

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4136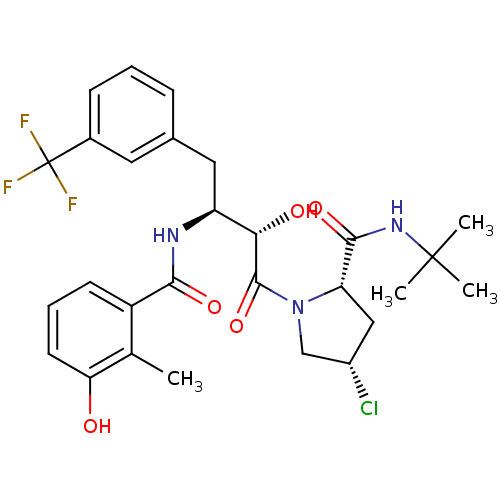 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4126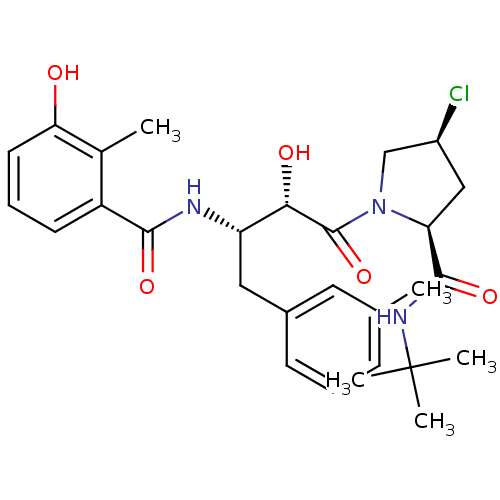 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4134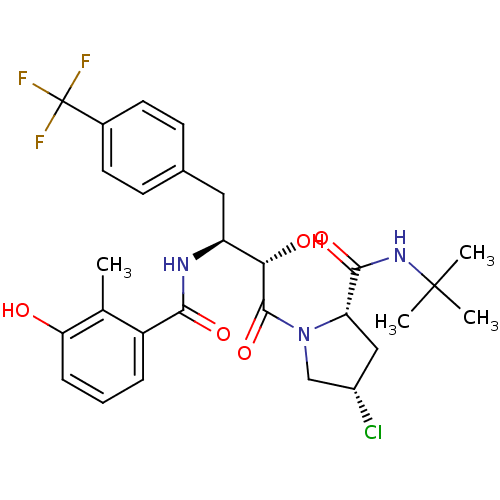 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4129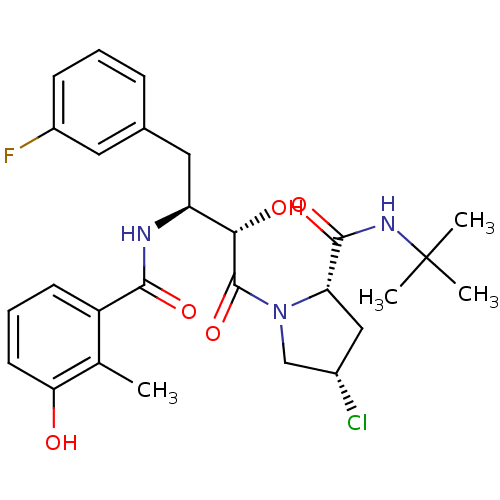 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29995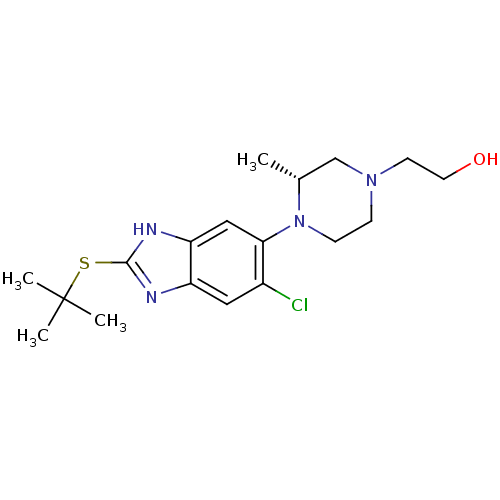 (CHEMBL494350 | benzimidazole-based antagonist, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4130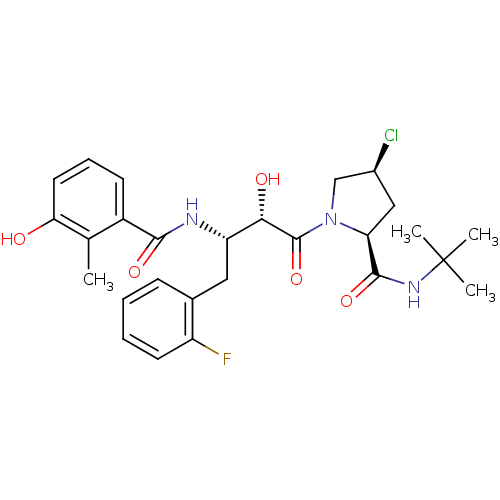 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(2-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4132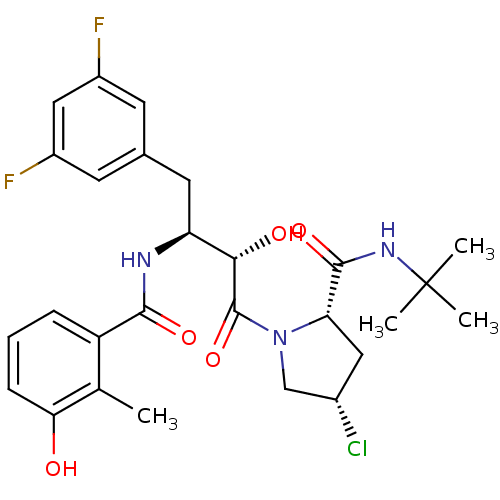 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29994 (2-Cyclohexylcarbonylbenzimidazole, 7e) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | 2.40 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4131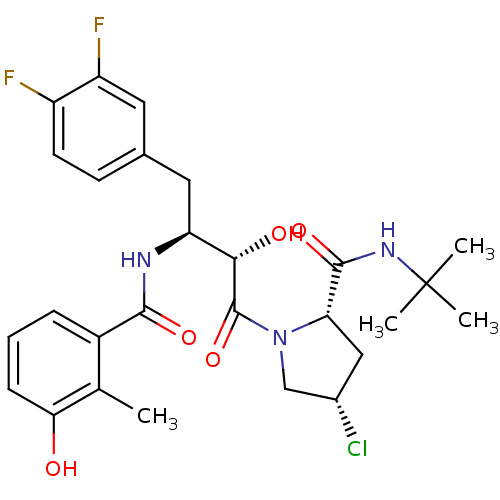 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4163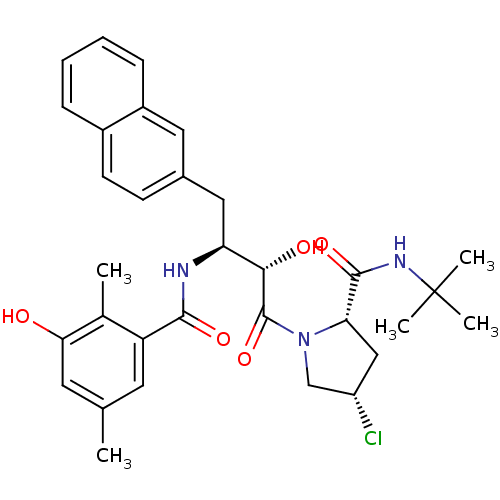 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29988 (benzimidazole analogue, 7h | benzimidazole derivat...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4138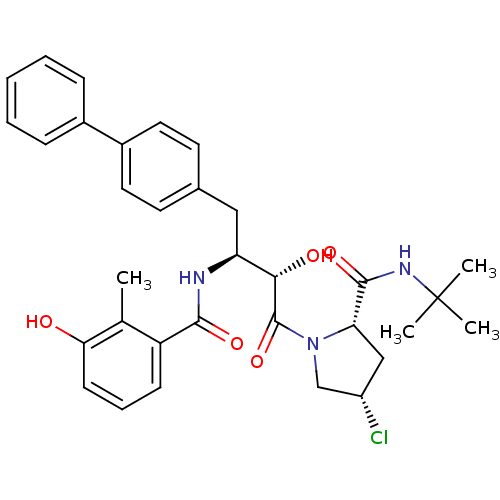 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4135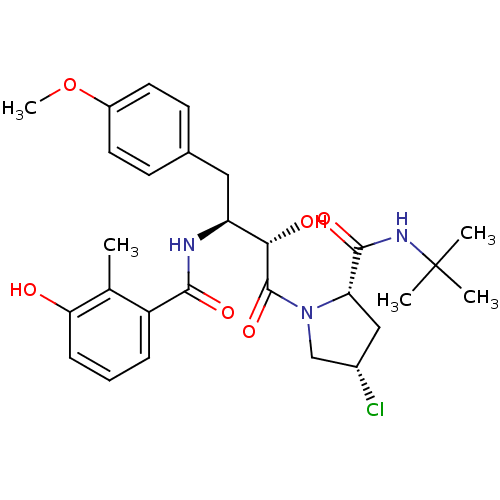 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4128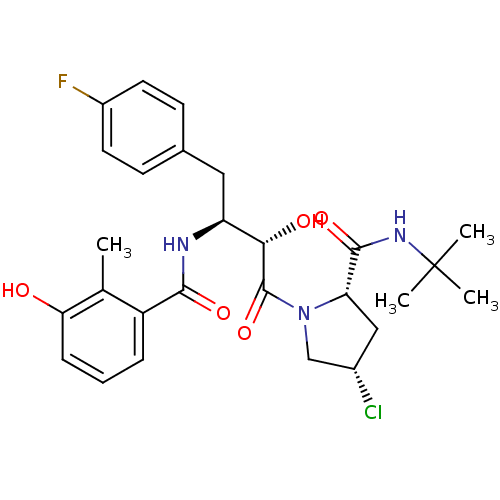 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(4-fluo...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4124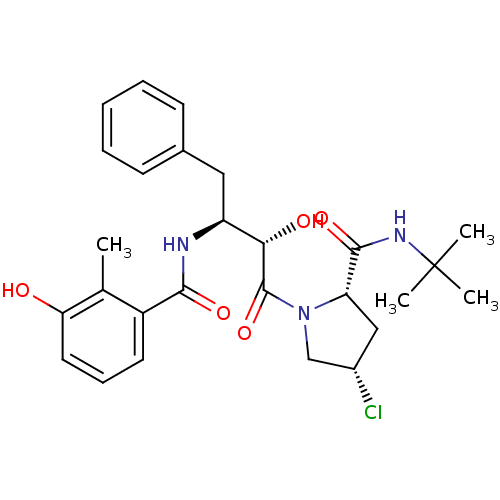 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4125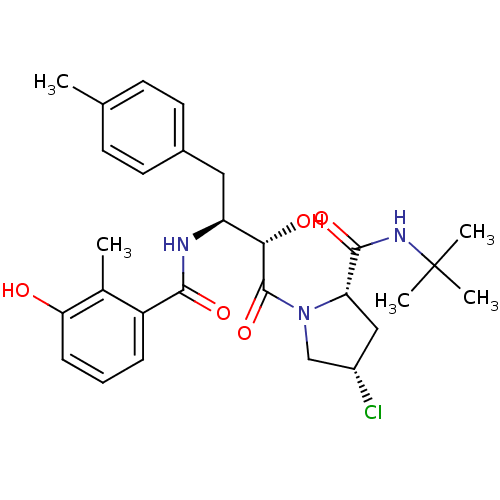 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4142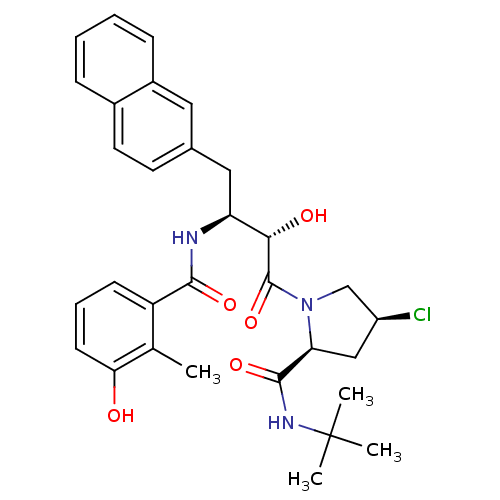 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30012 (benzimidazole analogue, 7k) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50260633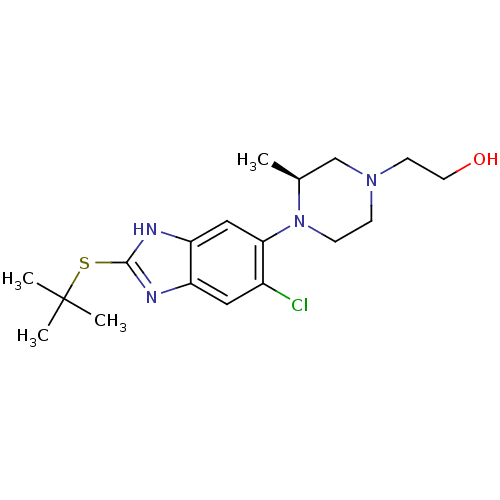 ((S)-2-(4-(2-(tert-butylthio)-6-chloro-3H-benzo[d]i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30017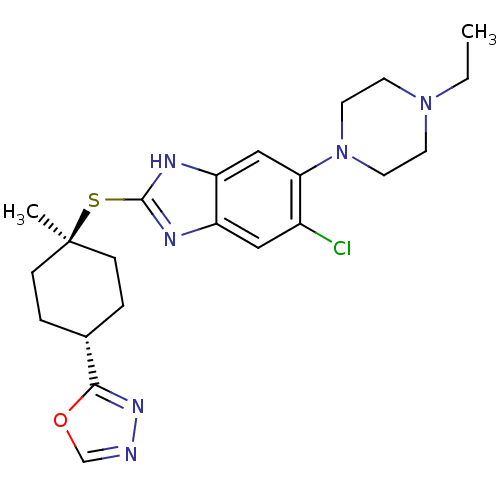 (benzimidazole analogue, 7p) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | 0.590 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC/OFQ from human ORL1 receptor | Bioorg Med Chem Lett 18: 3282-5 (2008) Article DOI: 10.1016/j.bmcl.2008.04.037 BindingDB Entry DOI: 10.7270/Q2542NDJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4165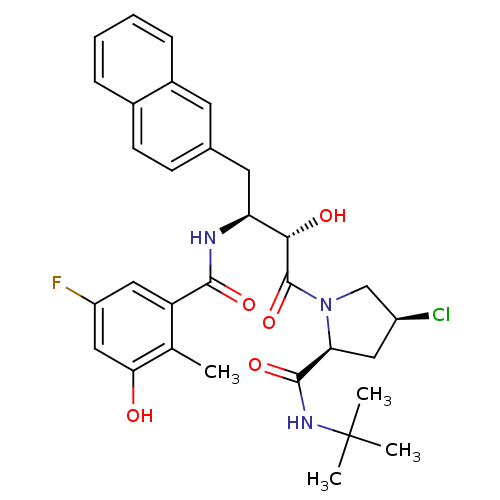 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(5-flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4161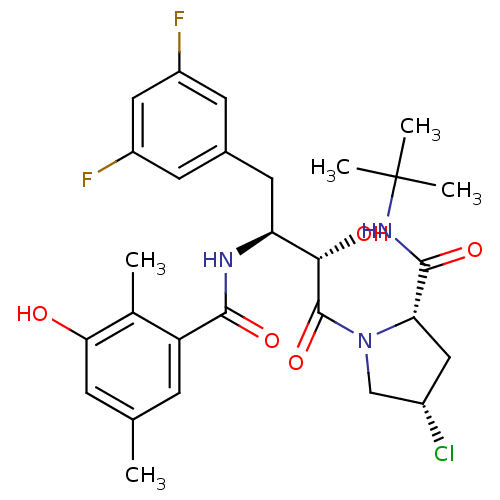 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,5-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50239749 (2-(4-(6-chloro-2-(3-methylpentan-3-ylthio)-3H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4139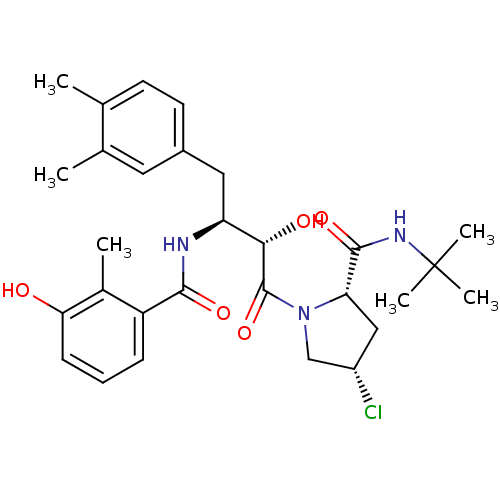 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(3,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4154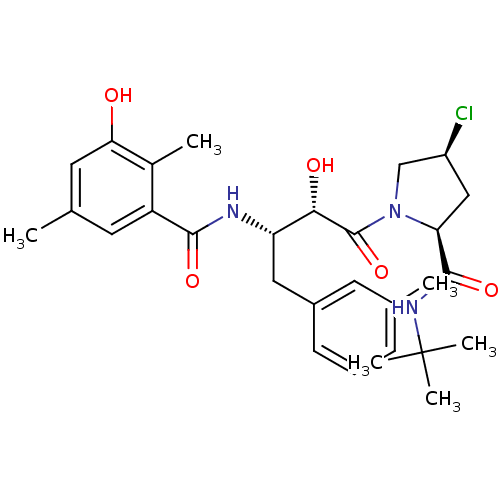 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4144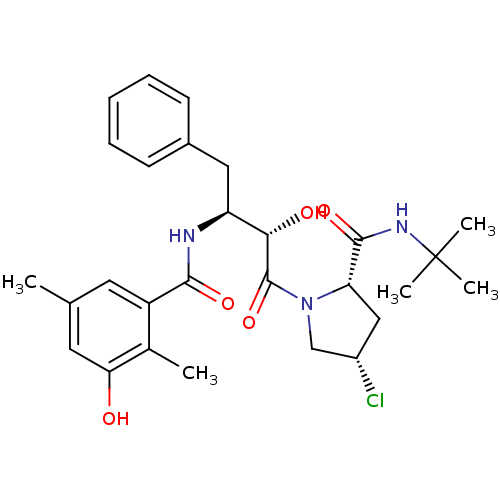 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4137 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4160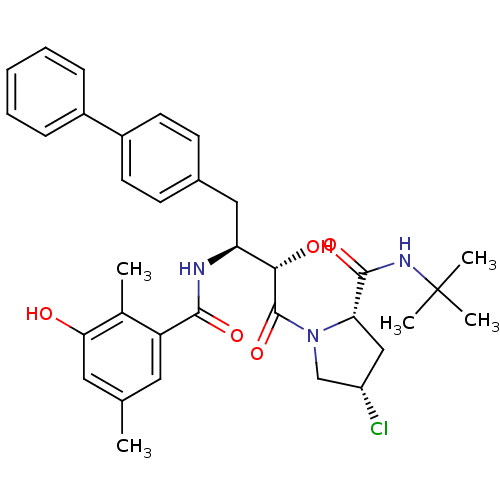 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4140 ((2S,4S)-1-[(2S,3S)-4-(1-benzothiophen-5-yl)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4123 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30011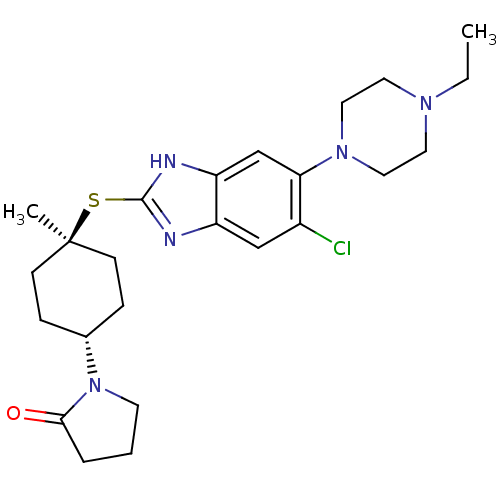 (benzimidazole analogue, 7j) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 0.550 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29992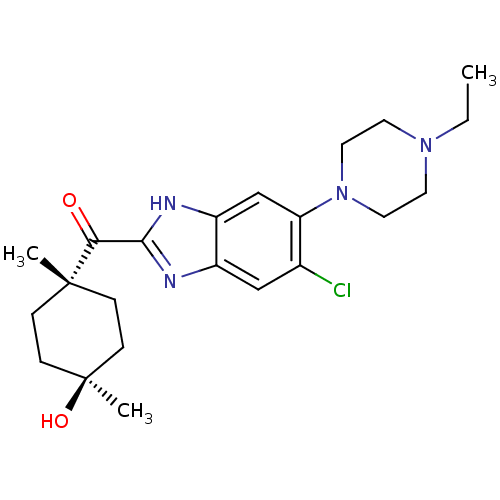 (2-Cyclohexylcarbonylbenzimidazole, 7c) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 1.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4166 ((2S,4S)-1-[(2S,3S)-4-(1-benzothiophen-5-yl)-2-hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4141 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4133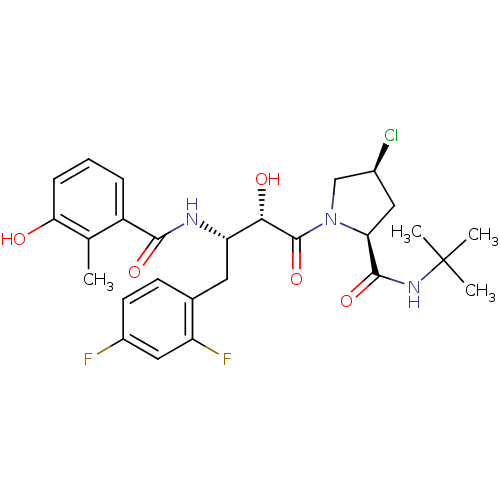 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-4-(2,4-di...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4145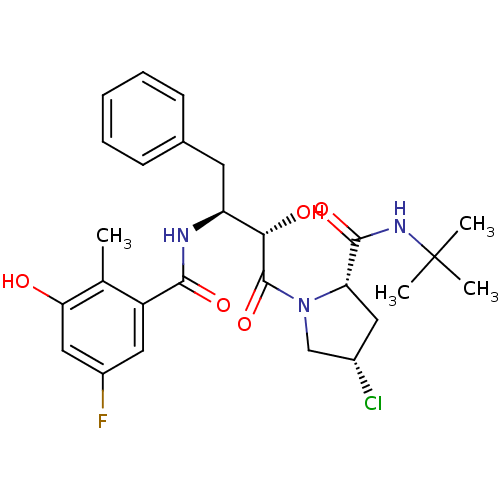 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-3-[(5-flu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30016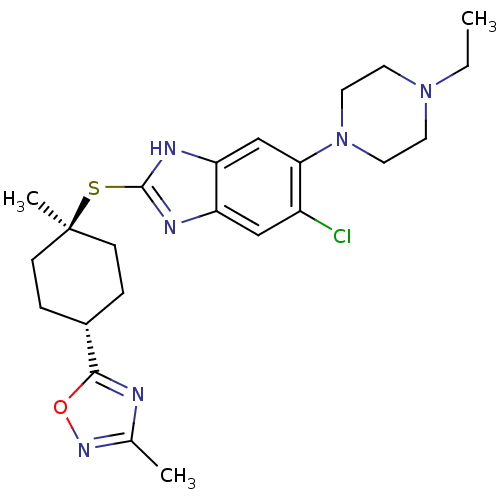 (benzimidazole analogue, 7o) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.70 | n/a | 0.910 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373360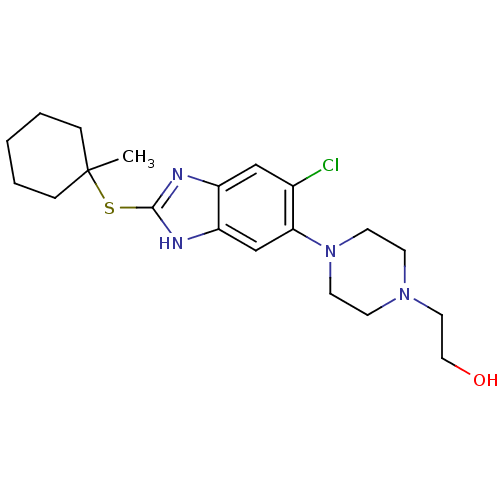 (CHEMBL263917) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Antagonist activity at human ORL1 receptor expressed in CHO cell membrane by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373360 (CHEMBL263917) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30018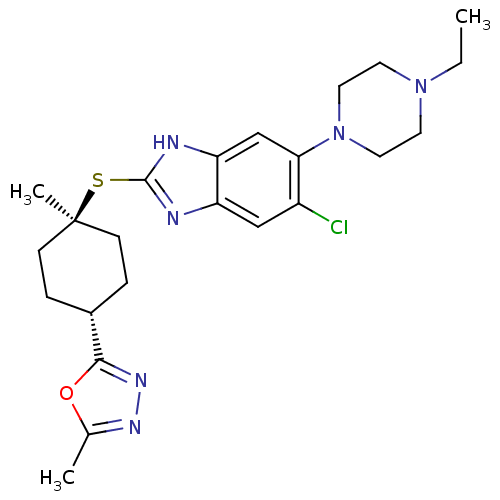 (benzimidazole analogue, 7q) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | 7.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4148 ((2S,4S)-N-tert-butyl-4-chloro-1-[(2S,3S)-2-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4167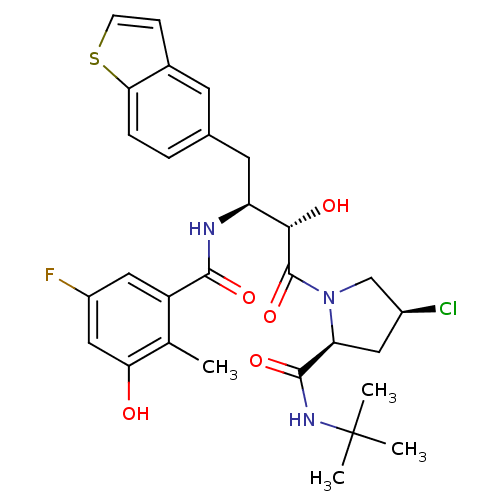 ((2S,4S)-1-[(2S,3S)-4-(1-benzothiophen-5-yl)-3-[(5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 7: 2063-72 (1999) Article DOI: 10.1016/s0968-0896(99)00127-3 BindingDB Entry DOI: 10.7270/Q23T9FD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30008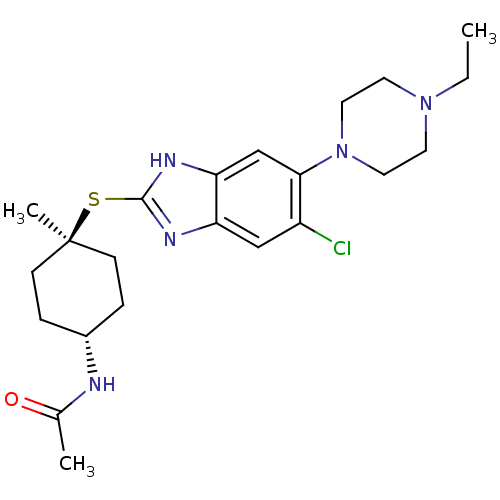 (benzimidazole analogue, 7g) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 1.10 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM29990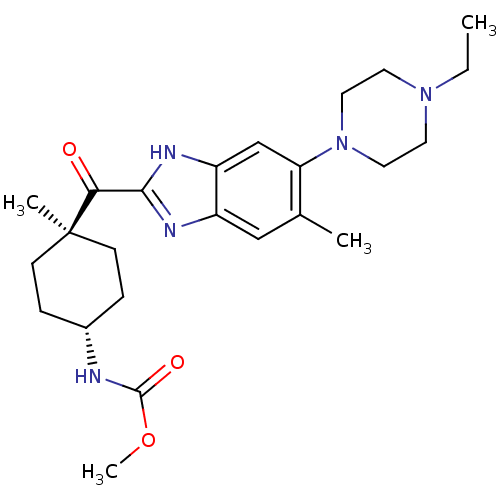 (2-Cyclohexylcarbonylbenzimidazole, 7b) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | 5.30 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3096-9 (2009) Article DOI: 10.1016/j.bmcl.2009.04.023 BindingDB Entry DOI: 10.7270/Q24J0CFR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50373365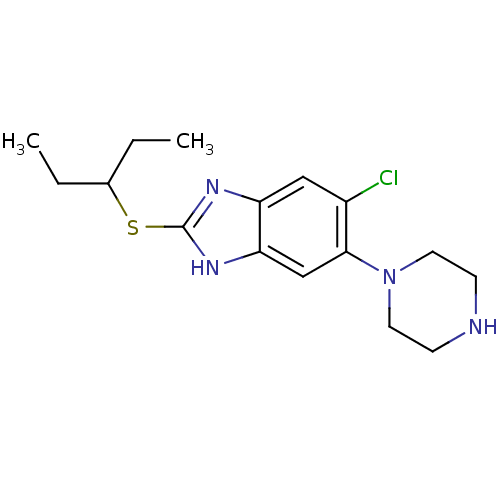 (CHEMBL258710) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Banyu Tsukuba Research Institute Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-NC from human ORL1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 18: 3278-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.054 BindingDB Entry DOI: 10.7270/Q2HT2Q57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM30010 (benzimidazole analogue, 7i) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | 1.80 | n/a | n/a | 7.4 | 37 |
Banyu Pharmaceutical Co. | Assay Description Compounds were tested for their inhibitory effects on ligand binding to the human ORL1 receptor. Bound and free radioligands are separated by filtra... | Bioorg Med Chem Lett 19: 3100-3 (2009) Article DOI: 10.1016/j.bmcl.2009.04.022 BindingDB Entry DOI: 10.7270/Q20R9MRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 235 total ) | Next | Last >> |