

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290221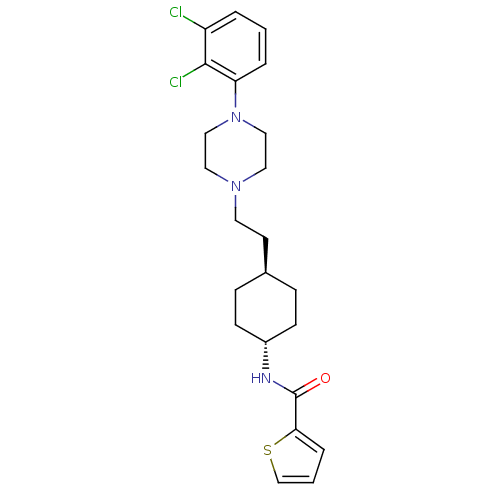 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Rattus norvegicus (Rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]- prazosin binding against Alpha-1A adrenergic receptor from rat submaxillary gland | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279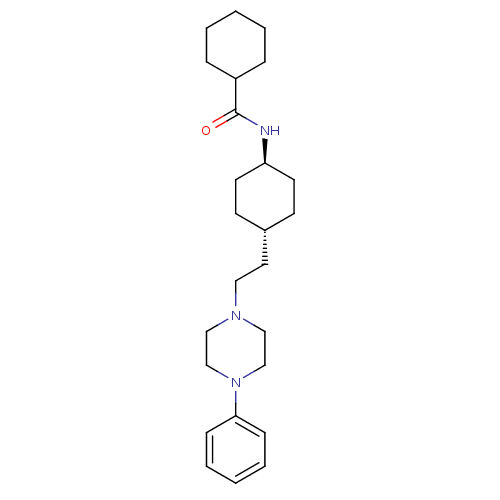 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Rattus norvegicus (rat)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]- prazosin binding against Alpha-1B adrenergic receptor from rat liver | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260806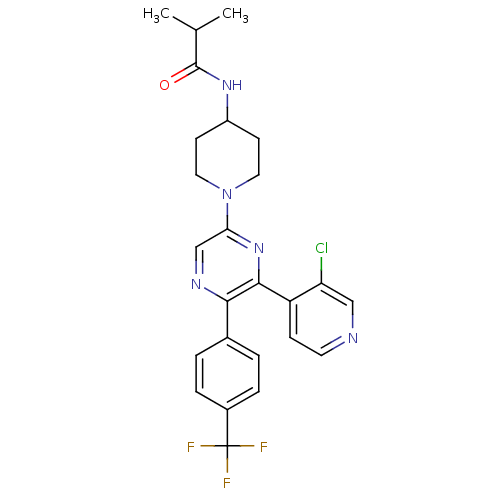 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50202412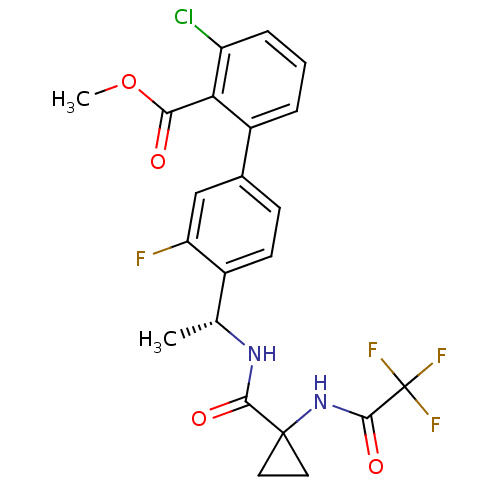 (3-Chloro-3'-fluoro-4'-((R)-1-{[1-(2,2,2-trifluoro-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inhibition of human bradykinin B1 receptor | Bioorg Med Chem Lett 18: 5027-31 (2008) Article DOI: 10.1016/j.bmcl.2008.08.014 BindingDB Entry DOI: 10.7270/Q2NG4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50281503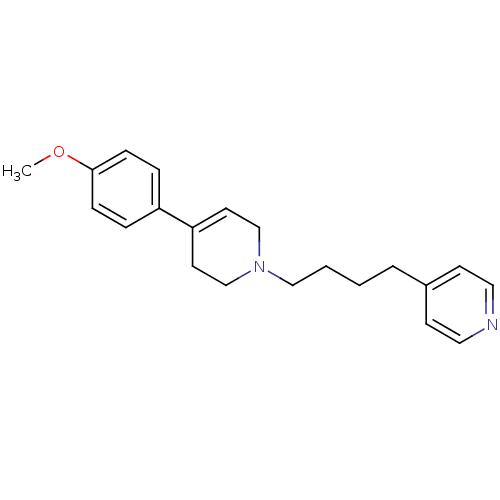 (4-{4-[4-(4-Methoxy-phenyl)-3,6-dihydro-2H-pyridin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in guinea pig brain preparation was estimated using [3H]-3-PPP as radioligand | Bioorg Med Chem Lett 3: 277-280 (1993) Article DOI: 10.1016/S0960-894X(01)80892-1 BindingDB Entry DOI: 10.7270/Q2HH6K0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50054067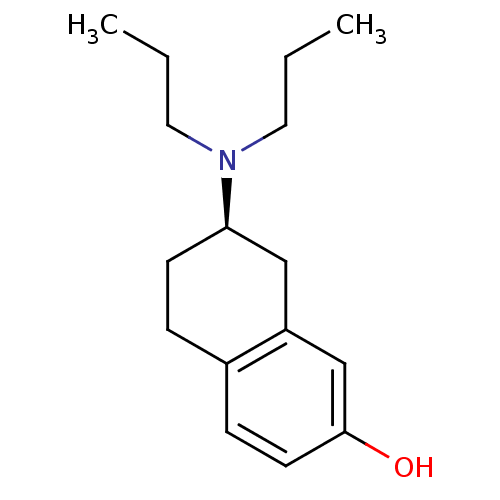 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290206 (CHEMBL78800 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50127438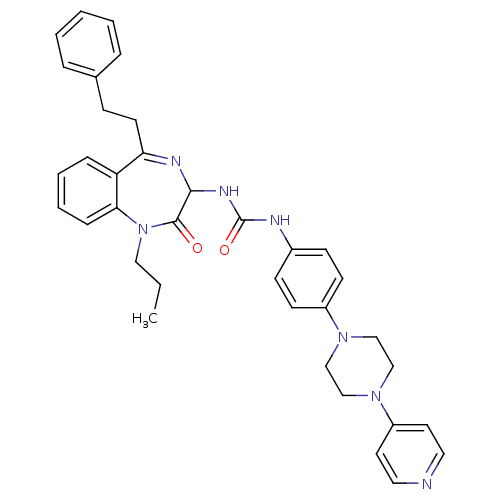 (1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inhibition of human bradykinin B1 receptor | Bioorg Med Chem Lett 18: 5027-31 (2008) Article DOI: 10.1016/j.bmcl.2008.08.014 BindingDB Entry DOI: 10.7270/Q2NG4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290221 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50054067 ((2R)-7-Dipropylamino-5,6,7,8-tetrahydro-naphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290225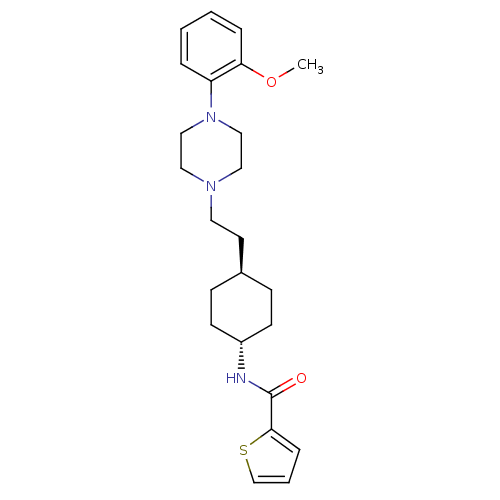 (CHEMBL78950 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260767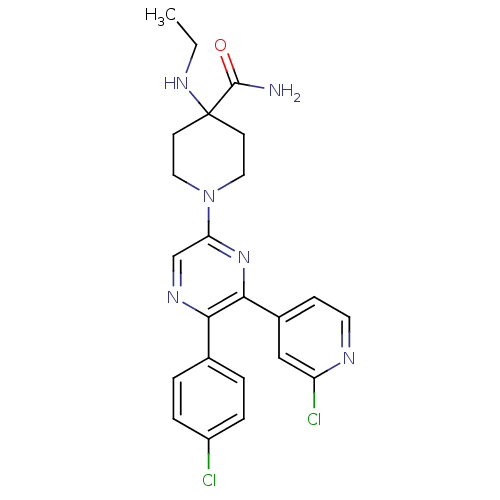 (1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063291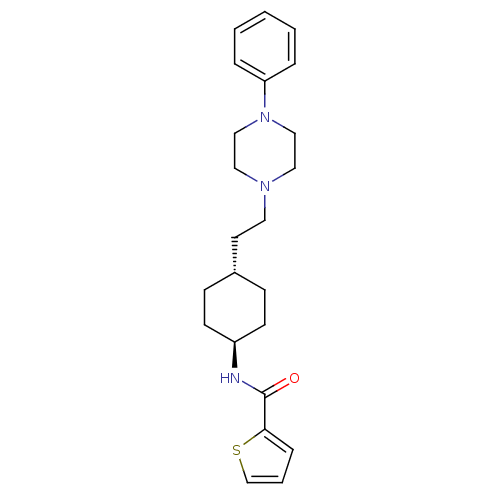 (CHEMBL78791 | Thiophene-2-carboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260805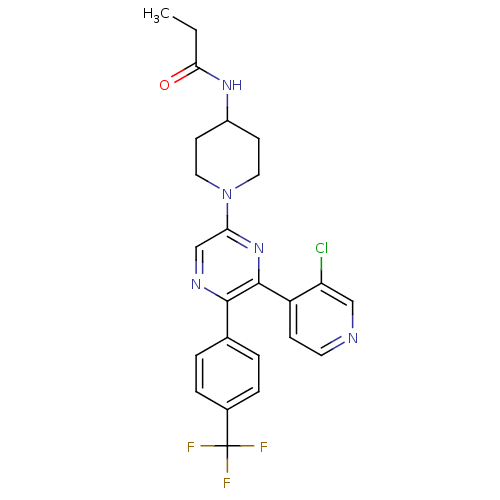 (CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260681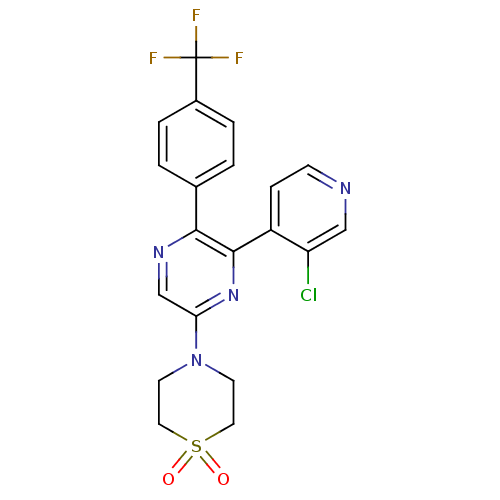 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260768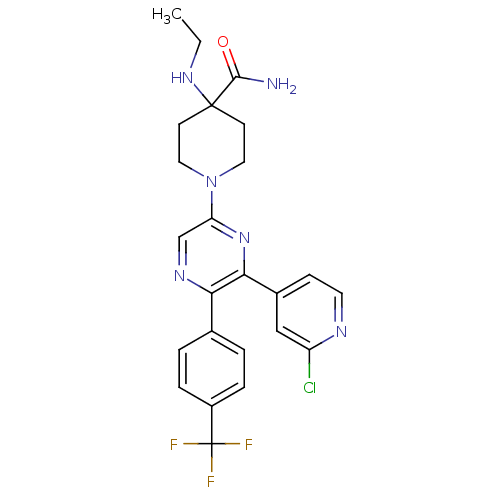 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093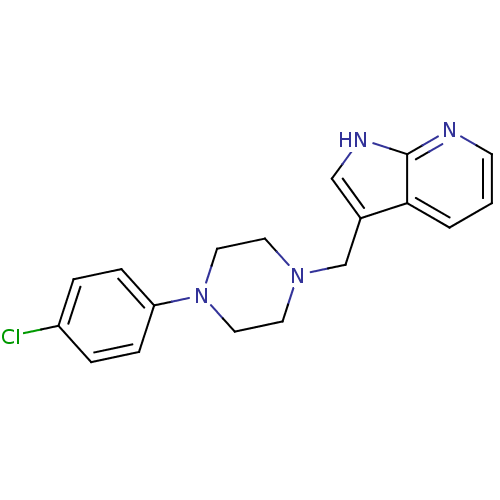 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D4 in CHO cells by radioligand displacement. | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260682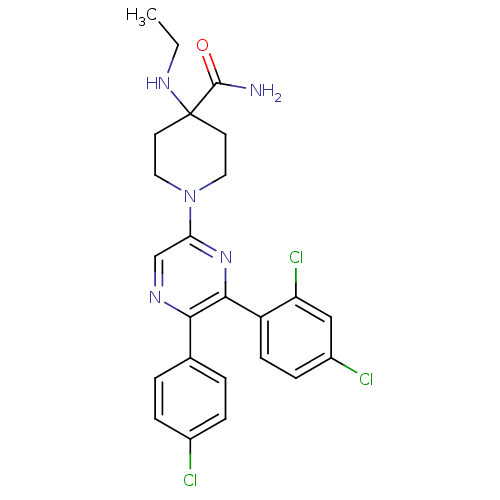 (1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM85093 (CAS_3853 | CHEMBL267014 | CHEMBL555670 | L 745,870...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Parke-Davis Pharmaceutical Research Curated by ChEMBL | Assay Description In vitro binding affinity at human dopamine D4 receptor expressed in CHO cells by [3H]spiperone displacement. | Bioorg Med Chem Lett 8: 1499-502 (1999) BindingDB Entry DOI: 10.7270/Q2WH2P54 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (RAT) | BDBM50127438 (1-(2-Oxo-5-phenethyl-1-propyl-2,3-dihydro-1H-benzo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat bradykinin B1 receptor | Bioorg Med Chem Lett 18: 5027-31 (2008) Article DOI: 10.1016/j.bmcl.2008.08.014 BindingDB Entry DOI: 10.7270/Q2NG4QDX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290206 (CHEMBL78800 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50281500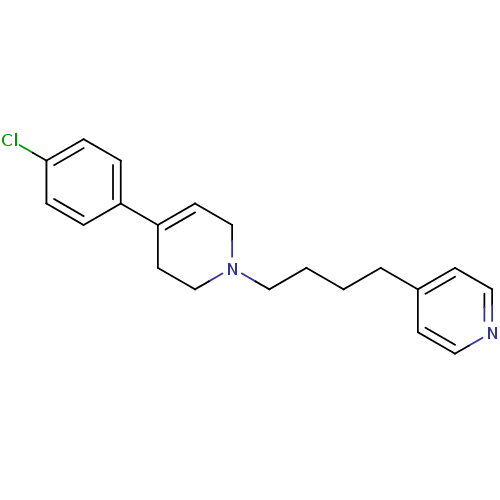 (4-{4-[4-(4-Chloro-phenyl)-3,6-dihydro-2H-pyridin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.04 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in guinea pig brain preparation was estimated using [3H]-3-PPP as radioligand | Bioorg Med Chem Lett 3: 277-280 (1993) Article DOI: 10.1016/S0960-894X(01)80892-1 BindingDB Entry DOI: 10.7270/Q2HH6K0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260803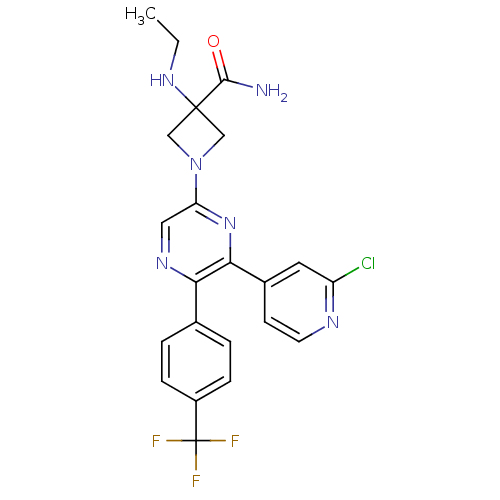 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290225 (CHEMBL78950 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290209 (CHEMBL310577 | Thiophene-2-carboxylic acid (4-{2-[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290214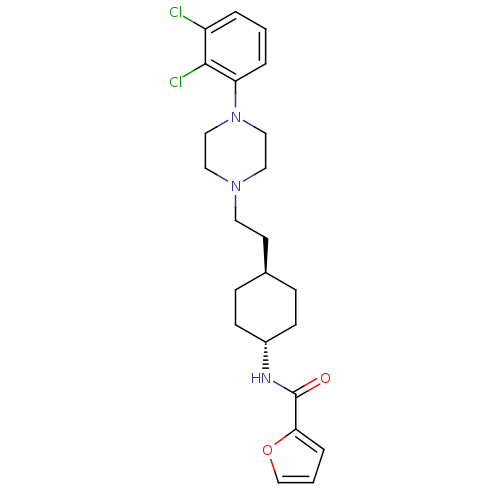 (CHEMBL312430 | Furan-2-carboxylic acid (4-{2-[4-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260718 (1-(6-(2-chlorophenyl)-5-(4-chlorophenyl)pyrazin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50290218 (CHEMBL78617 | Cyclopentanecarboxylic acid (4-{2-[4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]N-0437 from human dopamine D2 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260717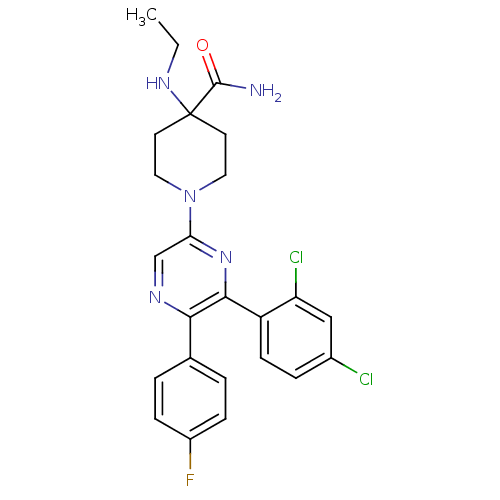 (1-(6-(2,4-dichlorophenyl)-5-(4-fluorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50281497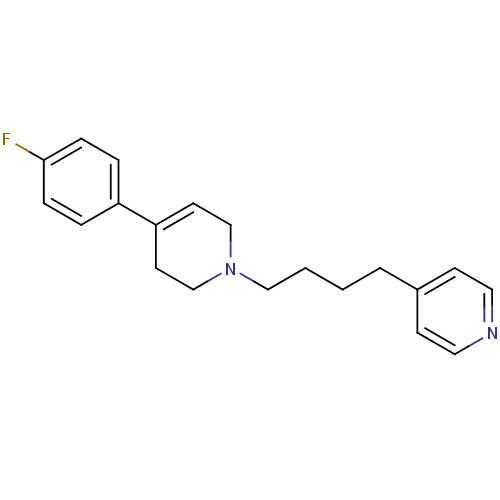 (4-{4-[4-(4-Fluoro-phenyl)-3,6-dihydro-2H-pyridin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in guinea pig brain preparation was estimated using [3H]-3-PPP as radioligand | Bioorg Med Chem Lett 3: 277-280 (1993) Article DOI: 10.1016/S0960-894X(01)80892-1 BindingDB Entry DOI: 10.7270/Q2HH6K0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]- pentazocine binding against alpha-1 from guinea pig brain | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50083349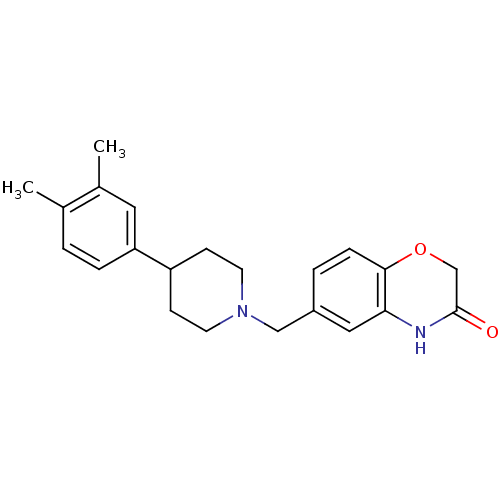 (6-[4-(3,4-Dimethyl-phenyl)-piperidin-1-ylmethyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D4 in CHO cells by radioligand displacement. | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290221 (CHEMBL80919 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260804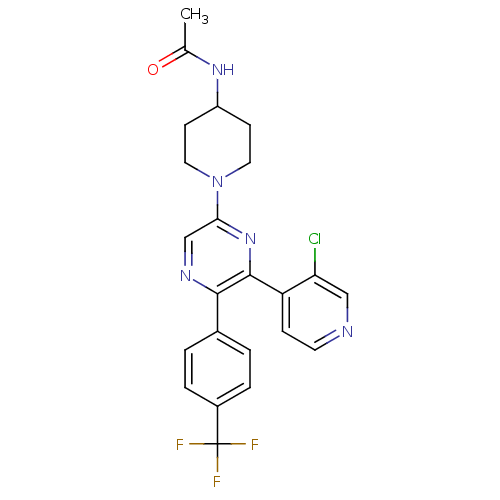 (CHEMBL497556 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (BOVINE) | BDBM50123627 ((S)-6-Dipropylamino-5,6,7,8-tetrahydro-naphthalene...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Centre for Pharmacy Curated by ChEMBL | Assay Description Binding affinity of compound for Dopamine receptor D2 using [3H]-N-0437 | J Med Chem 46: 584-90 (2003) Article DOI: 10.1021/jm020990u BindingDB Entry DOI: 10.7270/Q2H41QS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290210 (CHEMBL78368 | Thiophene-2-carboxylic acid (4-{2-[4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (NEONATAL RAT) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibition of [3H]- Yohimbine binding against rat kidney cortex Alpha-2B adrenergic receptor | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50063279 (CHEMBL309623 | Cyclohexanecarboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50281504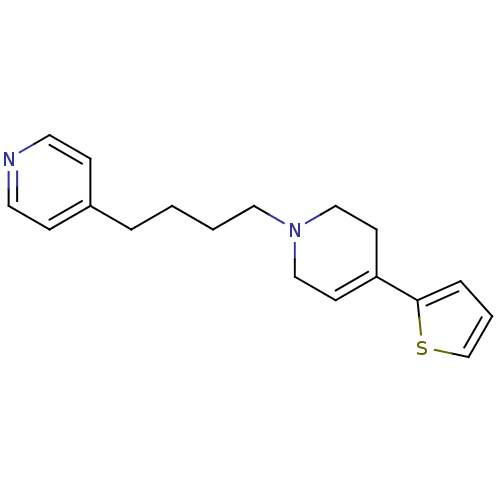 (4-[4-(4-Thiophen-2-yl-3,6-dihydro-2H-pyridin-1-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article | 2.13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against sigma receptor in guinea pig brain preparation was estimated using [3H]-3-PPP as radioligand | Bioorg Med Chem Lett 3: 277-280 (1993) Article DOI: 10.1016/S0960-894X(01)80892-1 BindingDB Entry DOI: 10.7270/Q2HH6K0R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290213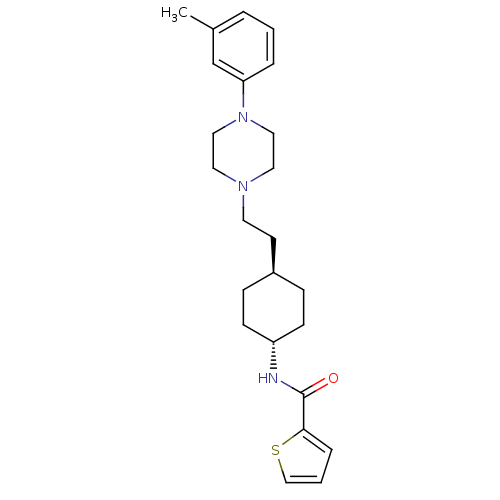 (CHEMBL80875 | Thiophene-2-carboxylic acid {4-[2-(4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50290217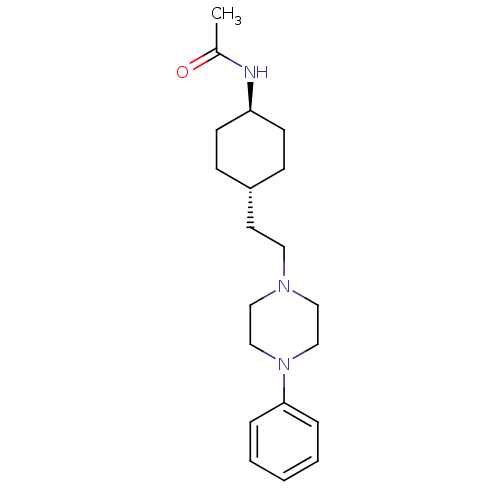 (CHEMBL78916 | N-{4-[2-(4-Phenyl-piperazin-1-yl)-et...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Ability to displace radioligand [3H]spiperone from human dopamine D3 receptor transfected chinese hamster ovary cell membranes. | Bioorg Med Chem Lett 7: 2403-2408 (1997) Article DOI: 10.1016/S0960-894X(97)00443-5 BindingDB Entry DOI: 10.7270/Q27W6CQ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50083337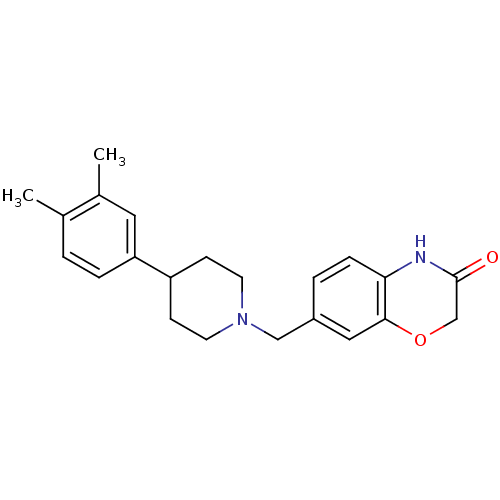 (7-[4-(3,4-Dimethyl-phenyl)-piperidin-1-ylmethyl]-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Binding affinity at Dopamine receptor D4 in CHO cells by radioligand displacement. | J Med Chem 42: 5181-7 (2000) BindingDB Entry DOI: 10.7270/Q23T9HZ0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50055721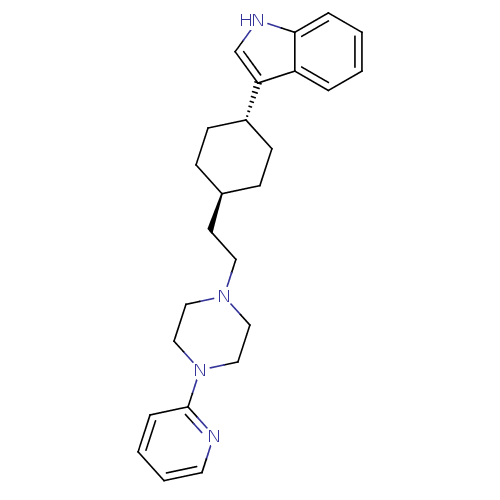 (3-{4-[2-(4-Pyridin-2-yl-piperazin-1-yl)-ethyl]-cyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Division of Warner Lambert Company Curated by ChEMBL | Assay Description Binding affinity was determined in vitro on rat striatum using [3H]-N-propylnorapomorphine against Dopamine receptor D2 | J Med Chem 40: 250-9 (1997) Article DOI: 10.1021/jm960597m BindingDB Entry DOI: 10.7270/Q28W3CD4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 913 total ) | Next | Last >> |