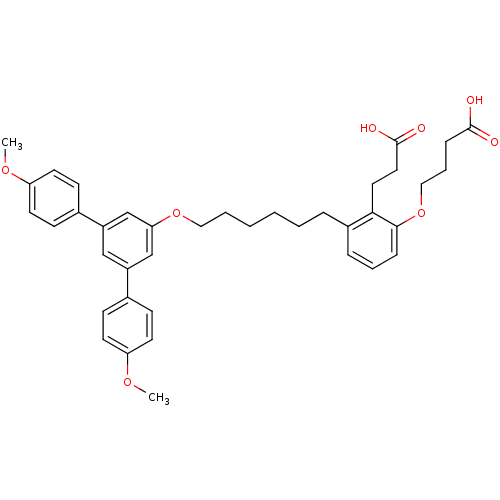 (4-{2-(2-Carboxy-ethyl)-3-[6-(4,4''-dimethoxy-[1.1'...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441334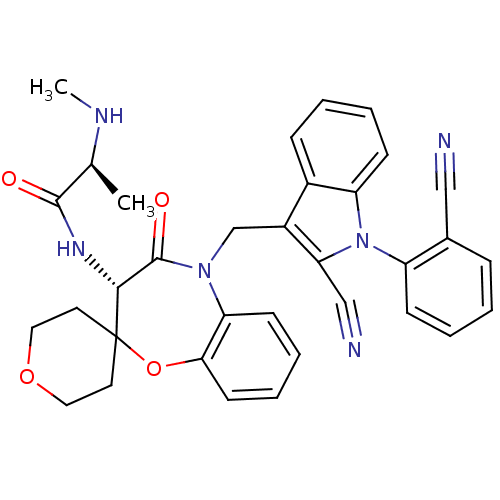 (CHEMBL2431755 | CHEMBL2436227) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441813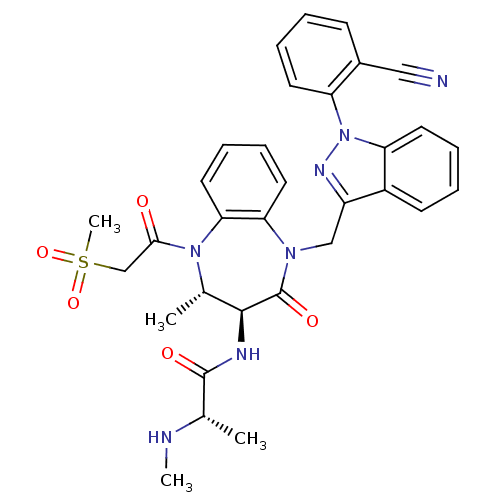 (CHEMBL2436222 | US9422331, 44) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441812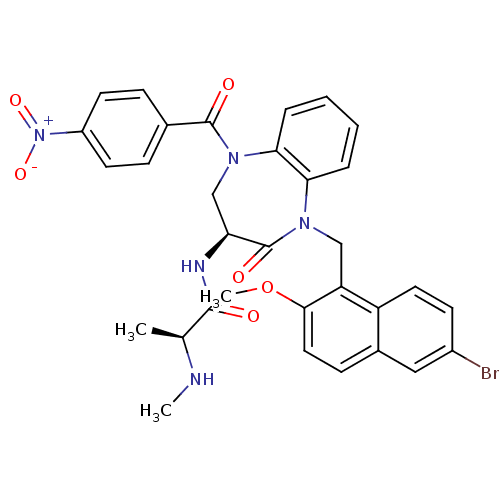 (CHEMBL2436207 | US10053431, 13m) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317625 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 38.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at FLAG-tagged human BLT1 receptor expressed in HEK293 cells assessed as inhibition of LTB4-stimulated calcium mobilization prein... | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441806 (CHEMBL2436226 | US9422331, 32) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441824 (CHEMBL2436224 | US9422331, 51) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 160 total ) | Next | Last >> |