

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM50051653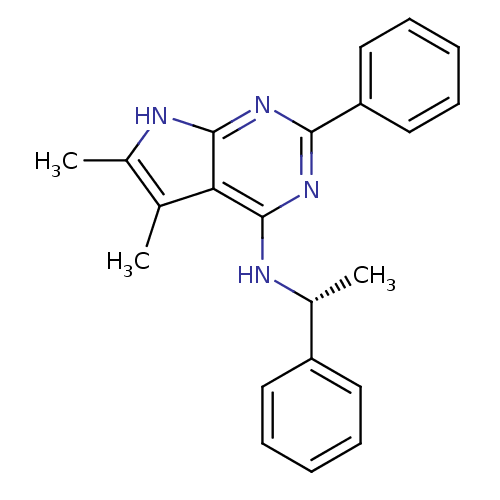 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Binding affinity towards rat A1 receptor was determined | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080288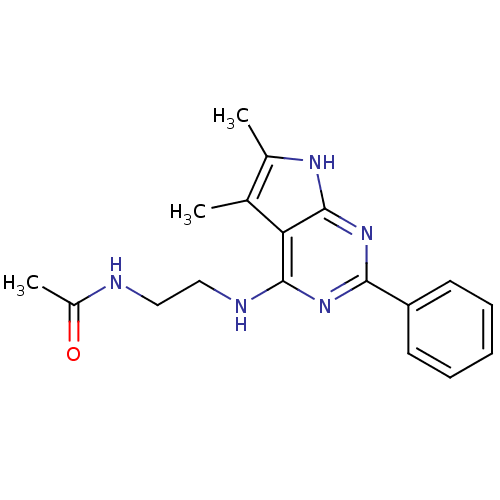 (CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080288 (CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080292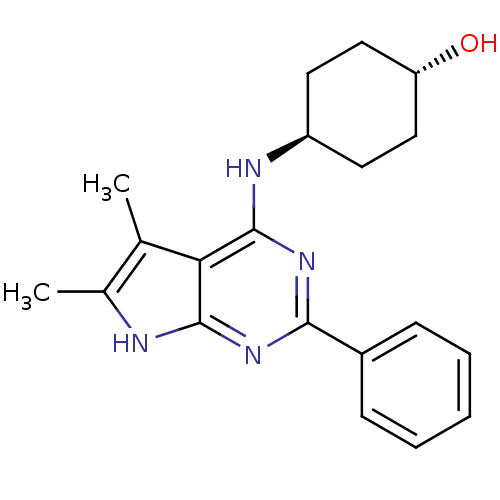 (4-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21173 (1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080291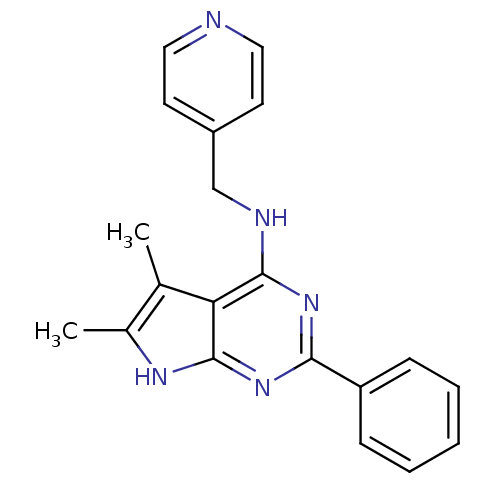 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080287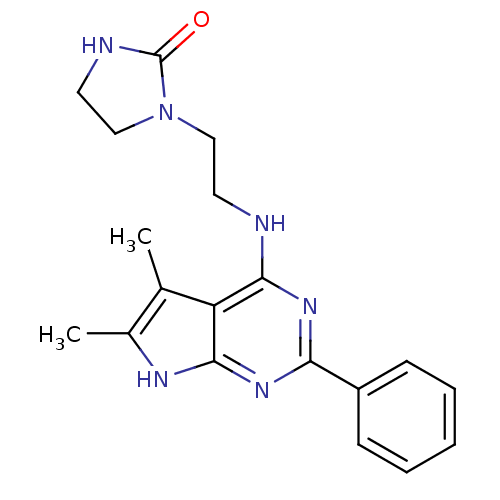 (1-[2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080287 (1-[2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrim...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016945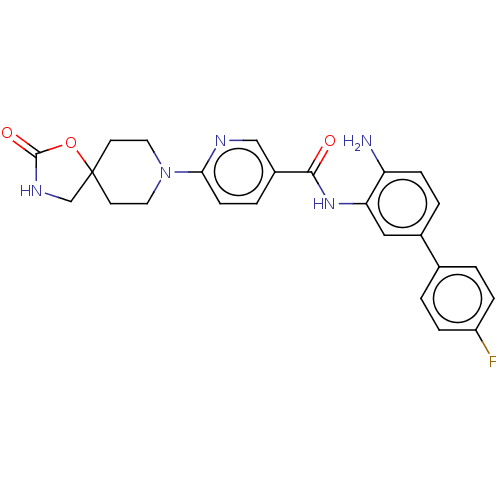 (CHEMBL3286736) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080292 (4-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 754 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-CGS-21,680 to human A2a receptor (hA2a) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080289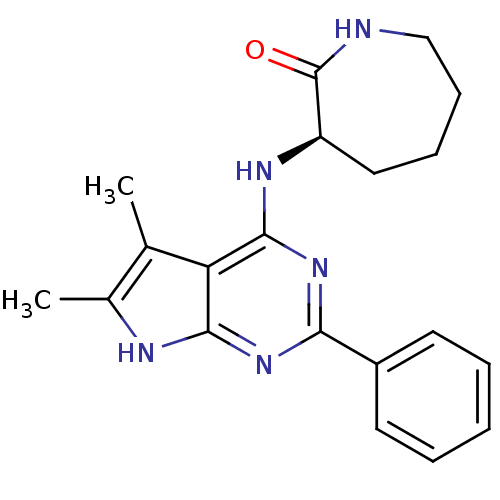 ((R)-3-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 771 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080291 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 811 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080290 ((1R,2R)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 896 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50080286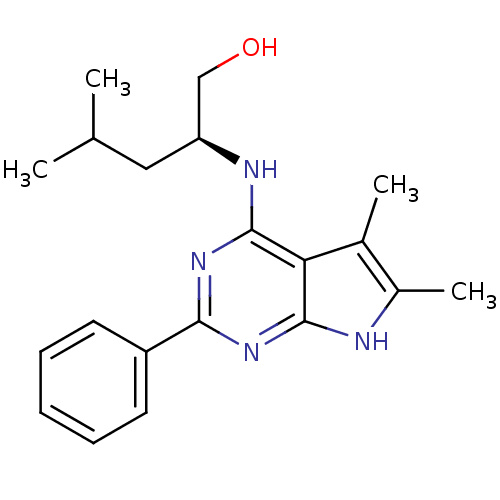 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 951 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080289 ((R)-3-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50051653 ((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 981 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50080286 ((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation Curated by ChEMBL | Assay Description Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay | Bioorg Med Chem Lett 9: 2413-8 (1999) BindingDB Entry DOI: 10.7270/Q2PR7V5N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016943 (CHEMBL3286734) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016944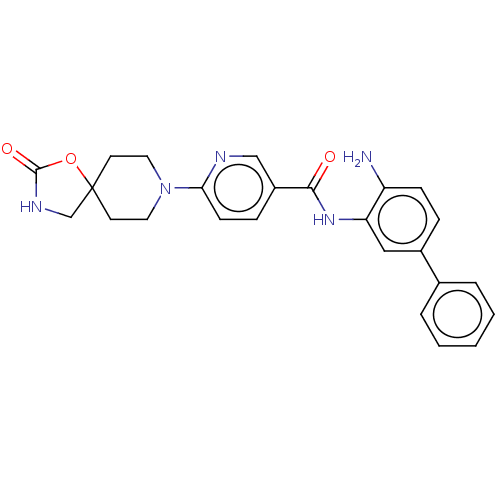 (CHEMBL3286735) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016948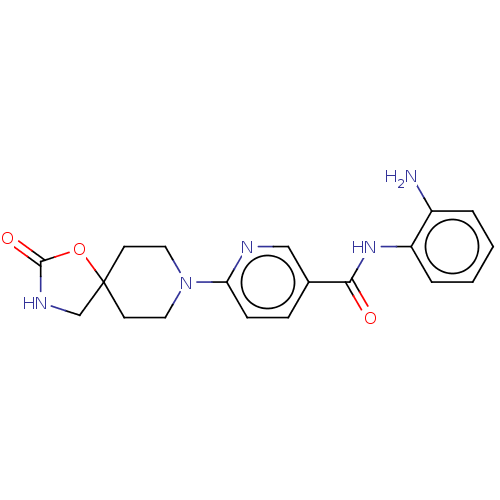 (CHEMBL3286739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016946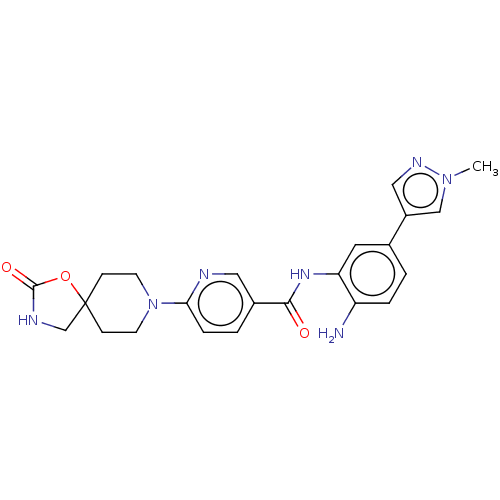 (CHEMBL3286737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016947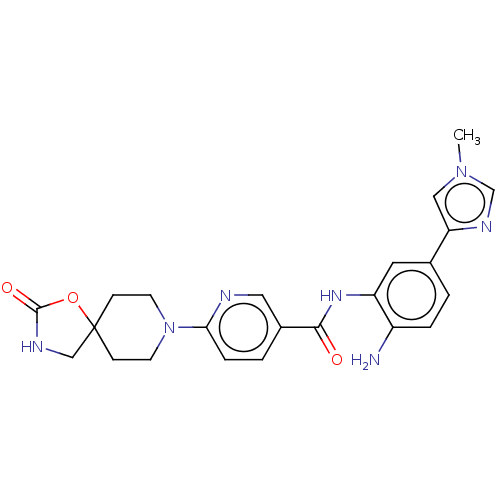 (CHEMBL3286738) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016941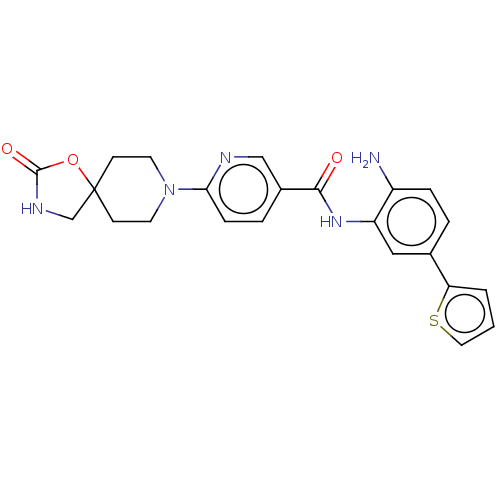 (CHEMBL3286732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50016942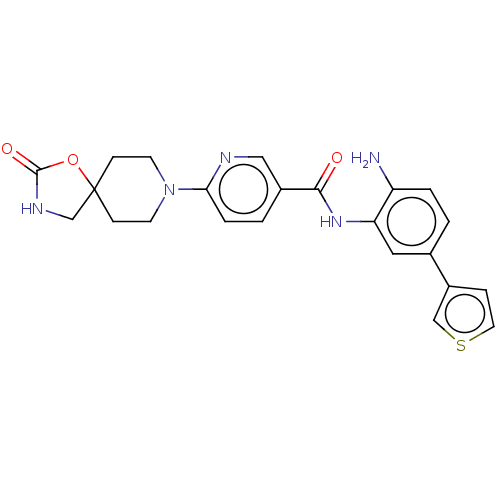 (CHEMBL3286733) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG | ACS Med Chem Lett 5: 340-5 (2014) Article DOI: 10.1021/ml4004233 BindingDB Entry DOI: 10.7270/Q2HH6MM7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153166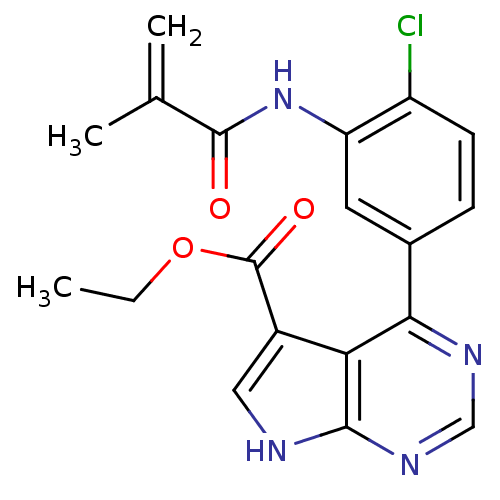 (US8993756, 4-9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153198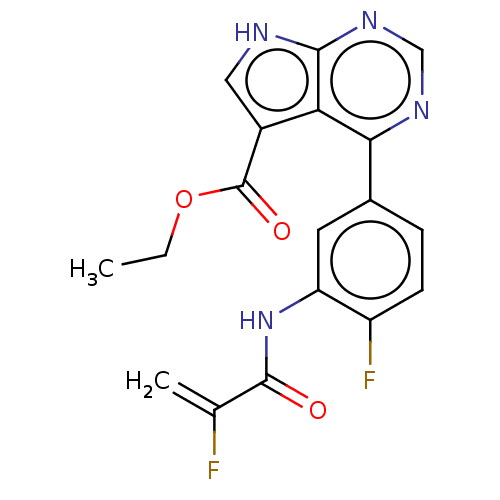 (US8993756, 5-10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0130 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153227 (US10421760, Comparative Example 12 | US8993756, 20...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153218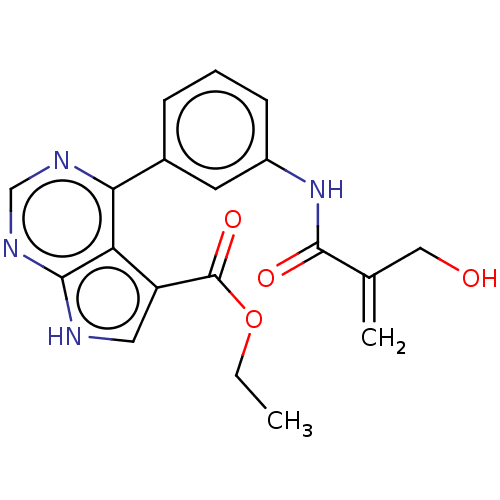 (US8993756, 15-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM47231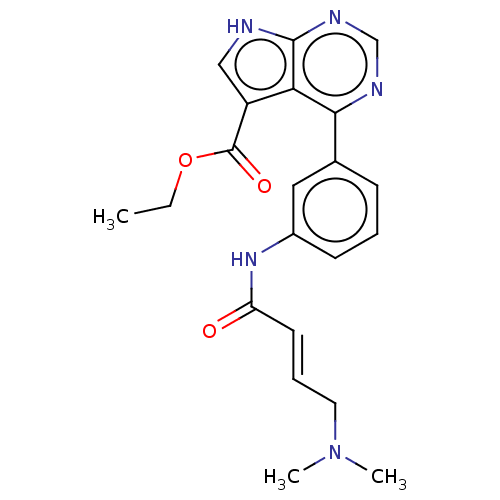 (US8993756, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153191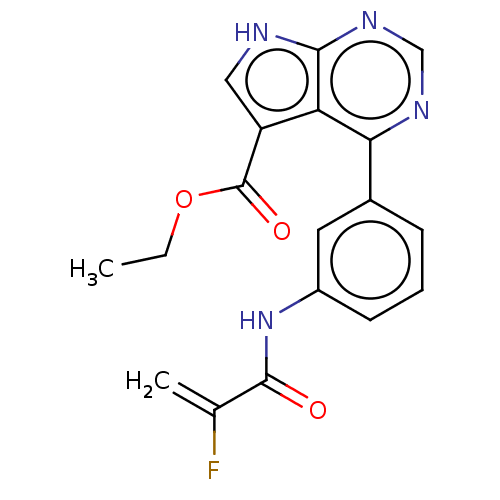 (US8993756, 5-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153217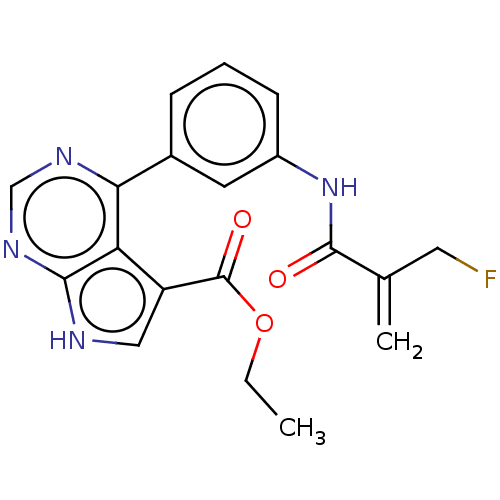 (US8993756, 15-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153207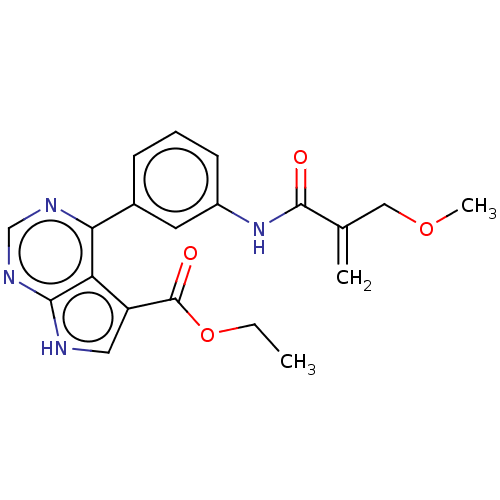 (US8993756, 5-45) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0250 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153168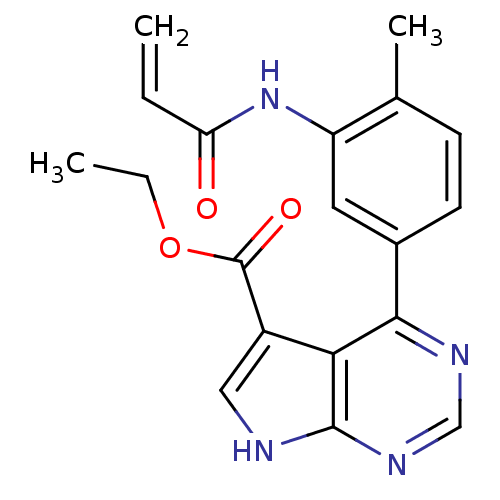 (US8993756, 4-11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153209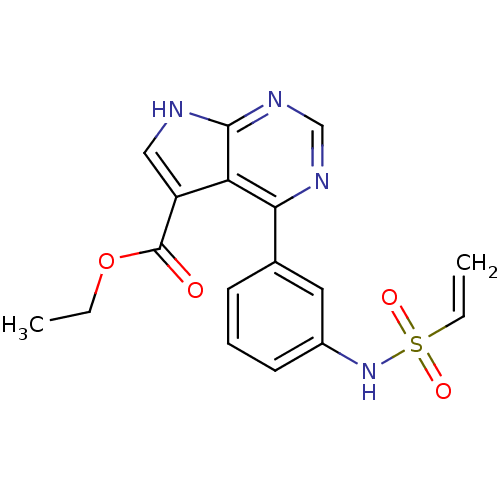 (US8993756, 10-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153163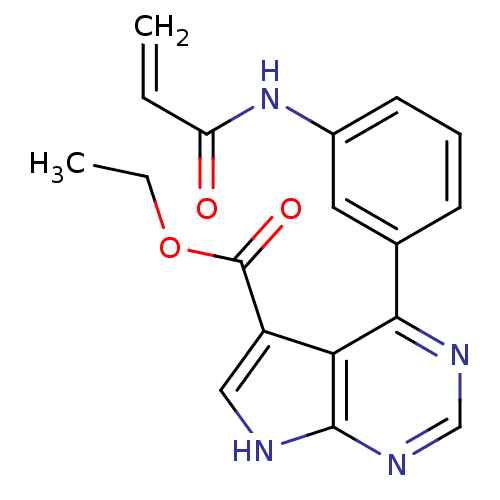 (US8993756, 4-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153194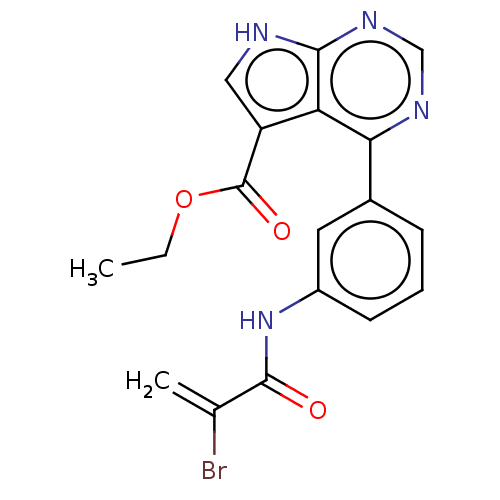 (US8993756, 5-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153190 (US8993756, 4-35) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153162 (US8993756, 4-4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0490 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153175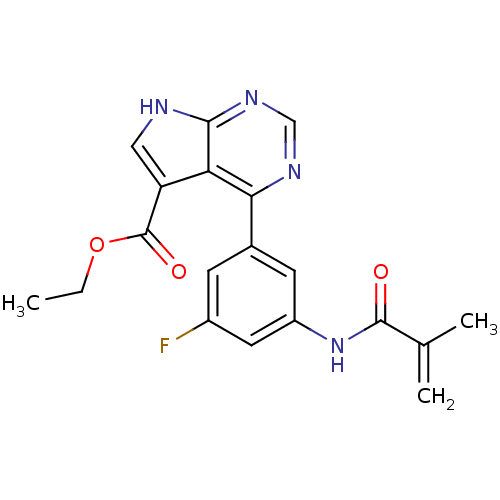 (US8993756, 4-19) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153214 (US8993756, 13-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0520 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153219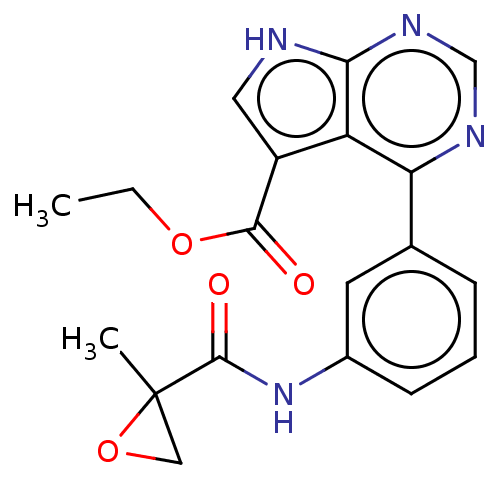 (US8993756, 18-1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153170 (US8993756, 4-14) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0660 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153179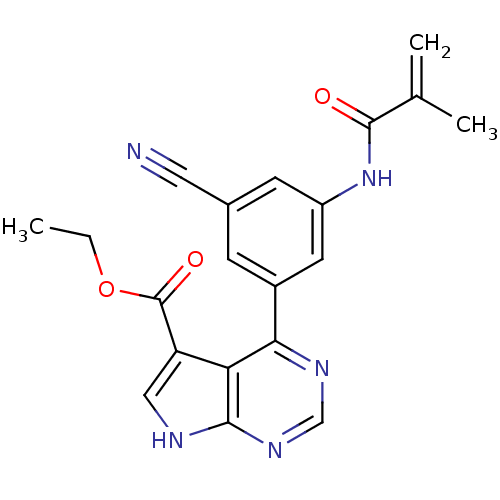 (US8993756, 4-24) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153172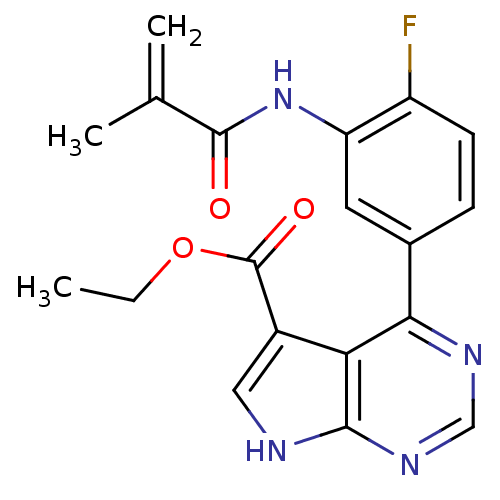 (US8993756, 4-16) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153193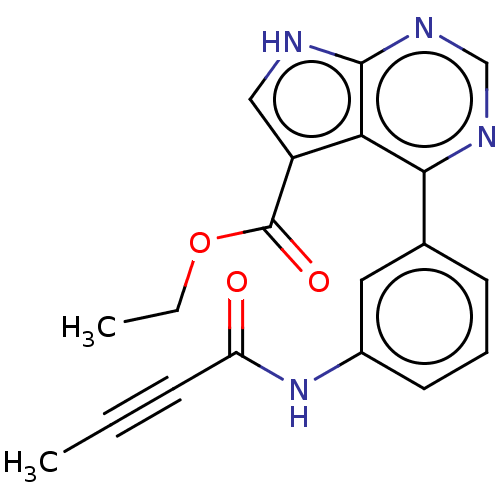 (US8993756, 5-3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0720 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153176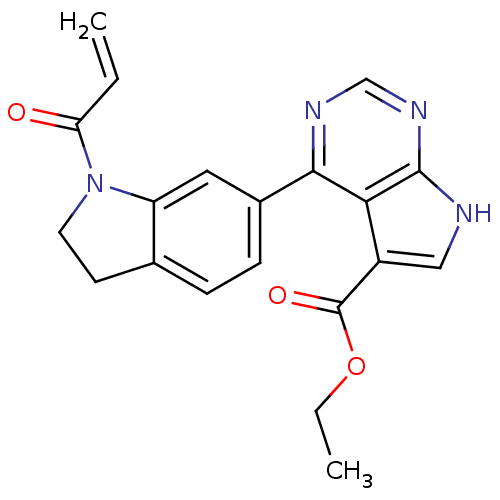 (US8993756, 4-20) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0810 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153161 (US8993756, 4-3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0850 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153184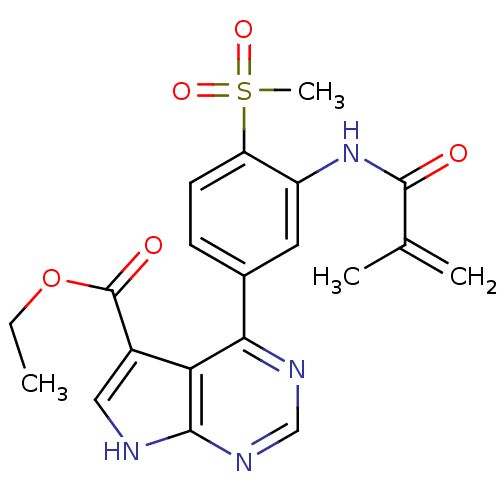 (US8993756, 4-29) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM153171 (US8993756, 4-15) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The ability of compounds to inhibit the activity of JAKl, JAK2, JAK3, and Tyk2 was measured using a recombinant purified GST-tagged catalytic domain ... | US Patent US8993756 (2015) BindingDB Entry DOI: 10.7270/Q2VQ31DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4224 total ) | Next | Last >> |