 Found 3307 hits with Last Name = 'thornberry' and Initial = 'na'
Found 3307 hits with Last Name = 'thornberry' and Initial = 'na' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Caspase-3
(Homo sapiens (Human)) | BDBM10246
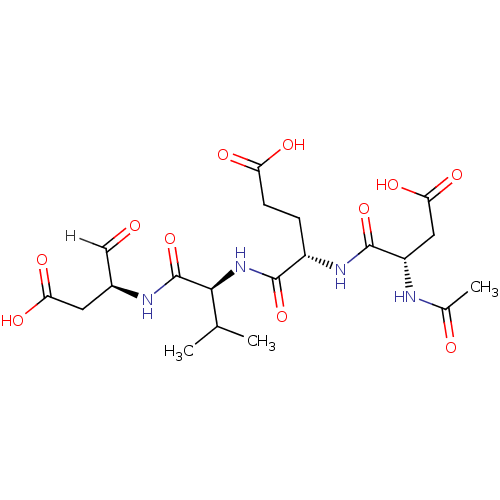
((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C20H30N4O11/c1-9(2)17(20(35)22-11(8-25)6-15(29)30)24-18(33)12(4-5-14(27)28)23-19(34)13(7-16(31)32)21-10(3)26/h8-9,11-13,17H,4-7H2,1-3H3,(H,21,26)(H,22,35)(H,23,34)(H,24,33)(H,27,28)(H,29,30)(H,31,32)/t11-,12-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.230 | -55.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Research Laboratories
| Assay Description
The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... |
J Biol Chem 273: 32608-13 (1998)
Article DOI: 10.1074/jbc.273.49.32608
BindingDB Entry DOI: 10.7270/Q26D5R5V |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50003020
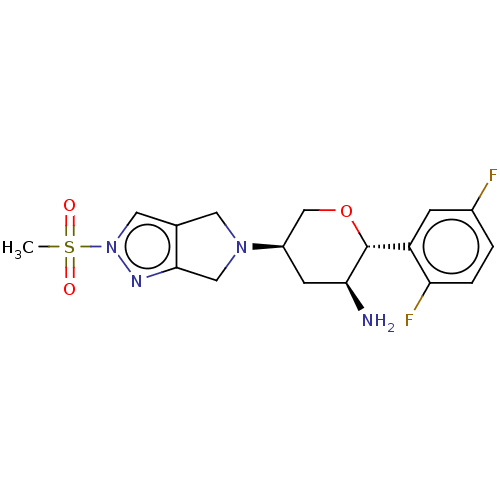
(MK-3102 | OMARIGLIPTIN | US10155775, Omarigliptin ...)Show SMILES CS(=O)(=O)n1cc2CN(Cc2n1)[C@H]1CO[C@@H]([C@@H](N)C1)c1cc(F)ccc1F |r| Show InChI InChI=1S/C17H20F2N4O3S/c1-27(24,25)23-7-10-6-22(8-16(10)21-23)12-5-15(20)17(26-9-12)13-4-11(18)2-3-14(13)19/h2-4,7,12,15,17H,5-6,8-9,20H2,1H3/t12-,15+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co., Inc.
Curated by ChEMBL
| Assay Description
Competitive reversible inhibition of DPP4 (unknown origin) |
J Med Chem 57: 3205-12 (2014)
Article DOI: 10.1021/jm401992e
BindingDB Entry DOI: 10.7270/Q2WD423H |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282978
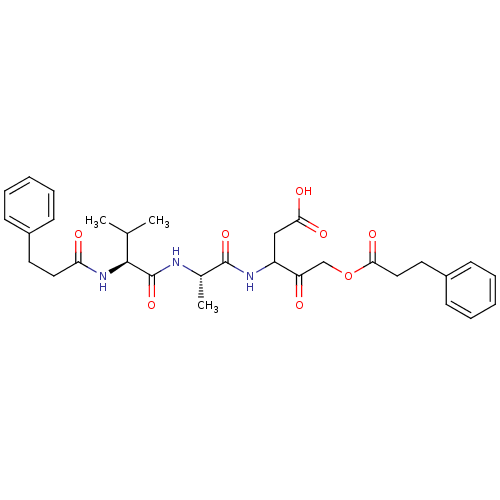
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COC(=O)CCc1ccccc1 Show InChI InChI=1S/C31H39N3O8/c1-20(2)29(34-26(36)16-14-22-10-6-4-7-11-22)31(41)32-21(3)30(40)33-24(18-27(37)38)25(35)19-42-28(39)17-15-23-12-8-5-9-13-23/h4-13,20-21,24,29H,14-19H2,1-3H3,(H,32,41)(H,33,40)(H,34,36)(H,37,38)/t21-,24?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282981
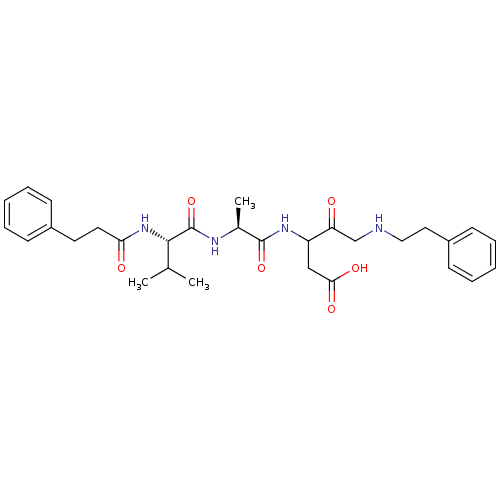
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CNCCc1ccccc1 Show InChI InChI=1S/C30H40N4O6/c1-20(2)28(34-26(36)15-14-22-10-6-4-7-11-22)30(40)32-21(3)29(39)33-24(18-27(37)38)25(35)19-31-17-16-23-12-8-5-9-13-23/h4-13,20-21,24,28,31H,14-19H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t21-,24?,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116262
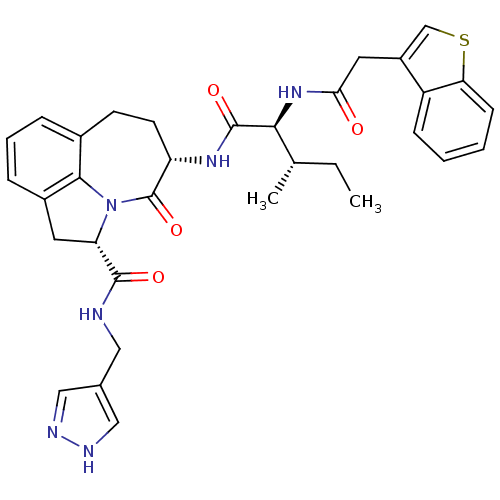
((2S,5S)-5-[(S)-2-(2-Benzo[b]thiophen-3-yl-acetylam...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1csc2ccccc12)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C33H36N6O4S/c1-3-19(2)29(38-28(40)14-23-18-44-27-10-5-4-9-24(23)27)32(42)37-25-12-11-21-7-6-8-22-13-26(39(30(21)22)33(25)43)31(41)34-15-20-16-35-36-17-20/h4-10,16-19,25-26,29H,3,11-15H2,1-2H3,(H,34,41)(H,35,36)(H,37,42)(H,38,40)/t19-,25-,26-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116260
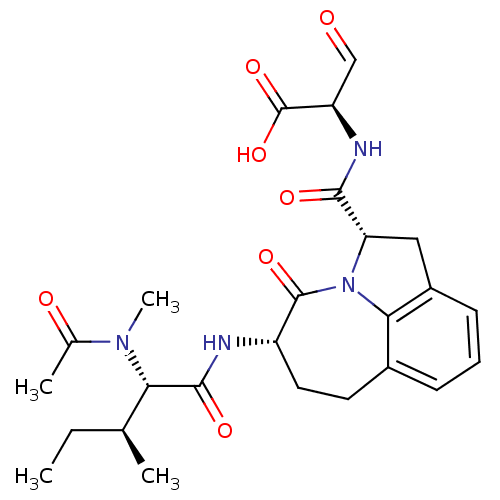
((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282984
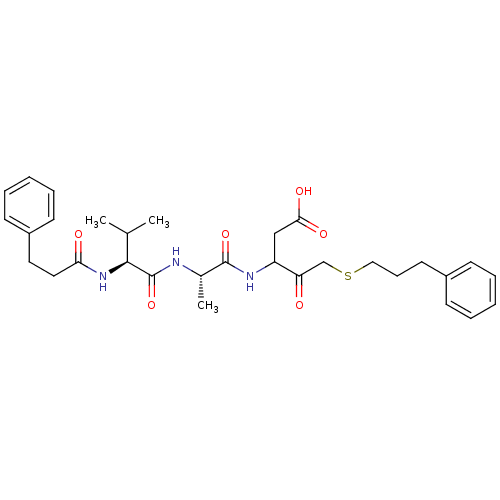
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CSCCCc1ccccc1 Show InChI InChI=1S/C31H41N3O6S/c1-21(2)29(34-27(36)17-16-24-13-8-5-9-14-24)31(40)32-22(3)30(39)33-25(19-28(37)38)26(35)20-41-18-10-15-23-11-6-4-7-12-23/h4-9,11-14,21-22,25,29H,10,15-20H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t22-,25?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116255
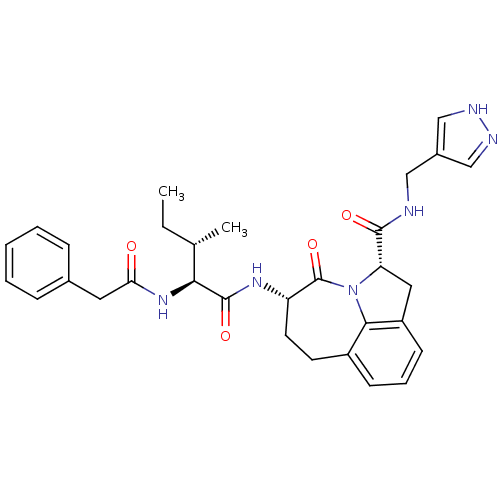
((2S,5S)-5-((S)-3-(S)-Methyl-2-phenylacetylamino-pe...)Show SMILES CC[C@H](C)[C@H](NC(=O)Cc1ccccc1)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cn[nH]c1 Show InChI InChI=1S/C31H36N6O4/c1-3-19(2)27(36-26(38)14-20-8-5-4-6-9-20)30(40)35-24-13-12-22-10-7-11-23-15-25(37(28(22)23)31(24)41)29(39)32-16-21-17-33-34-18-21/h4-11,17-19,24-25,27H,3,12-16H2,1-2H3,(H,32,39)(H,33,34)(H,35,40)(H,36,38)/t19-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116256
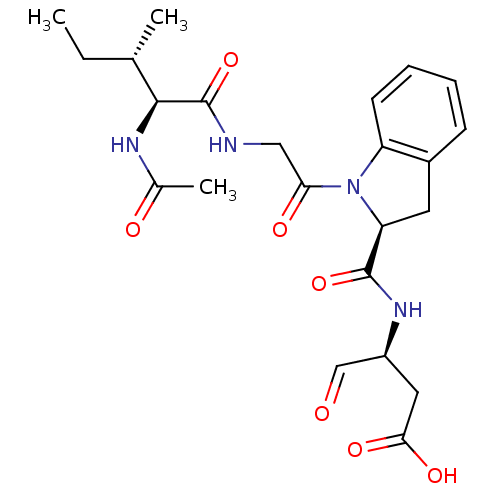
((S)-3-({1-[(S)-2-((3S,4S)-2-Acetylamino-3-methyl-p...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)NCC(=O)N1[C@@H](Cc2ccccc12)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C23H30N4O7/c1-4-13(2)21(25-14(3)29)23(34)24-11-19(30)27-17-8-6-5-7-15(17)9-18(27)22(33)26-16(12-28)10-20(31)32/h5-8,12-13,16,18,21H,4,9-11H2,1-3H3,(H,24,34)(H,25,29)(H,26,33)(H,31,32)/t13-,16-,18-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116267
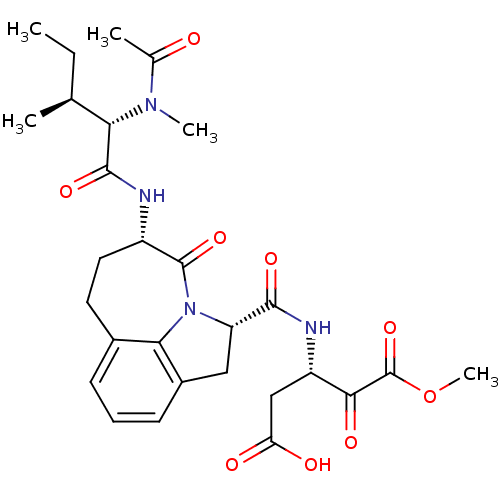
((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)C(=O)OC Show InChI InChI=1S/C28H36N4O9/c1-6-14(2)22(31(4)15(3)33)26(38)29-18-11-10-16-8-7-9-17-12-20(32(23(16)17)27(18)39)25(37)30-19(13-21(34)35)24(36)28(40)41-5/h7-9,14,18-20,22H,6,10-13H2,1-5H3,(H,29,38)(H,30,37)(H,34,35)/t14-,18-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM10246

((4S)-4-{[(1S)-1-{[(2S)-1-carboxy-3-oxopropan-2-yl]...)Show SMILES CC(C)[C@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CC(O)=O)NC(C)=O)C(=O)N[C@@H](CC(O)=O)C=O |r| Show InChI InChI=1S/C20H30N4O11/c1-9(2)17(20(35)22-11(8-25)6-15(29)30)24-18(33)12(4-5-14(27)28)23-19(34)13(7-16(31)32)21-10(3)26/h8-9,11-13,17H,4-7H2,1-3H3,(H,21,26)(H,22,35)(H,23,34)(H,24,33)(H,27,28)(H,29,30)(H,31,32)/t11-,12-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 18 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Merck Research Laboratories
| Assay Description
The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... |
J Biol Chem 273: 32608-13 (1998)
Article DOI: 10.1074/jbc.273.49.32608
BindingDB Entry DOI: 10.7270/Q26D5R5V |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM12197
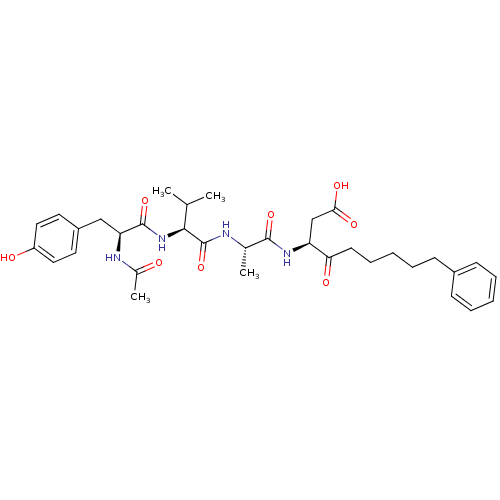
((3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydr...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCCc1ccccc1 |r| Show InChI InChI=1S/C34H46N4O8/c1-21(2)31(38-33(45)28(36-23(4)39)19-25-15-17-26(40)18-16-25)34(46)35-22(3)32(44)37-27(20-30(42)43)29(41)14-10-6-9-13-24-11-7-5-8-12-24/h5,7-8,11-12,15-18,21-22,27-28,31,40H,6,9-10,13-14,19-20H2,1-4H3,(H,35,46)(H,36,39)(H,37,44)(H,38,45)(H,42,43)/t22-,27-,28-,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 3: 2689-2692 (1993)
Article DOI: 10.1016/S0960-894X(01)80743-5
BindingDB Entry DOI: 10.7270/Q2BV7GJZ |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116264

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nnc(o1)-c1ccccc1 Show InChI InChI=1S/C34H38N6O8/c1-5-18(2)27(39(4)19(3)41)31(46)35-23-15-14-20-12-9-13-22-16-25(40(28(20)22)34(23)47)30(45)36-24(17-26(42)43)29(44)33-38-37-32(48-33)21-10-7-6-8-11-21/h6-13,18,23-25,27H,5,14-17H2,1-4H3,(H,35,46)(H,36,45)(H,42,43)/t18-,23-,24-,25-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282979
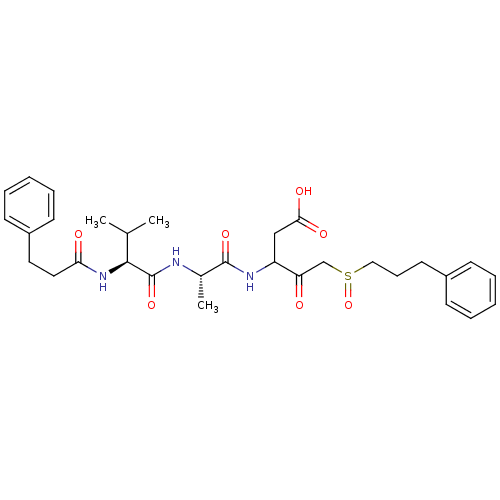
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CS(=O)CCCc1ccccc1 Show InChI InChI=1S/C31H41N3O7S/c1-21(2)29(34-27(36)17-16-24-13-8-5-9-14-24)31(40)32-22(3)30(39)33-25(19-28(37)38)26(35)20-42(41)18-10-15-23-11-6-4-7-12-23/h4-9,11-14,21-22,25,29H,10,15-20H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t22-,25?,29-,42?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116268
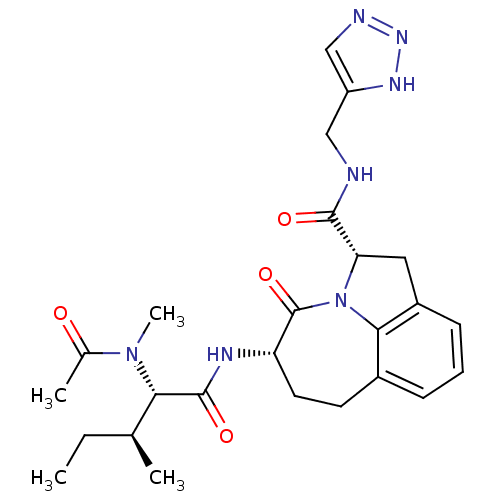
((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cnn[nH]1 Show InChI InChI=1S/C25H33N7O4/c1-5-14(2)21(31(4)15(3)33)24(35)28-19-10-9-16-7-6-8-17-11-20(32(22(16)17)25(19)36)23(34)26-12-18-13-27-30-29-18/h6-8,13-14,19-21H,5,9-12H2,1-4H3,(H,26,34)(H,28,35)(H,27,29,30)/t14-,19-,20-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50281182
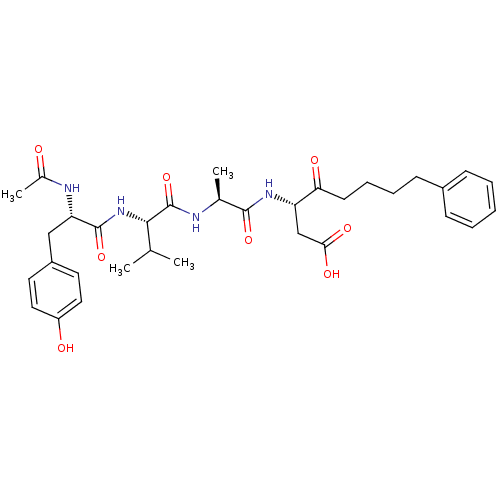
(3-(2-{2-[2-Acetylamino-3-(4-hydroxy-phenyl)-propio...)Show SMILES CC(C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCCc1ccccc1 Show InChI InChI=1S/C33H44N4O8/c1-20(2)30(37-32(44)27(35-22(4)38)18-24-14-16-25(39)17-15-24)33(45)34-21(3)31(43)36-26(19-29(41)42)28(40)13-9-8-12-23-10-6-5-7-11-23/h5-7,10-11,14-17,20-21,26-27,30,39H,8-9,12-13,18-19H2,1-4H3,(H,34,45)(H,35,38)(H,36,43)(H,37,44)(H,41,42)/t21-,26-,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 3: 2689-2692 (1993)
Article DOI: 10.1016/S0960-894X(01)80743-5
BindingDB Entry DOI: 10.7270/Q2BV7GJZ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282980
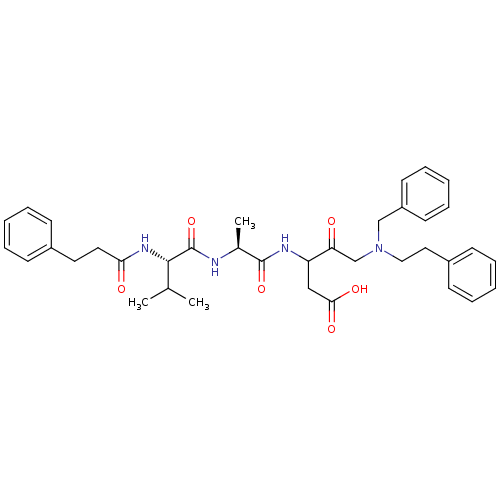
(5-(Benzyl-phenethyl-amino)-3-{(S)-2-[(S)-3-methyl-...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CN(CCc1ccccc1)Cc1ccccc1 Show InChI InChI=1S/C37H46N4O6/c1-26(2)35(40-33(43)20-19-28-13-7-4-8-14-28)37(47)38-27(3)36(46)39-31(23-34(44)45)32(42)25-41(24-30-17-11-6-12-18-30)22-21-29-15-9-5-10-16-29/h4-18,26-27,31,35H,19-25H2,1-3H3,(H,38,47)(H,39,46)(H,40,43)(H,44,45)/t27-,31?,35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116260

((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116269
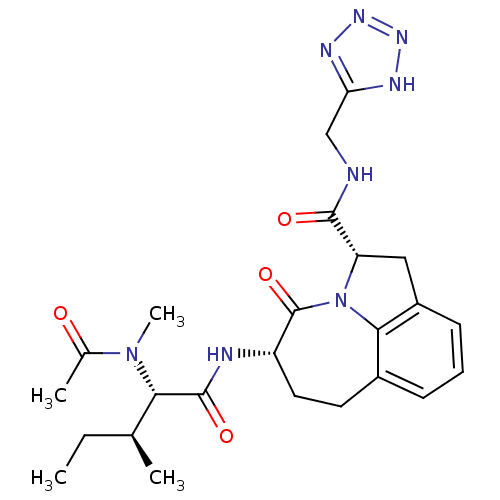
((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1nnn[nH]1 Show InChI InChI=1S/C24H32N8O4/c1-5-13(2)20(31(4)14(3)33)23(35)26-17-10-9-15-7-6-8-16-11-18(32(21(15)16)24(17)36)22(34)25-12-19-27-29-30-28-19/h6-8,13,17-18,20H,5,9-12H2,1-4H3,(H,25,34)(H,26,35)(H,27,28,29,30)/t13-,17-,18-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116261
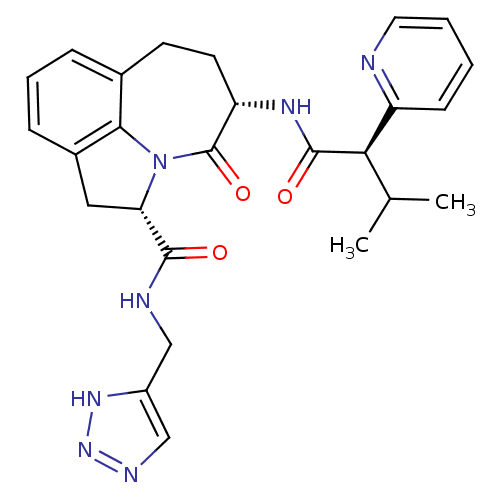
((2S,5S)-5-((R)-3-Methyl-2-pyridin-2-yl-butyrylamin...)Show SMILES CC(C)[C@@H](C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cnn[nH]1)c1ccccn1 Show InChI InChI=1S/C26H29N7O3/c1-15(2)22(19-8-3-4-11-27-19)25(35)30-20-10-9-16-6-5-7-17-12-21(33(23(16)17)26(20)36)24(34)28-13-18-14-29-32-31-18/h3-8,11,14-15,20-22H,9-10,12-13H2,1-2H3,(H,28,34)(H,30,35)(H,29,31,32)/t20-,21-,22+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116259
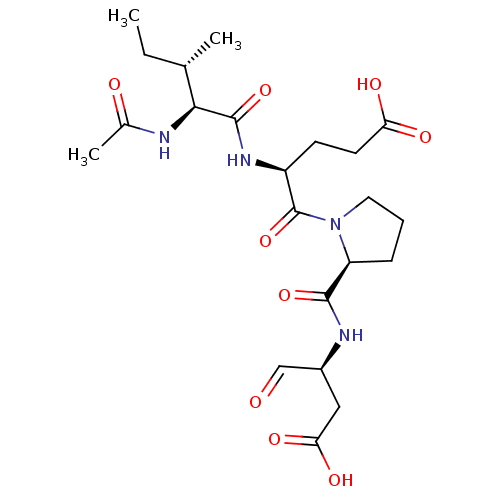
((S)-4-((3S,4S)-2-Acetylamino-3-methyl-pentanoylami...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@@H](CCC(O)=O)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C22H34N4O9/c1-4-12(2)19(23-13(3)28)21(34)25-15(7-8-17(29)30)22(35)26-9-5-6-16(26)20(33)24-14(11-27)10-18(31)32/h11-12,14-16,19H,4-10H2,1-3H3,(H,23,28)(H,24,33)(H,25,34)(H,29,30)(H,31,32)/t12-,14-,15-,16-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116265

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C33H37N5O7S/c1-5-17(2)27(37(4)18(3)39)31(44)34-22-14-13-19-9-8-10-20-15-24(38(28(19)20)33(22)45)30(43)35-23(16-26(40)41)29(42)32-36-21-11-6-7-12-25(21)46-32/h6-12,17,22-24,27H,5,13-16H2,1-4H3,(H,34,44)(H,35,43)(H,40,41)/t17-,22-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284744

(3-Allyloxycarbonylamino-5-[3-(1H-imidazol-2-yl)-na...)Show SMILES OC(=O)C[C@H](NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1-c1ncc[nH]1 Show InChI InChI=1S/C22H21N3O6/c1-2-9-30-22(29)25-17(12-20(27)28)18(26)13-31-19-11-15-6-4-3-5-14(15)10-16(19)21-23-7-8-24-21/h2-8,10-11,17H,1,9,12-13H2,(H,23,24)(H,25,29)(H,27,28)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282983

(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CCCCc1ccccc1 Show InChI InChI=1S/C31H41N3O6/c1-21(2)29(34-27(36)19-18-24-14-8-5-9-15-24)31(40)32-22(3)30(39)33-25(20-28(37)38)26(35)17-11-10-16-23-12-6-4-7-13-23/h4-9,12-15,21-22,25,29H,10-11,16-20H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t22-,25?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50281184
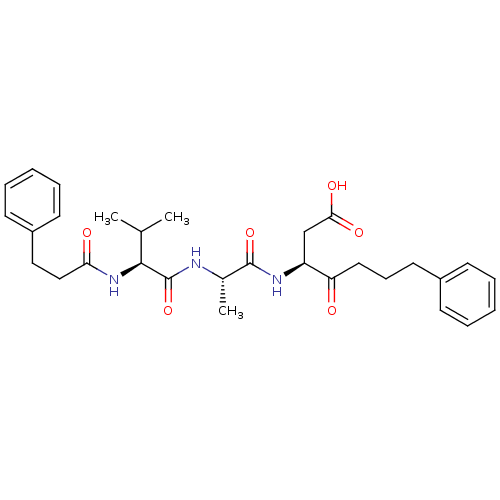
((S)-3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylam...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCCc1ccccc1 Show InChI InChI=1S/C30H39N3O6/c1-20(2)28(33-26(35)18-17-23-13-8-5-9-14-23)30(39)31-21(3)29(38)32-24(19-27(36)37)25(34)16-10-15-22-11-6-4-7-12-22/h4-9,11-14,20-21,24,28H,10,15-19H2,1-3H3,(H,31,39)(H,32,38)(H,33,35)(H,36,37)/t21-,24-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 3: 2689-2692 (1993)
Article DOI: 10.1016/S0960-894X(01)80743-5
BindingDB Entry DOI: 10.7270/Q2BV7GJZ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282977
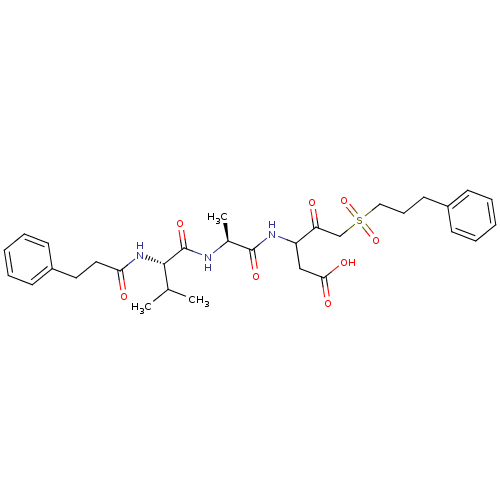
(3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylamino)...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)CS(=O)(=O)CCCc1ccccc1 Show InChI InChI=1S/C31H41N3O8S/c1-21(2)29(34-27(36)17-16-24-13-8-5-9-14-24)31(40)32-22(3)30(39)33-25(19-28(37)38)26(35)20-43(41,42)18-10-15-23-11-6-4-7-12-23/h4-9,11-14,21-22,25,29H,10,15-20H2,1-3H3,(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t22-,25?,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50282982
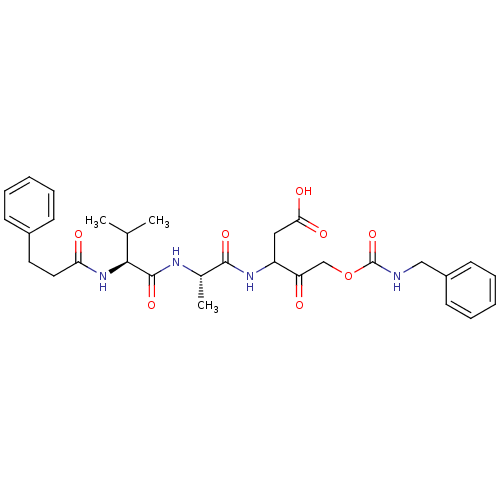
(5-Benzylcarbamoyloxy-3-{(S)-2-[(S)-3-methyl-2-(3-p...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)NC(CC(O)=O)C(=O)COC(=O)NCc1ccccc1 Show InChI InChI=1S/C30H38N4O8/c1-19(2)27(34-25(36)15-14-21-10-6-4-7-11-21)29(40)32-20(3)28(39)33-23(16-26(37)38)24(35)18-42-30(41)31-17-22-12-8-5-9-13-22/h4-13,19-20,23,27H,14-18H2,1-3H3,(H,31,41)(H,32,40)(H,33,39)(H,34,36)(H,37,38)/t20-,23?,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for inhibition of cysteine proteinase IL-1 beta converting enzyme (ICE) |
Bioorg Med Chem Lett 4: 1965-1968 (1994)
Article DOI: 10.1016/S0960-894X(01)80544-8
BindingDB Entry DOI: 10.7270/Q24M94GD |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116263

((S)-3-{[(S)-(5S,7S)-6-((3S,4S)-2-Acetylamino-3-met...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)N[C@H]1CC[C@H]2CC[C@H](N2C1=O)C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C21H32N4O7/c1-4-11(2)18(22-12(3)27)20(31)24-15-7-5-14-6-8-16(25(14)21(15)32)19(30)23-13(10-26)9-17(28)29/h10-11,13-16,18H,4-9H2,1-3H3,(H,22,27)(H,23,30)(H,24,31)(H,28,29)/t11-,13-,14-,15-,16-,18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116258
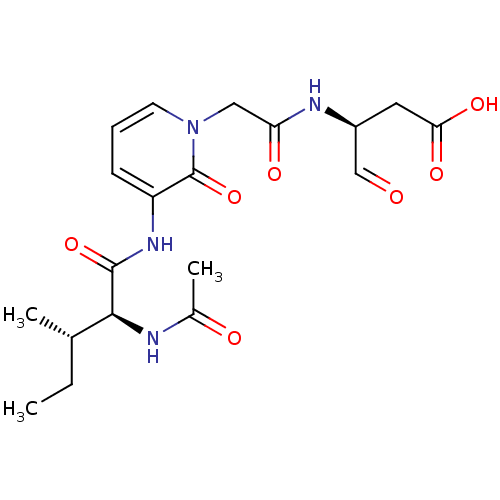
((S)-3-{2-[3-((2S,3S)-2-Acetylamino-3-methyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)Nc1cccn(CC(=O)N[C@@H](CC(O)=O)C=O)c1=O Show InChI InChI=1S/C19H26N4O7/c1-4-11(2)17(20-12(3)25)18(29)22-14-6-5-7-23(19(14)30)9-15(26)21-13(10-24)8-16(27)28/h5-7,10-11,13,17H,4,8-9H2,1-3H3,(H,20,25)(H,21,26)(H,22,29)(H,27,28)/t11-,13-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116265

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)c1nc2ccccc2s1 Show InChI InChI=1S/C33H37N5O7S/c1-5-17(2)27(37(4)18(3)39)31(44)34-22-14-13-19-9-8-10-20-15-24(38(28(19)20)33(22)45)30(43)35-23(16-26(40)41)29(42)32-36-21-11-6-7-12-25(21)46-32/h6-12,17,22-24,27H,5,13-16H2,1-4H3,(H,34,44)(H,35,43)(H,40,41)/t17-,22-,23-,24-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50116267

((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)C(=O)OC Show InChI InChI=1S/C28H36N4O9/c1-6-14(2)22(31(4)15(3)33)26(38)29-18-11-10-16-8-7-9-17-12-20(32(23(16)17)27(18)39)25(37)30-19(13-21(34)35)24(36)28(40)41-5/h7-9,14,18-20,22H,6,10-13H2,1-5H3,(H,29,38)(H,30,37)(H,34,35)/t14-,18-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 202 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-3 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116257
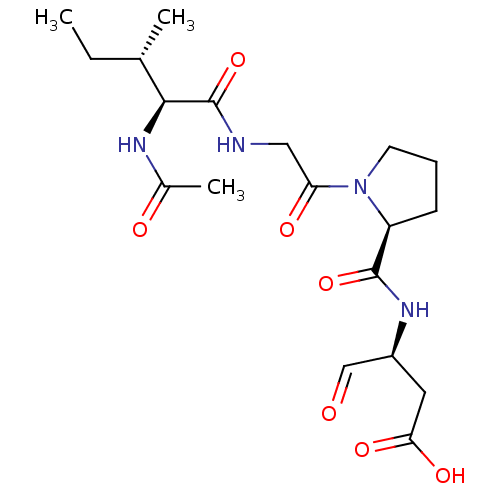
(3-[((S)-{1-[(S)-2-((3S,4S)-2-Acetylamino-3-methyl-...)Show SMILES CC[C@H](C)[C@H](NC(C)=O)C(=O)NCC(=O)N1CCC[C@H]1C(=O)N[C@@H](CC(O)=O)C=O Show InChI InChI=1S/C19H30N4O7/c1-4-11(2)17(21-12(3)25)19(30)20-9-15(26)23-7-5-6-14(23)18(29)22-13(10-24)8-16(27)28/h10-11,13-14,17H,4-9H2,1-3H3,(H,20,30)(H,21,25)(H,22,29)(H,27,28)/t11-,13-,14-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284735

(3-Allyloxycarbonylamino-5-(3-carbamoyl-naphthalen-...)Show SMILES NC(=O)c1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C20H20N2O7/c1-2-7-28-20(27)22-15(10-18(24)25)16(23)11-29-17-9-13-6-4-3-5-12(13)8-14(17)19(21)26/h2-6,8-9,15H,1,7,10-11H2,(H2,21,26)(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-8
(Homo sapiens (Human)) | BDBM50116260

((R)-2-({(2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@H](C=O)C(O)=O Show InChI InChI=1S/C25H32N4O7/c1-5-13(2)20(28(4)14(3)31)23(33)26-17-10-9-15-7-6-8-16-11-19(29(21(15)16)24(17)34)22(32)27-18(12-30)25(35)36/h6-8,12-13,17-20H,5,9-11H2,1-4H3,(H,26,33)(H,27,32)(H,35,36)/t13-,17-,18+,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibition against Caspase-8 |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116270

((2S,5S)-5-[(S)-2-(Acetyl-methyl-amino)-3-(S)-methy...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCc1cc(=O)[nH]o1 Show InChI InChI=1S/C26H33N5O6/c1-5-14(2)22(30(4)15(3)32)25(35)28-19-10-9-16-7-6-8-17-11-20(31(23(16)17)26(19)36)24(34)27-13-18-12-21(33)29-37-18/h6-8,12,14,19-20,22H,5,9-11,13H2,1-4H3,(H,27,34)(H,28,35)(H,29,33)/t14-,19-,20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284734
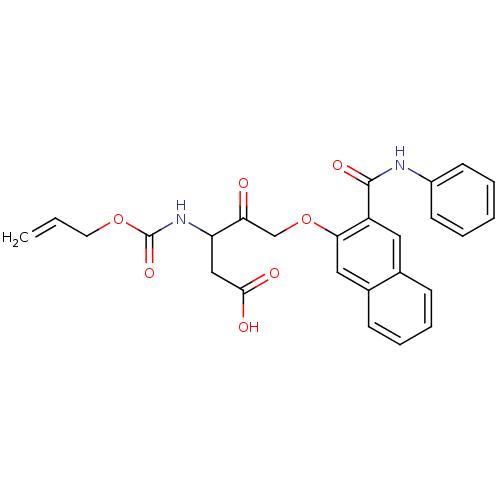
(3-Allyloxycarbonylamino-4-oxo-5-(3-phenylcarbamoyl...)Show SMILES OC(=O)CC(NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1C(=O)Nc1ccccc1 Show InChI InChI=1S/C26H24N2O7/c1-2-12-34-26(33)28-21(15-24(30)31)22(29)16-35-23-14-18-9-7-6-8-17(18)13-20(23)25(32)27-19-10-4-3-5-11-19/h2-11,13-14,21H,1,12,15-16H2,(H,27,32)(H,28,33)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity determined against ETA receptor in porcine aortic smooth muscle membrane |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116254

(3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)-3-m...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)NCCC(O)=O Show InChI InChI=1S/C25H34N4O6/c1-5-14(2)21(28(4)15(3)30)24(34)27-18-10-9-16-7-6-8-17-13-19(29(22(16)17)25(18)35)23(33)26-12-11-20(31)32/h6-8,14,18-19,21H,5,9-13H2,1-4H3,(H,26,33)(H,27,34)(H,31,32)/t14-,18-,19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50281183
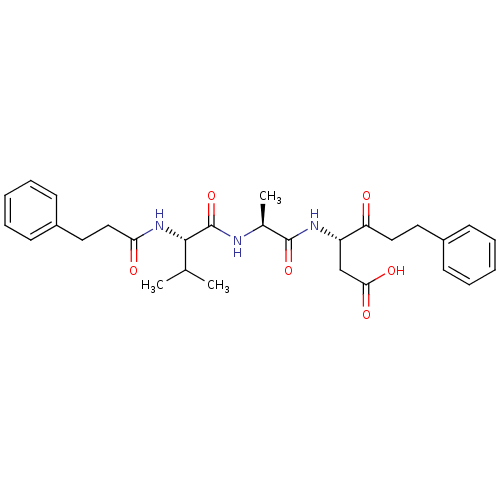
((S)-3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylam...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)CCc1ccccc1 Show InChI InChI=1S/C29H37N3O6/c1-19(2)27(32-25(34)17-15-22-12-8-5-9-13-22)29(38)30-20(3)28(37)31-23(18-26(35)36)24(33)16-14-21-10-6-4-7-11-21/h4-13,19-20,23,27H,14-18H2,1-3H3,(H,30,38)(H,31,37)(H,32,34)(H,35,36)/t20-,23-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 3: 2689-2692 (1993)
Article DOI: 10.1016/S0960-894X(01)80743-5
BindingDB Entry DOI: 10.7270/Q2BV7GJZ |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284733
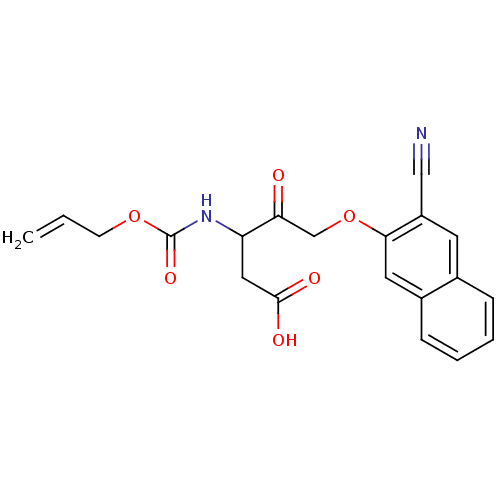
(3-Allyloxycarbonylamino-5-(3-cyano-naphthalen-2-yl...)Show SMILES OC(=O)CC(NC(=O)OCC=C)C(=O)COc1cc2ccccc2cc1C#N Show InChI InChI=1S/C20H18N2O6/c1-2-7-27-20(26)22-16(10-19(24)25)17(23)12-28-18-9-14-6-4-3-5-13(14)8-15(18)11-21/h2-6,8-9,16H,1,7,10,12H2,(H,22,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against ETA receptor in porcine aortic smooth muscle membrane |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284740
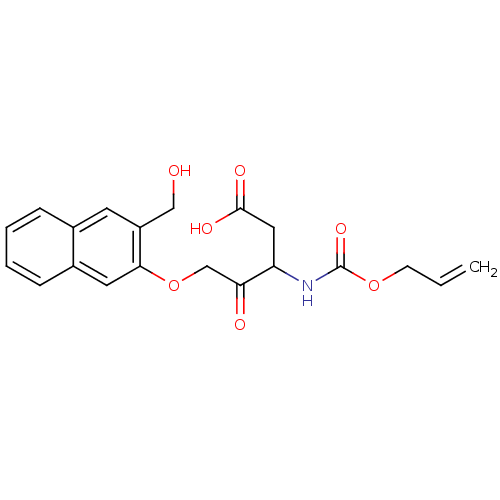
(3-Allyloxycarbonylamino-5-(3-hydroxymethyl-naphtha...)Show SMILES OCc1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C20H21NO7/c1-2-7-27-20(26)21-16(10-19(24)25)17(23)12-28-18-9-14-6-4-3-5-13(14)8-15(18)11-22/h2-6,8-9,16,22H,1,7,10-12H2,(H,21,26)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50213208
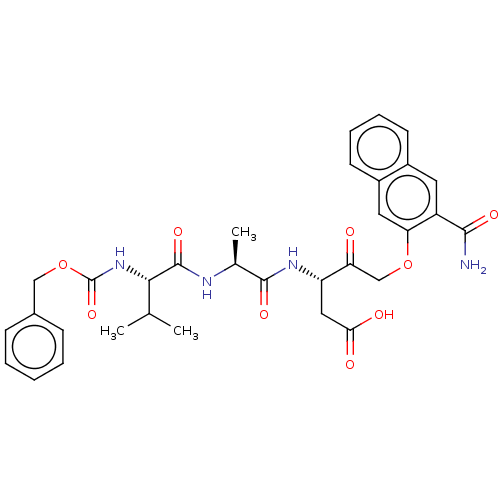
(CHEMBL3143890)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)COc1cc2ccccc2cc1C(N)=O |r| Show InChI InChI=1S/C32H36N4O9/c1-18(2)28(36-32(43)45-16-20-9-5-4-6-10-20)31(42)34-19(3)30(41)35-24(15-27(38)39)25(37)17-44-26-14-22-12-8-7-11-21(22)13-23(26)29(33)40/h4-14,18-19,24,28H,15-17H2,1-3H3,(H2,33,40)(H,34,42)(H,35,41)(H,36,43)(H,38,39)/t19-,24-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined against ETA receptor in porcine aortic smooth muscle membrane |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137257
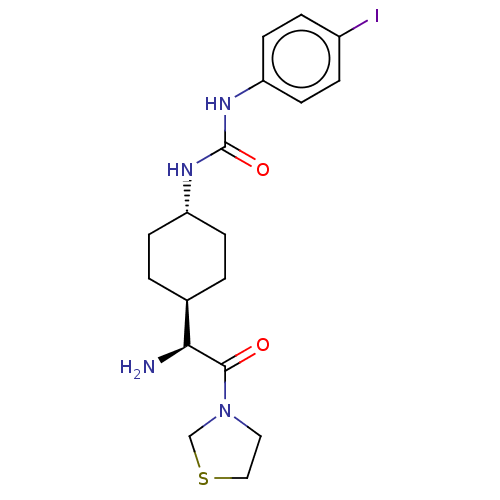
(1-[4-((S)-1-Amino-2-oxo-2-thiazolidin-3-yl-ethyl)-...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NC(=O)Nc1ccc(I)cc1)[C@H](N)C(=O)N1CCSC1 |wU:4.7,1.0,wD:18.20,(1.73,2.3,;3.06,1.54,;1.75,.76,;1.75,-.78,;3.06,-1.54,;4.39,-.78,;4.39,.76,;3.06,-3.08,;4.41,-3.85,;5.74,-3.08,;4.41,-5.39,;5.74,-6.16,;5.74,-7.7,;7.07,-8.47,;8.41,-7.7,;9.74,-8.47,;8.4,-6.14,;7.07,-5.39,;3.06,3.08,;1.73,3.85,;4.41,3.87,;4.41,5.41,;5.74,3.08,;7.15,3.71,;8.17,2.57,;7.4,1.23,;5.9,1.56,)| Show InChI InChI=1S/C18H25IN4O2S/c19-13-3-7-15(8-4-13)22-18(25)21-14-5-1-12(2-6-14)16(20)17(24)23-9-10-26-11-23/h3-4,7-8,12,14,16H,1-2,5-6,9-11,20H2,(H2,21,22,25)/t12-,14-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284745

(3-Allyloxycarbonylamino-5-(4-carbamoyl-biphenyl-3-...)Show SMILES NC(=O)c1ccc(cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C)-c1ccccc1 Show InChI InChI=1S/C22H22N2O7/c1-2-10-30-22(29)24-17(12-20(26)27)18(25)13-31-19-11-15(8-9-16(19)21(23)28)14-6-4-3-5-7-14/h2-9,11,17H,1,10,12-13H2,(H2,23,28)(H,24,29)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Granzyme B
(Homo sapiens (Human)) | BDBM50116266
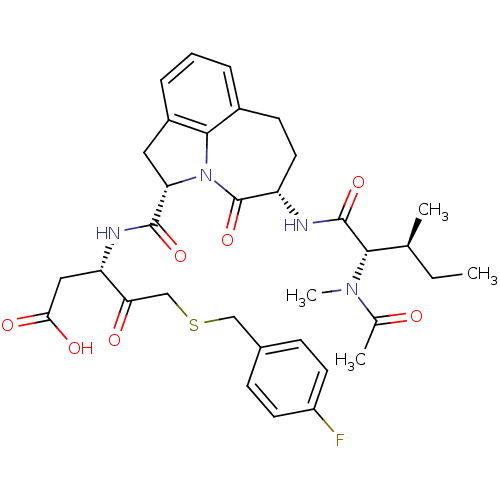
((S)-3-({(S)-(S)-5-[(S)-2-((S)-Acetyl-methyl-amino)...)Show SMILES CC[C@H](C)[C@H](N(C)C(C)=O)C(=O)N[C@H]1CCc2cccc3C[C@H](N(c23)C1=O)C(=O)N[C@@H](CC(O)=O)C(=O)CSCc1ccc(F)cc1 Show InChI InChI=1S/C34H41FN4O7S/c1-5-19(2)30(38(4)20(3)40)33(45)36-25-14-11-22-7-6-8-23-15-27(39(31(22)23)34(25)46)32(44)37-26(16-29(42)43)28(41)18-47-17-21-9-12-24(35)13-10-21/h6-10,12-13,19,25-27,30H,5,11,14-18H2,1-4H3,(H,36,45)(H,37,44)(H,42,43)/t19-,25-,26-,27-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition against human granzyme B |
Bioorg Med Chem Lett 12: 2197-200 (2002)
BindingDB Entry DOI: 10.7270/Q2WH2P9X |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284741
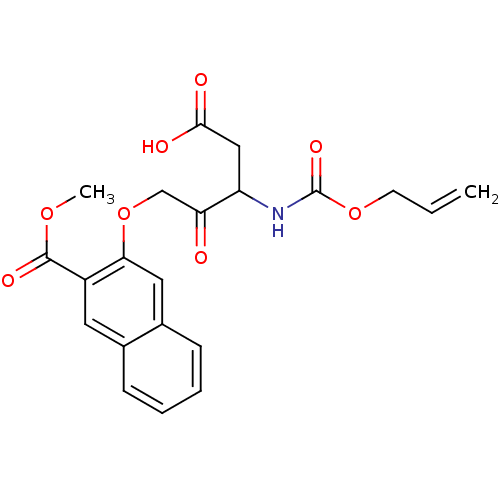
(3-(3-Allyloxycarbonylamino-4-carboxy-2-oxo-butoxy)...)Show SMILES COC(=O)c1cc2ccccc2cc1OCC(=O)C(CC(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C21H21NO8/c1-3-8-29-21(27)22-16(11-19(24)25)17(23)12-30-18-10-14-7-5-4-6-13(14)9-15(18)20(26)28-2/h3-7,9-10,16H,1,8,11-12H2,2H3,(H,22,27)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50284743
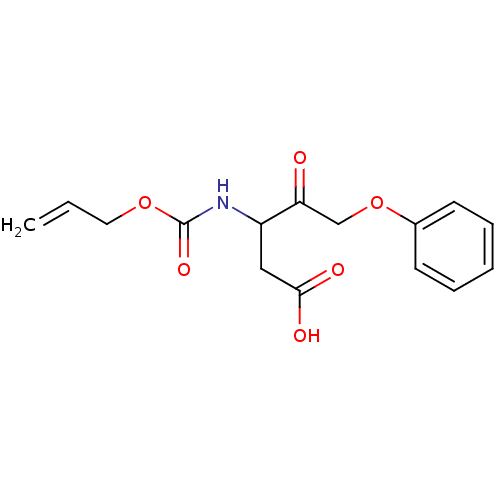
(3-Allyloxycarbonylamino-4-oxo-5-phenoxy-pentanoic ...)Show InChI InChI=1S/C15H17NO6/c1-2-8-21-15(20)16-12(9-14(18)19)13(17)10-22-11-6-4-3-5-7-11/h2-7,12H,1,8-10H2,(H,16,20)(H,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of IL-1 beta converting enzyme (ICE) in human blood monocytes expressed as Kon |
Bioorg Med Chem Lett 5: 1409-1414 (1995)
Article DOI: 10.1016/0960-894X(95)00232-I
BindingDB Entry DOI: 10.7270/Q2319VTD |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50137261
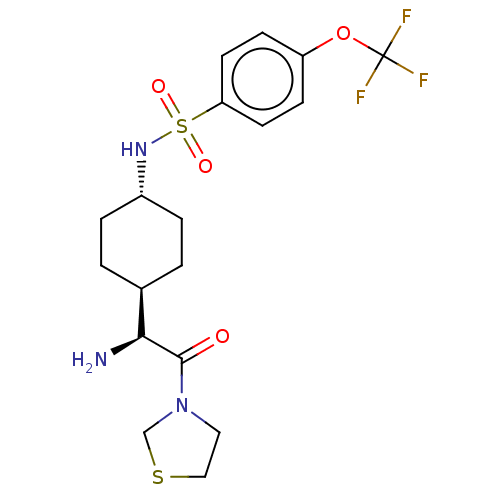
(CHEMBL25211 | N-[4-((S)-1-Amino-2-oxo-2-thiazolidi...)Show SMILES [H][C@@]1(CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)[C@H](N)C(=O)N1CCSC1 |wU:22.24,4.7,1.0,(4.31,-7.01,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;5.64,-6.24,;5.64,-7.78,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;9.64,-5.47,;10.79,-6.5,;10.15,-7.9,;8.62,-7.74,)| Show InChI InChI=1S/C18H24F3N3O4S2/c19-18(20,21)28-14-5-7-15(8-6-14)30(26,27)23-13-3-1-12(2-4-13)16(22)17(25)24-9-10-29-11-24/h5-8,12-13,16,23H,1-4,9-11,22H2/t12-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for Potassium channel HERG Kv11.1 |
Bioorg Med Chem Lett 14: 43-6 (2003)
BindingDB Entry DOI: 10.7270/Q26972ZM |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50140533
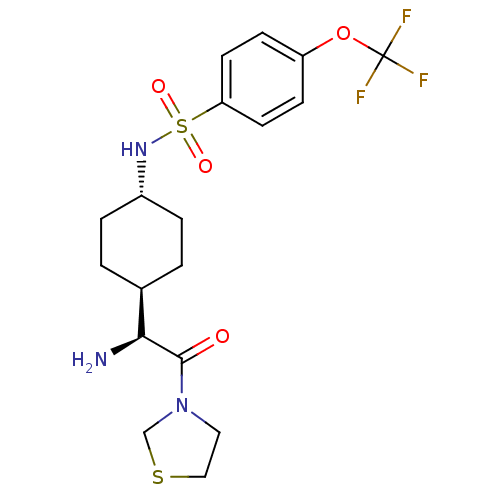
(CHEMBL25211 | N-[4-((S)-1-Amino-2-oxo-2-thiazolidi...)Show SMILES N[C@@H]([C@H]1CC[C@@H](CC1)NS(=O)(=O)c1ccc(OC(F)(F)F)cc1)C(=O)N1CCSC1 |wU:1.0,5.8,wD:2.7,(5.64,-7.78,;5.64,-6.24,;4.31,-5.47,;4.31,-3.92,;2.98,-3.15,;1.65,-3.92,;1.65,-5.47,;2.98,-6.24,;.31,-3.13,;-1.02,-3.9,;-.25,-5.25,;-2.37,-4.68,;-1.79,-2.56,;-1.02,-1.23,;-1.79,.11,;-3.34,.11,;-4.12,1.44,;-5.67,1.44,;-5.67,-.1,;-5.67,2.97,;-7.21,1.44,;-4.11,-1.23,;-3.34,-2.56,;6.97,-5.47,;6.97,-3.92,;8.3,-6.24,;9.64,-5.47,;10.79,-6.5,;10.15,-7.9,;8.62,-7.74,)| Show InChI InChI=1S/C18H24F3N3O4S2/c19-18(20,21)28-14-5-7-15(8-6-14)30(26,27)23-13-3-1-12(2-4-13)16(22)17(25)24-9-10-29-11-24/h5-8,12-13,16,23H,1-4,9-11,22H2/t12-,13-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards human ERG potassium ion channel was determined |
Bioorg Med Chem Lett 14: 1265-8 (2004)
Article DOI: 10.1016/j.bmcl.2003.12.040
BindingDB Entry DOI: 10.7270/Q2G73D5Q |
More data for this
Ligand-Target Pair | |
Caspase-1
(Homo sapiens (Human)) | BDBM50281181
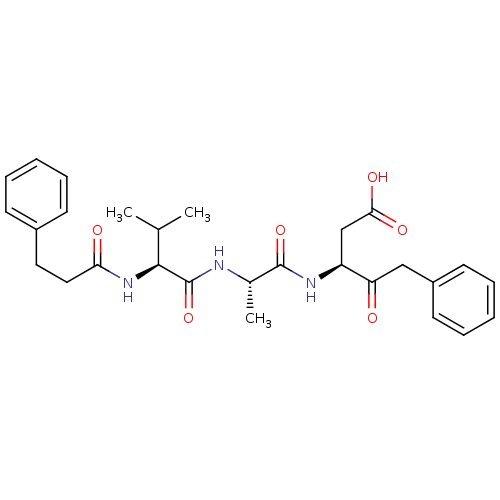
((S)-3-{(S)-2-[(S)-3-Methyl-2-(3-phenyl-propionylam...)Show SMILES CC(C)[C@H](NC(=O)CCc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](CC(O)=O)C(=O)Cc1ccccc1 Show InChI InChI=1S/C28H35N3O6/c1-18(2)26(31-24(33)15-14-20-10-6-4-7-11-20)28(37)29-19(3)27(36)30-22(17-25(34)35)23(32)16-21-12-8-5-9-13-21/h4-13,18-19,22,26H,14-17H2,1-3H3,(H,29,37)(H,30,36)(H,31,33)(H,34,35)/t19-,22-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
The compound was tested for its inhibitory activity against IL-1 beta converting enzyme |
Bioorg Med Chem Lett 3: 2689-2692 (1993)
Article DOI: 10.1016/S0960-894X(01)80743-5
BindingDB Entry DOI: 10.7270/Q2BV7GJZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data