

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399909 (CHEMBL2180945) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50287545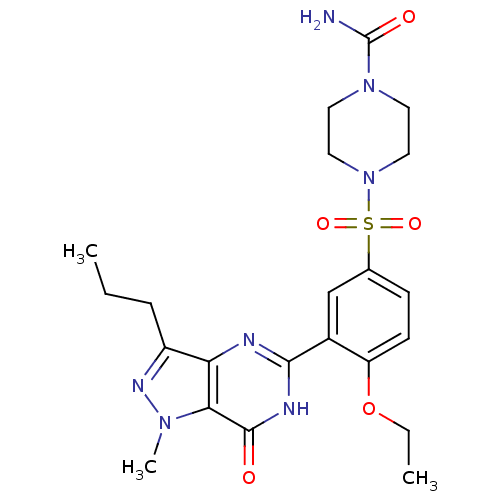 (4-[4-Ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Phosphodiesterase 5 from rabbit platelets | Bioorg Med Chem Lett 6: 1819-1824 (1996) Article DOI: 10.1016/0960-894X(96)00323-X BindingDB Entry DOI: 10.7270/Q2G73DQW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50161957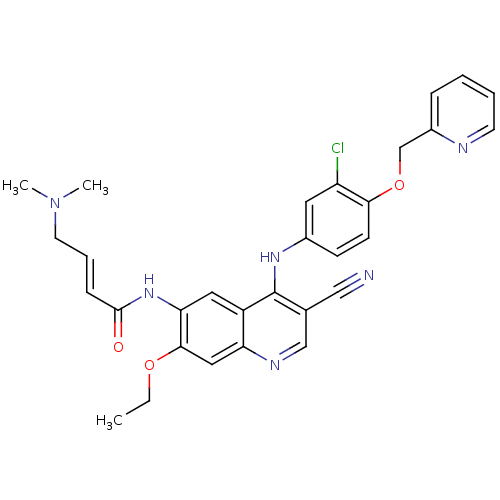 (4-Dimethylamino-but-2-enoic acid {4-[3-chloro-4-(p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of recombinant EGFR (unknown origin) after 10 mins by DELFIA/time-resolved fluorometry | Bioorg Med Chem 21: 3090-104 (2013) Article DOI: 10.1016/j.bmc.2013.03.053 BindingDB Entry DOI: 10.7270/Q2KP852S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50286792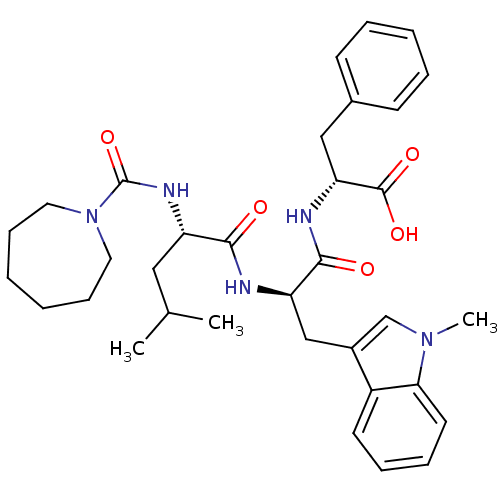 ((R)-2-[(R)-2-{(S)-2-[(Azepane-1-carbonyl)-amino]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against ETA receptor from dog spleen membranes using [125I]-ET1 as radioligand. | Bioorg Med Chem Lett 5: 917-922 (1995) Article DOI: 10.1016/0960-894X(95)00144-I BindingDB Entry DOI: 10.7270/Q28S4PWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399893 (CHEMBL2180948) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50112346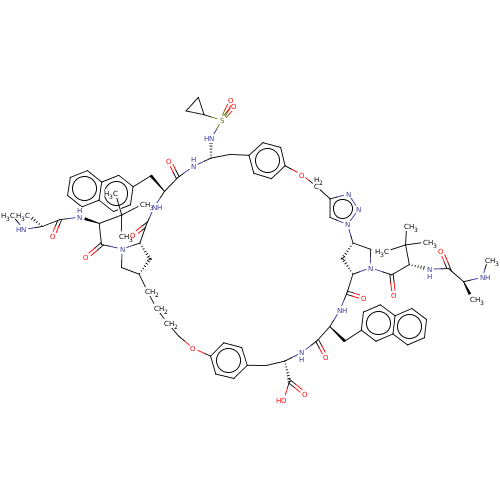 (CHEMBL3609325) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research Curated by ChEMBL | Assay Description Inhibition of cIAP BIR2-3 domain (unknown origin) | ACS Med Chem Lett 6: 770-5 (2015) Article DOI: 10.1021/acsmedchemlett.5b00091 BindingDB Entry DOI: 10.7270/Q29P33D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Phosphodiesterase 5 from human corpus cavernosum | Bioorg Med Chem Lett 6: 1819-1824 (1996) Article DOI: 10.1016/0960-894X(96)00323-X BindingDB Entry DOI: 10.7270/Q2G73DQW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Phosphodiesterase 5 from human corpus cavernosum | Bioorg Med Chem Lett 6: 1819-1824 (1996) Article DOI: 10.1016/0960-894X(96)00323-X BindingDB Entry DOI: 10.7270/Q2G73DQW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50078359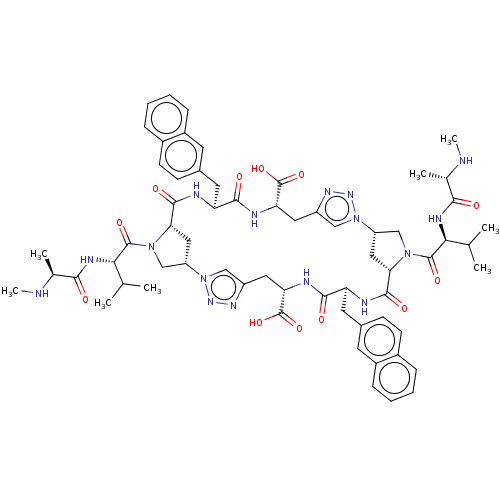 (CHEMBL3414729) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of cIAP1 BIR2-3 (154 to 352 residues) (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarization assay | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50112346 (CHEMBL3609325) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research Curated by ChEMBL | Assay Description Inhibition of XIAP BIR3 domain (unknown origin) | ACS Med Chem Lett 6: 770-5 (2015) Article DOI: 10.1021/acsmedchemlett.5b00091 BindingDB Entry DOI: 10.7270/Q29P33D2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM14390 (5-[2-ethoxy-5-(4-methyl-1-piperazinylsulfonyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399891 (CHEMBL2180950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399894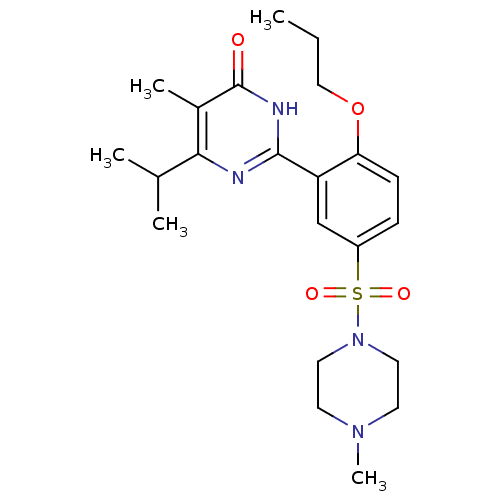 (CHEMBL2180947) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50078360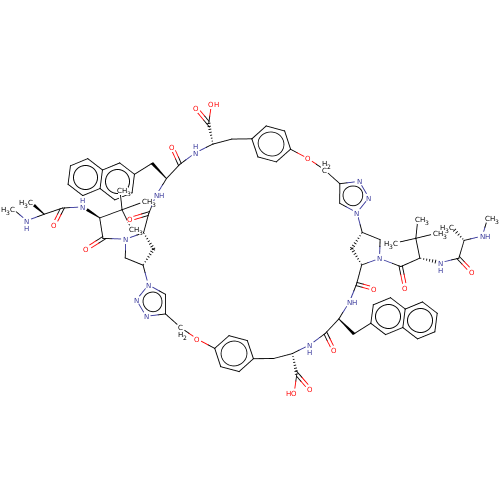 (CHEMBL3414728) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of cIAP1 BIR2-3 (154 to 352 residues) (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarization assay | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50286804 ((R)-2-[(R)-2-{(S)-2-[(Azepane-1-carbonyl)-amino]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against ETA receptor from dog spleen membranes using [125I]-ET1 as radioligand. | Bioorg Med Chem Lett 5: 917-922 (1995) Article DOI: 10.1016/0960-894X(95)00144-I BindingDB Entry DOI: 10.7270/Q28S4PWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50287543 (5-(2-ethoxy-5-(piperazin-1-ylsulfonyl)phenyl)-1-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Phosphodiesterase 5 from rabbit platelets | Bioorg Med Chem Lett 6: 1819-1824 (1996) Article DOI: 10.1016/0960-894X(96)00323-X BindingDB Entry DOI: 10.7270/Q2G73DQW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399896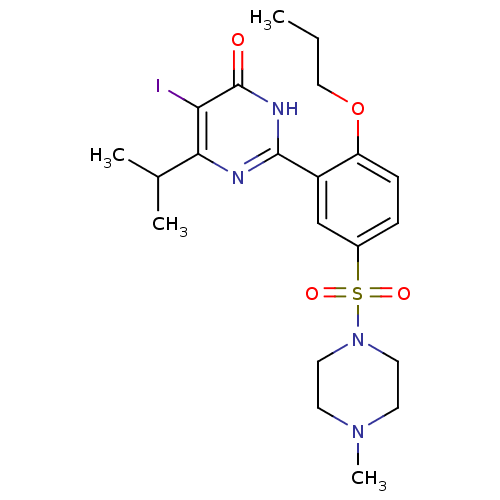 (CHEMBL2180943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129516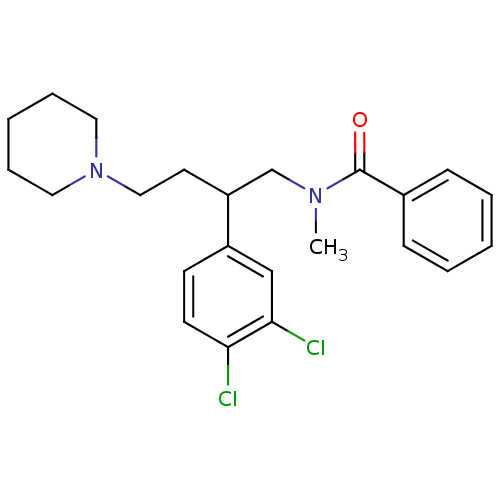 (CHEMBL71918 | N-[2-(3,4-Dichloro-phenyl)-4-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129511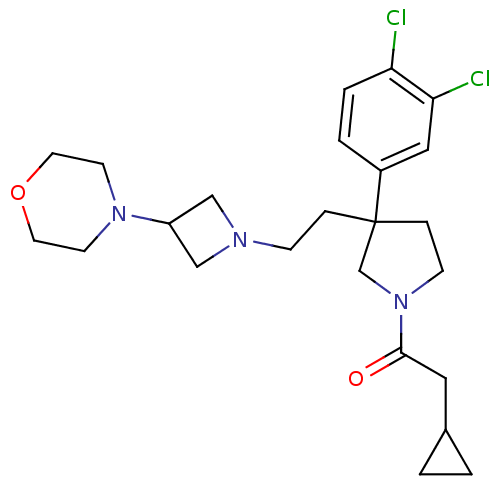 (2-Cyclopropyl-1-{3-(3,4-dichloro-phenyl)-3-[2-(3-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129509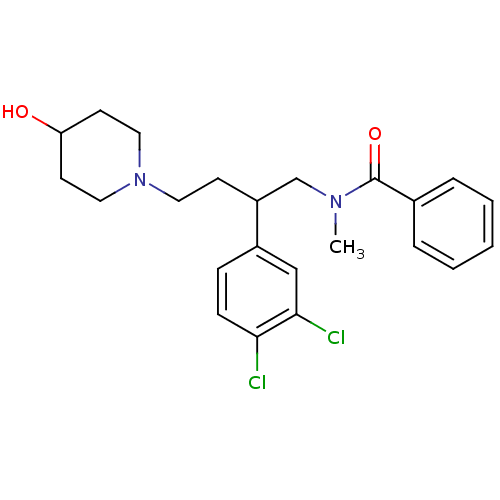 (CHEMBL69065 | N-[2-(3,4-Dichloro-phenyl)-4-(4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129506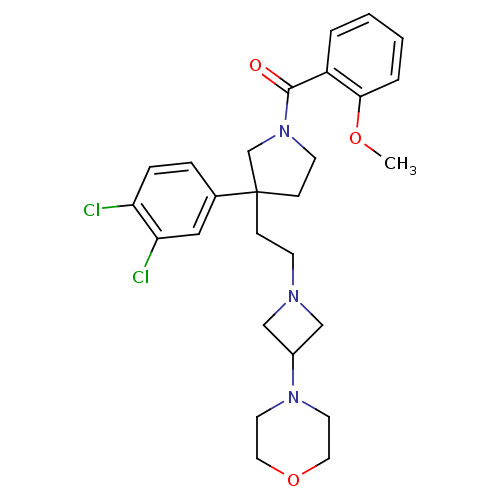 (CHEMBL308901 | {3-(3,4-Dichloro-phenyl)-3-[2-(3-mo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129517 (CHEMBL307338 | N-{2-(3,4-Dichloro-phenyl)-4-[4-hyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399898 (CHEMBL2180941) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129519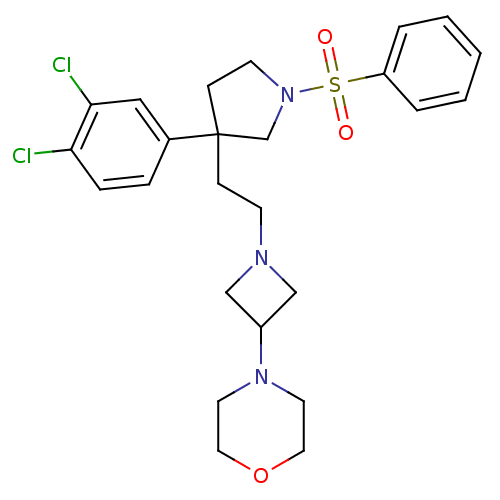 (4-(1-{2-[1-Benzenesulfonyl-3-(3,4-dichloro-phenyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129508 (CHEMBL305191 | N-[2-(3,4-Dichloro-phenyl)-4-(4-[1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129510 (CHEMBL71704 | N-[2-(3,4-Dichloro-phenyl)-4-(3-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50078354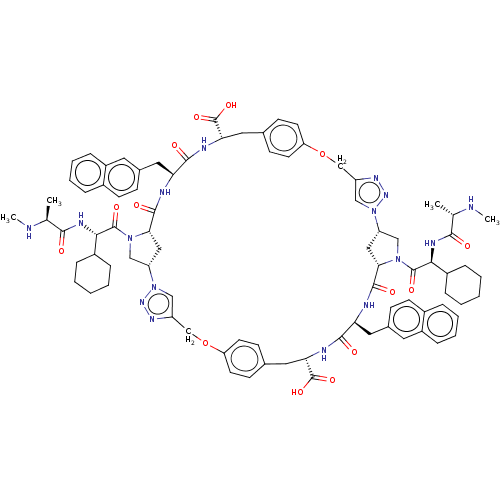 (CHEMBL3414727) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of cIAP1 BIR2-3 (154 to 352 residues) (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarization assay | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129520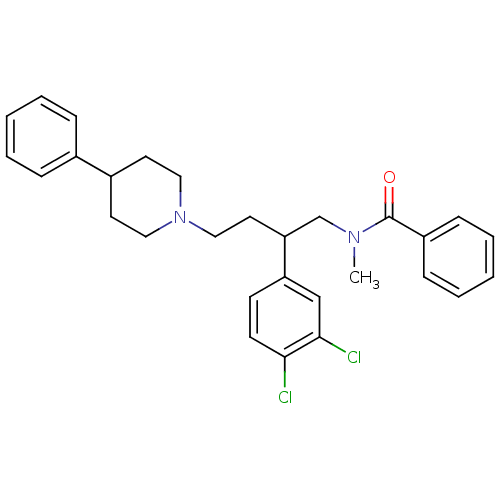 (CHEMBL69335 | N-[2-(3,4-Dichloro-phenyl)-4-(4-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | <7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129518 (CHEMBL71394 | N-{2-(3,4-Dichloro-phenyl)-4-[3-(2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399897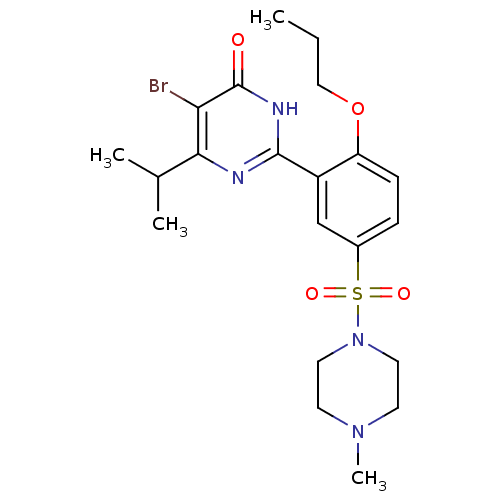 (CHEMBL2180942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129514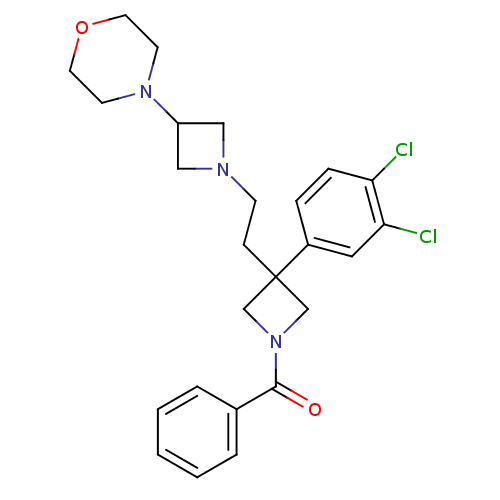 (CHEMBL71644 | {3-(3,4-Dichloro-phenyl)-3-[2-(3-mor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129512 (CHEMBL304807 | N-[4-[4-(1H-Benzoimidazol-2-yl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129515 (CHEMBL71226 | N-{2-(3,4-Dichloro-phenyl)-4-[3-(2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129505 (CHEMBL69889 | {3-(3,4-Dichloro-phenyl)-3-[2-(3-mor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129522 (CHEMBL68917 | N-[2-(3,4-Dichloro-phenyl)-4-(4-imid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129521 (CHEMBL71647 | N-[2-(3,4-Dichloro-phenyl)-4-(3-morp...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50286795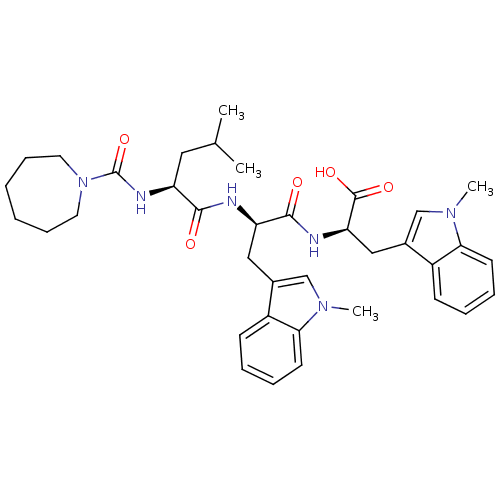 ((R)-2-[(R)-2-{(S)-2-[(Azepane-1-carbonyl)-amino]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against ETA receptor from dog spleen membranes using [125I]-ET1 as radioligand. | Bioorg Med Chem Lett 5: 917-922 (1995) Article DOI: 10.1016/0960-894X(95)00144-I BindingDB Entry DOI: 10.7270/Q28S4PWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50286800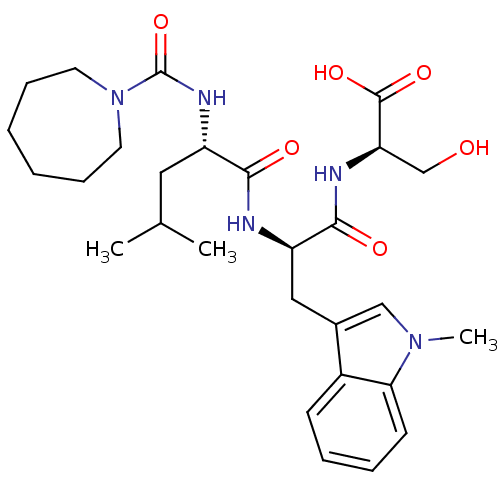 ((R)-2-[(R)-2-{(S)-2-[(Azepane-1-carbonyl)-amino]-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against ETA receptor from dog spleen membranes using [125I]-ET1 as radioligand. | Bioorg Med Chem Lett 5: 917-922 (1995) Article DOI: 10.1016/0960-894X(95)00144-I BindingDB Entry DOI: 10.7270/Q28S4PWG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50078358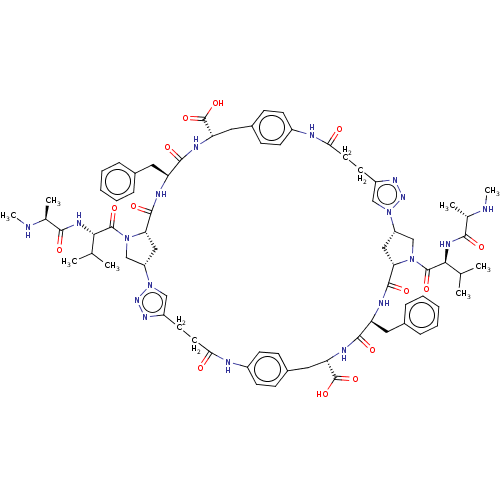 (CHEMBL3414723) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129507 (CHEMBL305236 | N-[4-(4-Benzoimidazol-1-yl-piperidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129524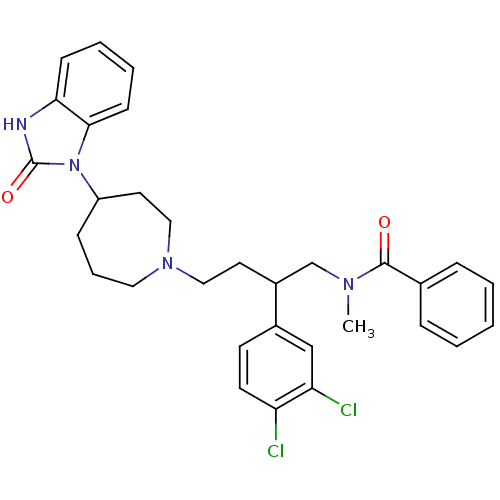 (CHEMBL74127 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399890 (CHEMBL2180951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129513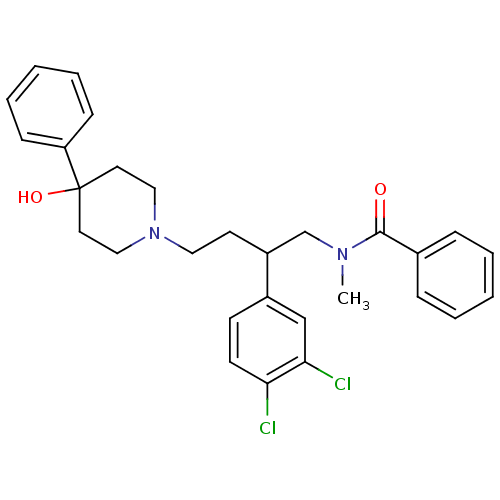 (CHEMBL71397 | N-[2-(3,4-Dichloro-phenyl)-4-(4-hydr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50129523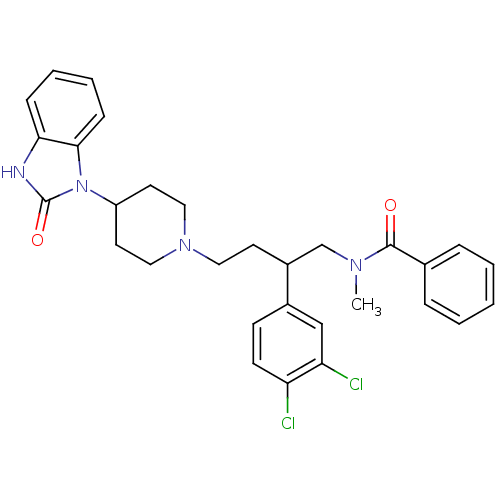 (CHEMBL68440 | N-{2-(3,4-Dichloro-phenyl)-4-[4-(2-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity for human tachykinin receptor 2 expressed in CHO cells using [125I]- NKA radioligand | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071112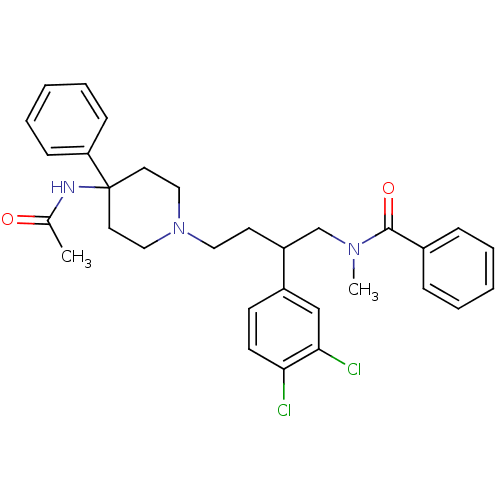 (CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity towards human Tachykinin receptor 2 using [125I]- NKA radioligand expressed in CHO cells | Bioorg Med Chem Lett 13: 2211-5 (2003) BindingDB Entry DOI: 10.7270/Q2D799TN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 2 (Homo sapiens (Human)) | BDBM50078359 (CHEMBL3414729) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of XIAP BIR2-3 (125 to 356 residues) C202A/C213G mutant (unknown origin) fluoresceinated dimeric SMAC peptide based fluorescence polarizat... | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50078359 (CHEMBL3414729) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Baculoviral IAP repeat-containing protein 3 (Homo sapiens (Human)) | BDBM50078357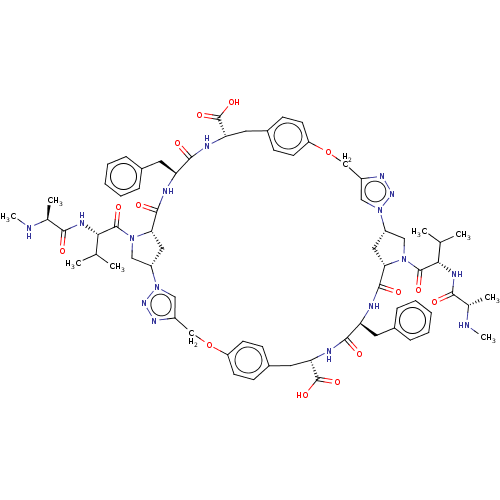 (CHEMBL3414724) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Ensemble Therapeutics Corp Curated by ChEMBL | Assay Description Inhibition of cIAP1 BIR3 (154 to 352 residues) (unknown origin) by fluoresceinated dimeric SMAC peptide based fluorescence polarization assay | J Med Chem 58: 2855-61 (2015) Article DOI: 10.1021/jm501892g BindingDB Entry DOI: 10.7270/Q2RF5WQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399887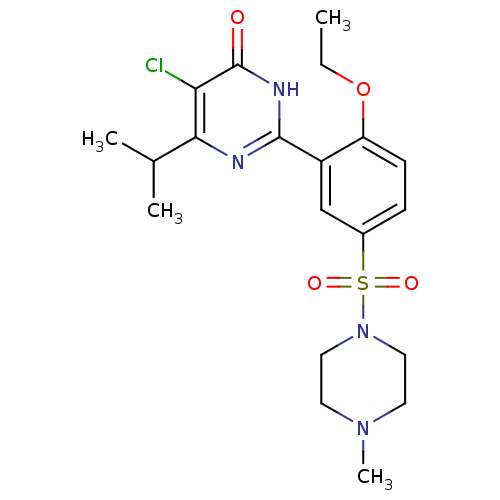 (CHEMBL2180954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50399910 (CHEMBL2180944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant PDE5A by [3H]cGMP based tritium scintillation proximity assay | J Med Chem 55: 10540-50 (2012) Article DOI: 10.1021/jm301159y BindingDB Entry DOI: 10.7270/Q2154J6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 204 total ) | Next | Last >> |