 Found 62 hits with Last Name = 'youssef' and Initial = 's'
Found 62 hits with Last Name = 'youssef' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175075
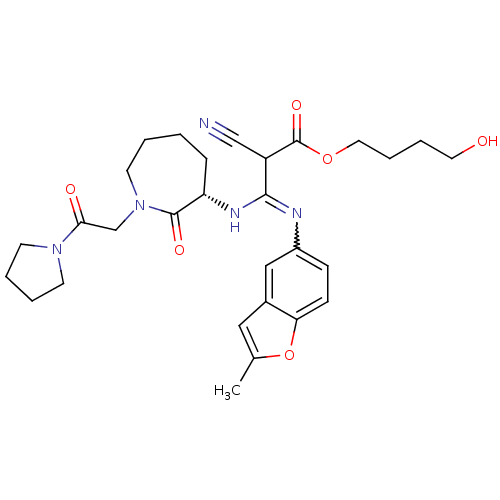
((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to chymotrypsin |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to factor IXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to urokinase |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to tPA |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to plasma kallikrein |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to activated protein C |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to plasmin |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to factor VIIa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26359
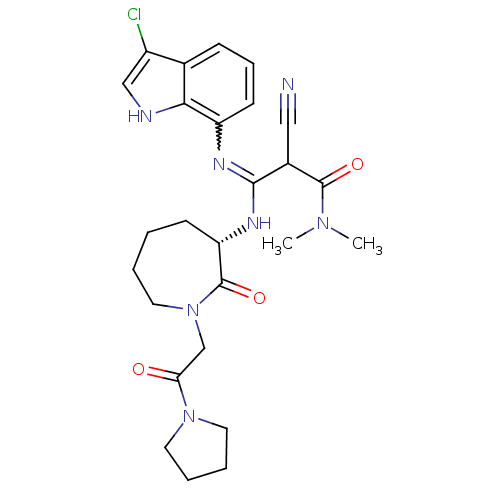
((2Z)-3-[(3-chloro-1H-indol-7-yl)amino]-2-cyano-N,N...)Show SMILES CN(C)C(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1cccc2c(Cl)c[nH]c12 |r,w:26.28| Show InChI InChI=1S/C26H32ClN7O3/c1-32(2)25(36)18(14-28)24(30-20-10-7-8-17-19(27)15-29-23(17)20)31-21-9-3-4-13-34(26(21)37)16-22(35)33-11-5-6-12-33/h7-8,10,15,18,21,29H,3-6,9,11-13,16H2,1-2H3,(H,30,31)/t18?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26357
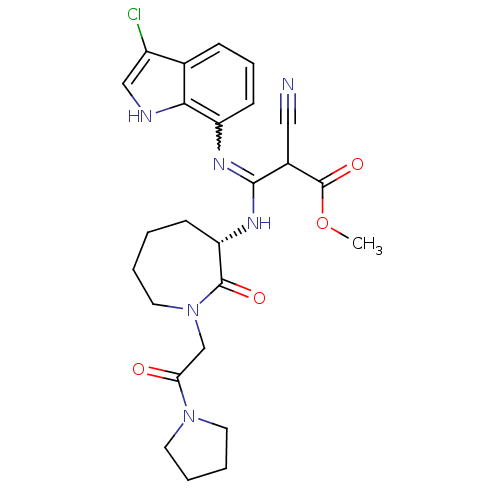
(3-chloroindole compound, 18 | methyl (2Z)-3-[(3-ch...)Show SMILES COC(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1cccc2c(Cl)c[nH]c12 |r,w:25.27| Show InChI InChI=1S/C25H29ClN6O4/c1-36-25(35)17(13-27)23(29-19-9-6-7-16-18(26)14-28-22(16)19)30-20-8-2-3-12-32(24(20)34)15-21(33)31-10-4-5-11-31/h6-7,9,14,17,20,28H,2-5,8,10-12,15H2,1H3,(H,29,30)/t17?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26360
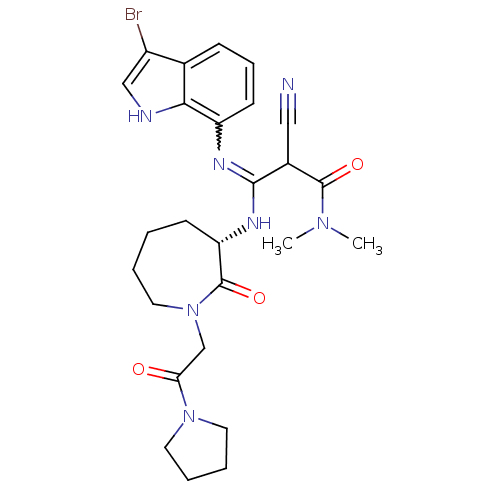
((2Z)-3-[(3-bromo-1H-indol-7-yl)amino]-2-cyano-N,N-...)Show SMILES CN(C)C(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1cccc2c(Br)c[nH]c12 |r,w:26.28| Show InChI InChI=1S/C26H32BrN7O3/c1-32(2)25(36)18(14-28)24(30-20-10-7-8-17-19(27)15-29-23(17)20)31-21-9-3-4-13-34(26(21)37)16-22(35)33-11-5-6-12-33/h7-8,10,15,18,21,29H,3-6,9,11-13,16H2,1-2H3,(H,30,31)/t18?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26354
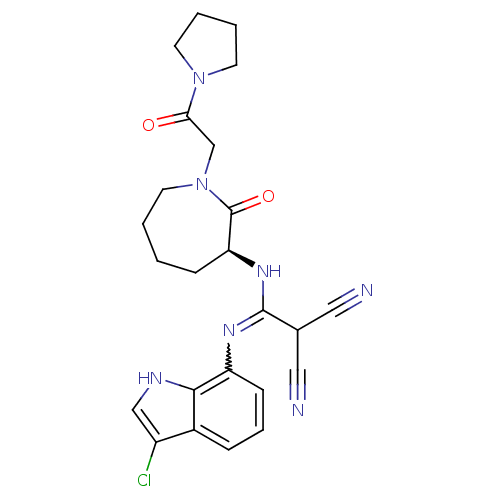
(2-{[(3-chloro-1H-indol-7-yl)amino]({[(3S)-2-oxo-1-...)Show SMILES Clc1c[nH]c2c(cccc12)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C#N |r,w:10.11| Show InChI InChI=1S/C24H26ClN7O2/c25-18-14-28-22-17(18)6-5-8-19(22)29-23(16(12-26)13-27)30-20-7-1-2-11-32(24(20)34)15-21(33)31-9-3-4-10-31/h5-6,8,14,16,20,28H,1-4,7,9-11,15H2,(H,29,30)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175078
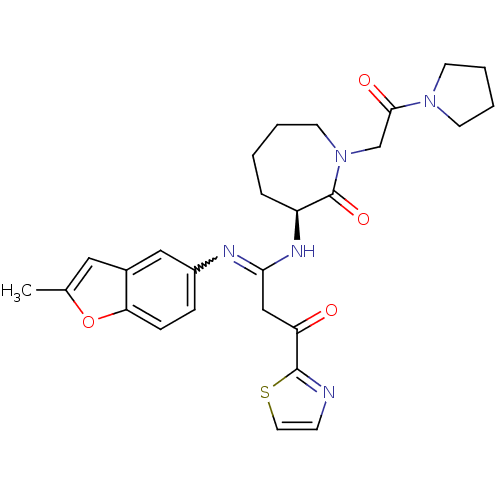
((S,Z)-3-(1-(2-methylbenzofuran-5-ylamino)-3-oxo-3-...)Show SMILES Cc1cc2cc(ccc2o1)N=C(CC(=O)c1nccs1)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |w:10.11| Show InChI InChI=1S/C27H31N5O4S/c1-18-14-19-15-20(7-8-23(19)36-18)29-24(16-22(33)26-28-9-13-37-26)30-21-6-2-3-12-32(27(21)35)17-25(34)31-10-4-5-11-31/h7-9,13-15,21H,2-6,10-12,16-17H2,1H3,(H,29,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175093
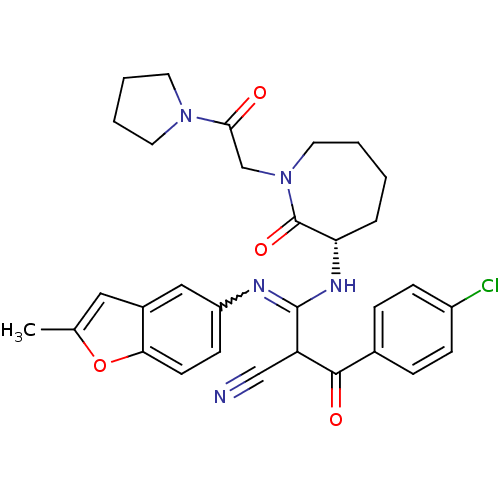
((S,Z)-2-(4-chlorobenzoyl)-3-(2-methylbenzofuran-5-...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)c1ccc(Cl)cc1 |w:10.11| Show InChI InChI=1S/C31H32ClN5O4/c1-20-16-22-17-24(11-12-27(22)41-20)34-30(25(18-33)29(39)21-7-9-23(32)10-8-21)35-26-6-2-3-15-37(31(26)40)19-28(38)36-13-4-5-14-36/h7-12,16-17,25-26H,2-6,13-15,19H2,1H3,(H,34,35)/t25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175076
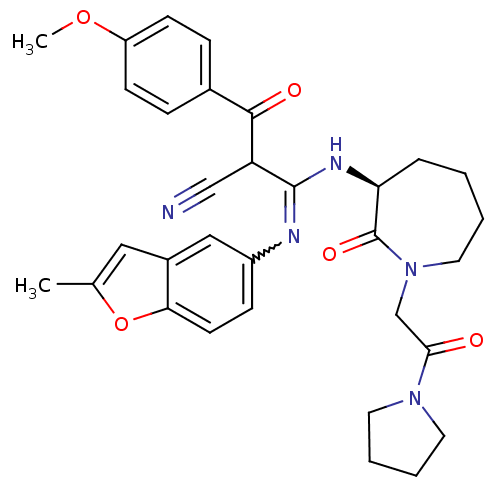
((S,Z)-2-(4-methoxybenzoyl)-3-(2-methylbenzofuran-5...)Show SMILES COc1ccc(cc1)C(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1ccc2oc(C)cc2c1 |w:31.34| Show InChI InChI=1S/C32H35N5O5/c1-21-17-23-18-24(10-13-28(23)42-21)34-31(26(19-33)30(39)22-8-11-25(41-2)12-9-22)35-27-7-3-4-16-37(32(27)40)20-29(38)36-14-5-6-15-36/h8-13,17-18,26-27H,3-7,14-16,20H2,1-2H3,(H,34,35)/t26?,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175097

((S,Z)-1-(2-cyano-3-(2-methylbenzofuran-5-ylamino)-...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)NC(N)=O |w:10.11| Show InChI InChI=1S/C26H31N7O5/c1-16-12-17-13-18(7-8-21(17)38-16)29-23(19(14-27)24(35)31-26(28)37)30-20-6-2-3-11-33(25(20)36)15-22(34)32-9-4-5-10-32/h7-8,12-13,19-20H,2-6,9-11,15H2,1H3,(H,29,30)(H3,28,31,35,37)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175095
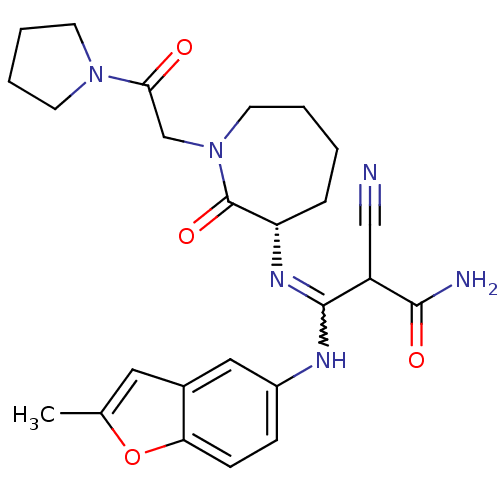
((S,Z)-2-cyano-3-(2-methylbenzofuran-5-ylamino)-3-(...)Show SMILES Cc1cc2cc(NC(=N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)C(C#N)C(N)=O)ccc2o1 |w:7.6| Show InChI InChI=1S/C25H30N6O4/c1-16-12-17-13-18(7-8-21(17)35-16)28-24(19(14-26)23(27)33)29-20-6-2-3-11-31(25(20)34)15-22(32)30-9-4-5-10-30/h7-8,12-13,19-20H,2-6,9-11,15H2,1H3,(H2,27,33)(H,28,29)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175079

((S,Z)-methyl 2-cyano-3-(2-methylbenzofuran-5-ylami...)Show SMILES COC(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1ccc2oc(C)cc2c1 |w:25.27| Show InChI InChI=1S/C26H31N5O5/c1-17-13-18-14-19(8-9-22(18)36-17)28-24(20(15-27)26(34)35-2)29-21-7-3-4-12-31(25(21)33)16-23(32)30-10-5-6-11-30/h8-9,13-14,20-21H,3-7,10-12,16H2,1-2H3,(H,28,29)/t20?,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175081

((S,Z)-ethyl 2-cyano-3-(2-methylbenzofuran-5-ylamin...)Show SMILES CCOC(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1ccc2oc(C)cc2c1 |w:26.28| Show InChI InChI=1S/C27H33N5O5/c1-3-36-27(35)21(16-28)25(29-20-9-10-23-19(15-20)14-18(2)37-23)30-22-8-4-5-13-32(26(22)34)17-24(33)31-11-6-7-12-31/h9-10,14-15,21-22H,3-8,11-13,17H2,1-2H3,(H,29,30)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26351

(2-methylbenzofuran compound, 2 | 2-{[(2-methyl-1-b...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C#N |r,w:10.11| Show InChI InChI=1S/C25H28N6O3/c1-17-12-18-13-20(7-8-22(18)34-17)28-24(19(14-26)15-27)29-21-6-2-3-11-31(25(21)33)16-23(32)30-9-4-5-10-30/h7-8,12-13,19,21H,2-6,9-11,16H2,1H3,(H,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26351

(2-methylbenzofuran compound, 2 | 2-{[(2-methyl-1-b...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C#N |r,w:10.11| Show InChI InChI=1S/C25H28N6O3/c1-17-12-18-13-20(7-8-22(18)34-17)28-24(19(14-26)15-27)29-21-6-2-3-11-31(25(21)33)16-23(32)30-9-4-5-10-30/h7-8,12-13,19,21H,2-6,9-11,16H2,1H3,(H,28,29)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26364
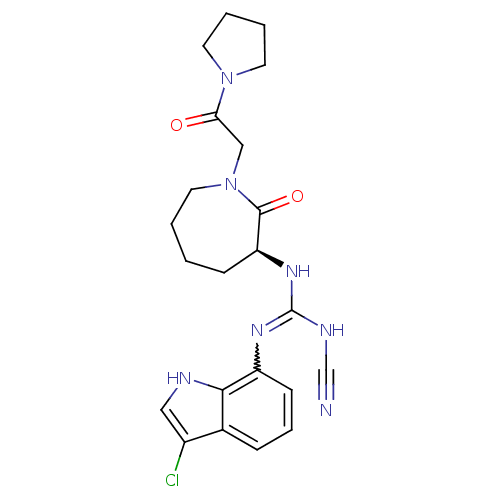
((3S)-1-(3-chloro-1H-indol-7-yl)-2-cyano-2-oxo-1-[2...)Show SMILES Clc1c[nH]c2c(cccc12)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C22H26ClN7O2/c23-16-12-25-20-15(16)6-5-8-17(20)27-22(26-14-24)28-18-7-1-2-11-30(21(18)32)13-19(31)29-9-3-4-10-29/h5-6,8,12,18,25H,1-4,7,9-11,13H2,(H2,26,27,28)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175080
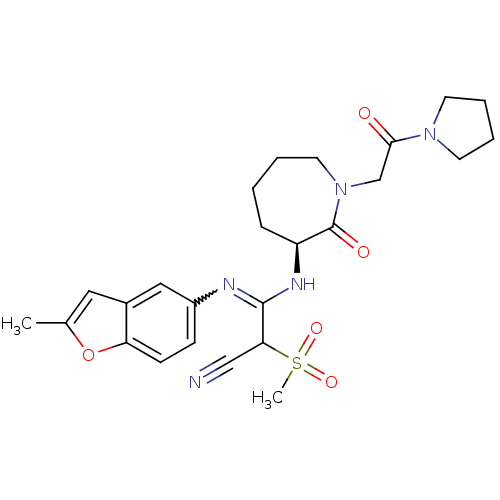
((S,Z)-3-(2-methylbenzofuran-5-ylamino)-2-(methylsu...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)S(C)(=O)=O |w:10.11| Show InChI InChI=1S/C25H31N5O5S/c1-17-13-18-14-19(8-9-21(18)35-17)27-24(22(15-26)36(2,33)34)28-20-7-3-4-12-30(25(20)32)16-23(31)29-10-5-6-11-29/h8-9,13-14,20,22H,3-7,10-12,16H2,1-2H3,(H,27,28)/t20-,22?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM24777

(5-hydroxy-1,4-dihydronaphthalene-1,4-dione | 5-hyd...)Show InChI InChI=1S/C10H6O3/c11-7-4-5-9(13)10-6(7)2-1-3-8(10)12/h1-5,12H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175083
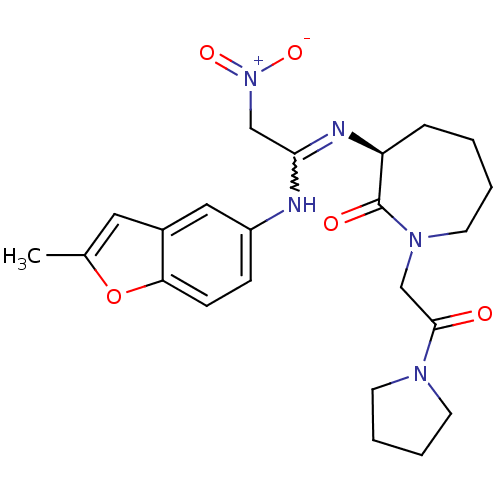
((S,Z)-3-(1-(2-methylbenzofuran-5-ylamino)-2-nitrov...)Show SMILES Cc1cc2cc(NC(C[N+]([O-])=O)=N[C@H]3CCCCN(CC(=O)N4CCCC4)C3=O)ccc2o1 |w:7.6| Show InChI InChI=1S/C23H29N5O5/c1-16-12-17-13-18(7-8-20(17)33-16)24-21(14-28(31)32)25-19-6-2-3-11-27(23(19)30)15-22(29)26-9-4-5-10-26/h7-8,12-13,19H,2-6,9-11,14-15H2,1H3,(H,24,25)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175099
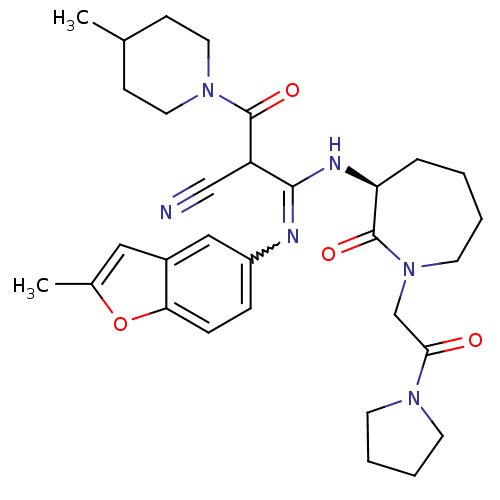
((S,Z)-3-(2-methylbenzofuran-5-ylamino)-2-(4-methyl...)Show SMILES CC1CCN(CC1)C(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1ccc2oc(C)cc2c1 |w:30.33| Show InChI InChI=1S/C31H40N6O4/c1-21-10-15-36(16-11-21)30(39)25(19-32)29(33-24-8-9-27-23(18-24)17-22(2)41-27)34-26-7-3-4-14-37(31(26)40)20-28(38)35-12-5-6-13-35/h8-9,17-18,21,25-26H,3-7,10-16,20H2,1-2H3,(H,33,34)/t25?,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175084
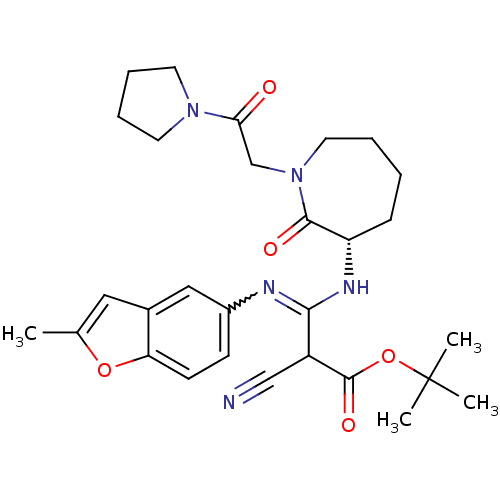
((S,Z)-tert-butyl 2-cyano-3-(2-methylbenzofuran-5-y...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OC(C)(C)C |w:10.11| Show InChI InChI=1S/C29H37N5O5/c1-19-15-20-16-21(10-11-24(20)38-19)31-26(22(17-30)28(37)39-29(2,3)4)32-23-9-5-6-14-34(27(23)36)18-25(35)33-12-7-8-13-33/h10-11,15-16,22-23H,5-9,12-14,18H2,1-4H3,(H,31,32)/t22?,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50060898
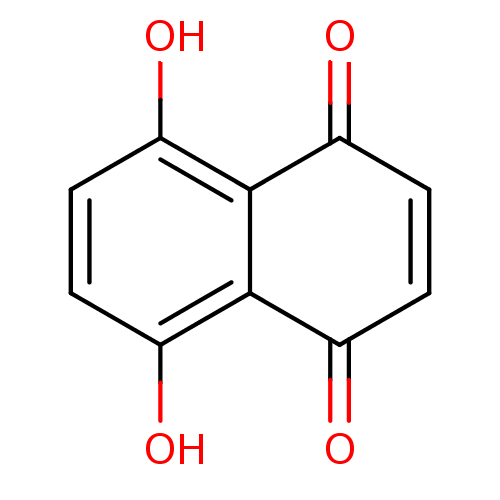
(5,8-Dihydroxy-[1,4]naphthoquinone | 5,8-dihydroxy-...)Show InChI InChI=1S/C10H6O4/c11-5-1-2-6(12)10-8(14)4-3-7(13)9(5)10/h1-4,11-12H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50594273
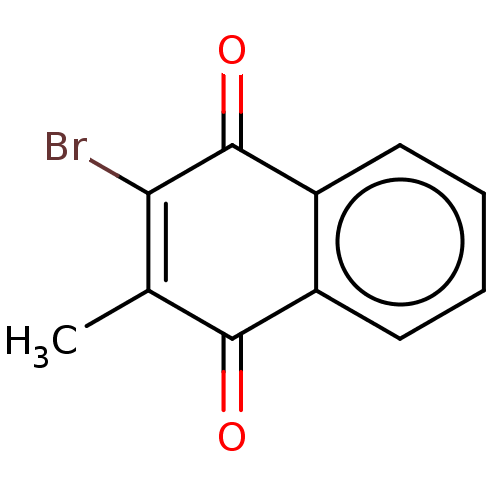
(CHEMBL181549) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26350
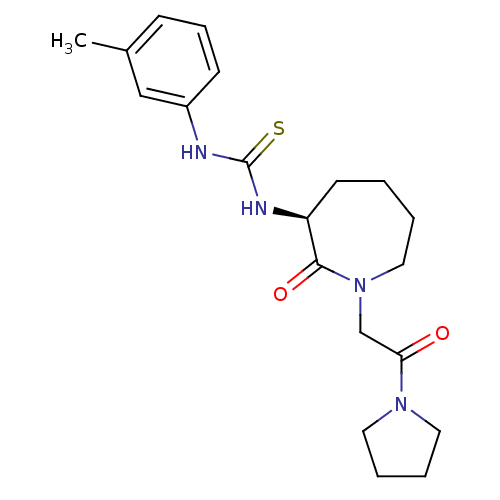
((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...)Show SMILES Cc1cccc(NC(=S)N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r| Show InChI InChI=1S/C20H28N4O2S/c1-15-7-6-8-16(13-15)21-20(27)22-17-9-2-3-12-24(19(17)26)14-18(25)23-10-4-5-11-23/h6-8,13,17H,2-5,9-12,14H2,1H3,(H2,21,22,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26350

((S)-1-(2-oxo-1-(2-oxo-2-(pyrrolidin-1-yl)ethyl)aze...)Show SMILES Cc1cccc(NC(=S)N[C@H]2CCCCN(CC(=O)N3CCCC3)C2=O)c1 |r| Show InChI InChI=1S/C20H28N4O2S/c1-15-7-6-8-16(13-15)21-20(27)22-17-9-2-3-12-24(19(17)26)14-18(25)23-10-4-5-11-23/h6-8,13,17H,2-5,9-12,14H2,1H3,(H2,21,22,27)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26352
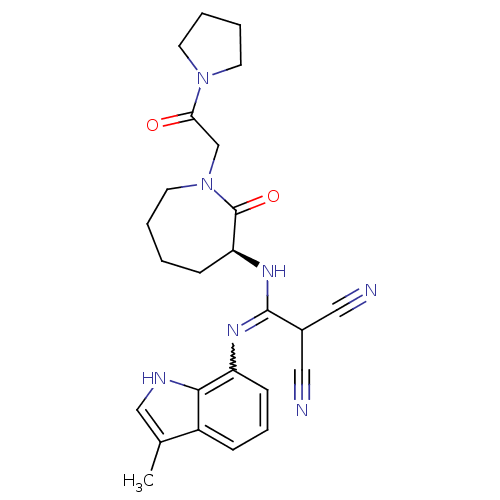
(2-{[(3-methyl-1H-indol-7-yl)amino]({[(3S)-2-oxo-1-...)Show SMILES Cc1c[nH]c2c(cccc12)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C#N |r,w:10.11| Show InChI InChI=1S/C25H29N7O2/c1-17-15-28-23-19(17)7-6-9-20(23)29-24(18(13-26)14-27)30-21-8-2-3-12-32(25(21)34)16-22(33)31-10-4-5-11-31/h6-7,9,15,18,21,28H,2-5,8,10-12,16H2,1H3,(H,29,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26358
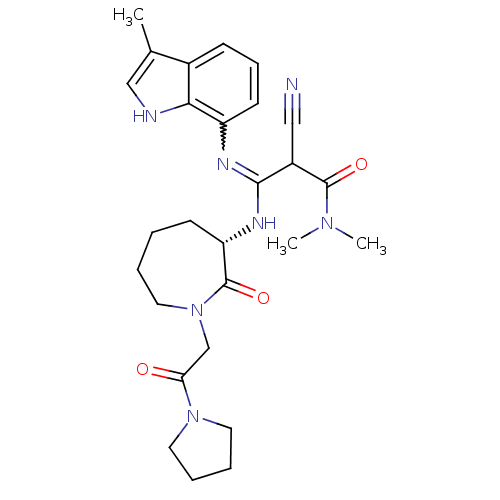
((2Z)-2-cyano-N,N-dimethyl-3-[(3-methyl-1H-indol-7-...)Show SMILES CN(C)C(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1cccc2c(C)c[nH]c12 |r,w:26.28| Show InChI InChI=1S/C27H35N7O3/c1-18-16-29-24-19(18)9-8-11-21(24)30-25(20(15-28)26(36)32(2)3)31-22-10-4-5-14-34(27(22)37)17-23(35)33-12-6-7-13-33/h8-9,11,16,20,22,29H,4-7,10,12-14,17H2,1-3H3,(H,30,31)/t20?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 118 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50594274
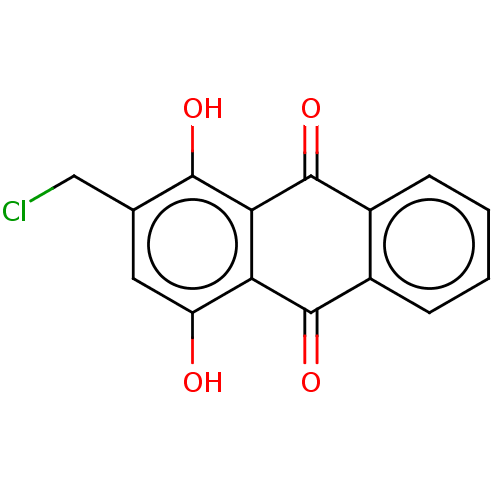
(CHEMBL5182750) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50594272
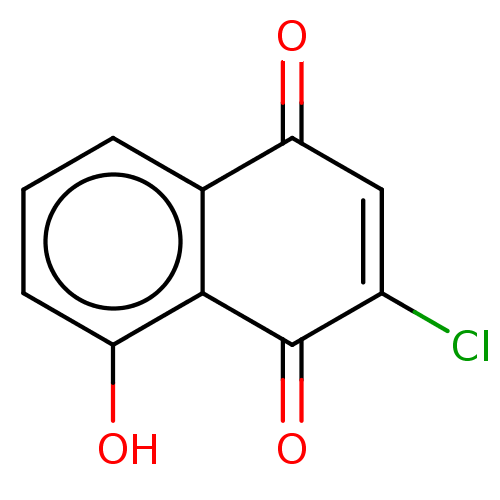
(CHEMBL5179592) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26361
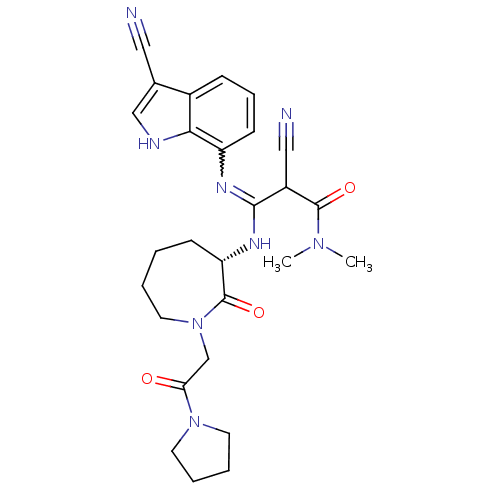
((2Z)-2-cyano-3-[(3-cyano-1H-indol-7-yl)amino]-N,N-...)Show SMILES CN(C)C(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1cccc2c(c[nH]c12)C#N |r,w:26.28| Show InChI InChI=1S/C27H32N8O3/c1-33(2)26(37)20(15-29)25(31-21-10-7-8-19-18(14-28)16-30-24(19)21)32-22-9-3-4-13-35(27(22)38)17-23(36)34-11-5-6-12-34/h7-8,10,16,20,22,30H,3-6,9,11-13,17H2,1-2H3,(H,31,32)/t20?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26363
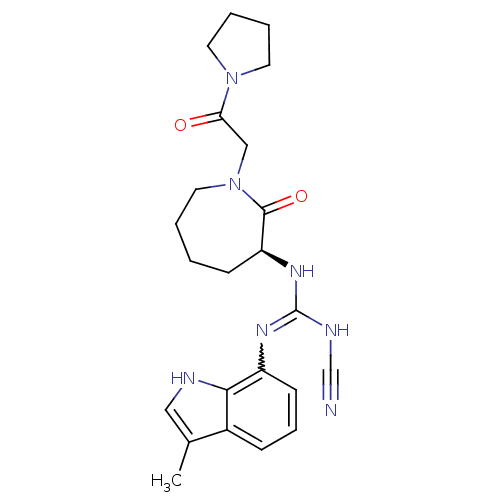
((3S)-2-cyano-1-(3-methyl-1H-indol-7-yl)-2-oxo-1-[2...)Show SMILES Cc1c[nH]c2c(cccc12)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H29N7O2/c1-16-13-25-21-17(16)7-6-9-18(21)27-23(26-15-24)28-19-8-2-3-12-30(22(19)32)14-20(31)29-10-4-5-11-29/h6-7,9,13,19,25H,2-5,8,10-12,14H2,1H3,(H2,26,27,28)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 336 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50060898

(5,8-Dihydroxy-[1,4]naphthoquinone | 5,8-dihydroxy-...)Show InChI InChI=1S/C10H6O4/c11-5-1-2-6(12)10-8(14)4-3-7(13)9(5)10/h1-4,11-12H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50594274

(CHEMBL5182750) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26353
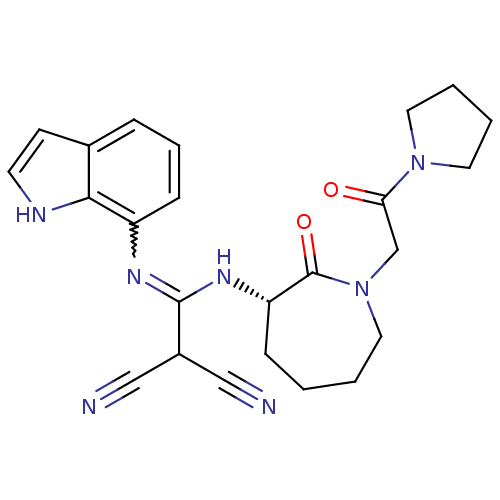
(2-[(1H-indol-7-ylamino)({[(3S)-2-oxo-1-[2-oxo-2-(p...)Show SMILES O=C(CN1CCCC[C@H](NC(=Nc2cccc3cc[nH]c23)C(C#N)C#N)C1=O)N1CCCC1 |r,w:11.11| Show InChI InChI=1S/C24H27N7O2/c25-14-18(15-26)23(28-19-8-5-6-17-9-10-27-22(17)19)29-20-7-1-2-13-31(24(20)33)16-21(32)30-11-3-4-12-30/h5-6,8-10,18,20,27H,1-4,7,11-13,16H2,(H,28,29)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175077

((S,Z)-3-(1-(benzofuran-5-ylamino)-2-nitrovinylamin...)Show SMILES [O-][N+](=O)CC(Nc1ccc2occc2c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |w:4.4| Show InChI InChI=1S/C22H27N5O5/c28-21(25-9-3-4-10-25)15-26-11-2-1-5-18(22(26)29)24-20(14-27(30)31)23-17-6-7-19-16(13-17)8-12-32-19/h6-8,12-13,18H,1-5,9-11,14-15H2,(H,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 539 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM24777

(5-hydroxy-1,4-dihydronaphthalene-1,4-dione | 5-hyd...)Show InChI InChI=1S/C10H6O3/c11-7-4-5-9(13)10-6(7)2-1-3-8(10)12/h1-5,12H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175088

((S,Z)-2-(isopropylsulfonyl)-3-(2-methylbenzofuran-...)Show SMILES CC(C)S(=O)(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1ccc2oc(C)cc2c1 |w:27.29| Show InChI InChI=1S/C27H35N5O5S/c1-18(2)38(35,36)24(16-28)26(29-21-9-10-23-20(15-21)14-19(3)37-23)30-22-8-4-5-13-32(27(22)34)17-25(33)31-11-6-7-12-31/h9-10,14-15,18,22,24H,4-8,11-13,17H2,1-3H3,(H,29,30)/t22-,24?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 647 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM26355
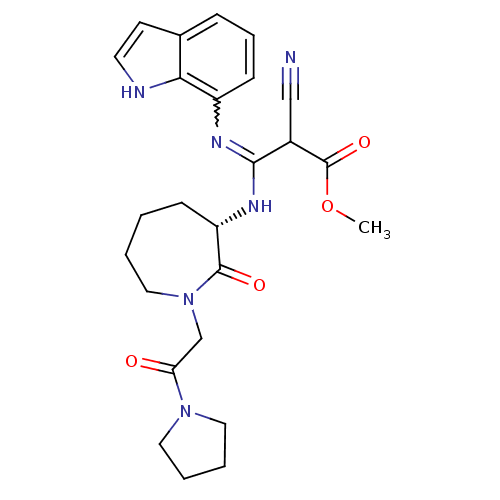
(indole-7-yl compound, 16 | methyl (2Z)-2-cyano-3-(...)Show SMILES COC(=O)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1cccc2cc[nH]c12 |r,w:25.27| Show InChI InChI=1S/C25H30N6O4/c1-35-25(34)18(15-26)23(28-19-9-6-7-17-10-11-27-22(17)19)29-20-8-2-3-14-31(24(20)33)16-21(32)30-12-4-5-13-30/h6-7,9-11,18,20,27H,2-5,8,12-14,16H2,1H3,(H,28,29)/t18?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Time-dependent optical density change was followed at 405 nm using a kinetic microplate reader at room temperature. Enzyme activity in the presence o... |
J Med Chem 51: 7541-51 (2008)
Article DOI: 10.1021/jm800855x
BindingDB Entry DOI: 10.7270/Q2V12332 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
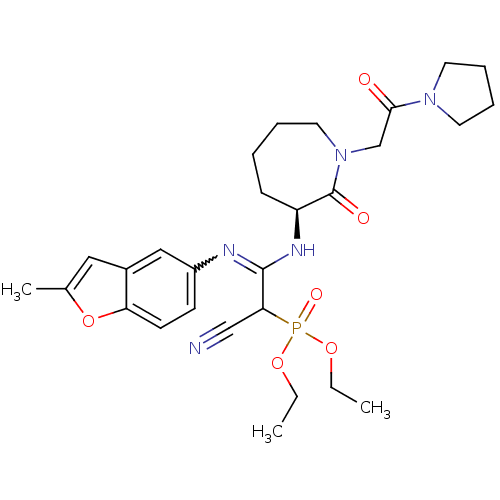
(Homo sapiens (Human)) | BDBM50175082
((S,Z)-diethyl 1-cyano-2-(2-methylbenzofuran-5-ylam...)Show SMILES CCOP(=O)(OCC)C(C#N)C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)=Nc1ccc2oc(C)cc2c1 |w:29.31| Show InChI InChI=1S/C28H38N5O6P/c1-4-37-40(36,38-5-2)25(18-29)27(30-22-11-12-24-21(17-22)16-20(3)39-24)31-23-10-6-7-15-33(28(23)35)19-26(34)32-13-8-9-14-32/h11-12,16-17,23,25H,4-10,13-15,19H2,1-3H3,(H,30,31)/t23-,25?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 658 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM50594273

(CHEMBL181549) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.1c01222
BindingDB Entry DOI: 10.7270/Q2T72NFX |
More data for this
Ligand-Target Pair | |
Coagulation factor X
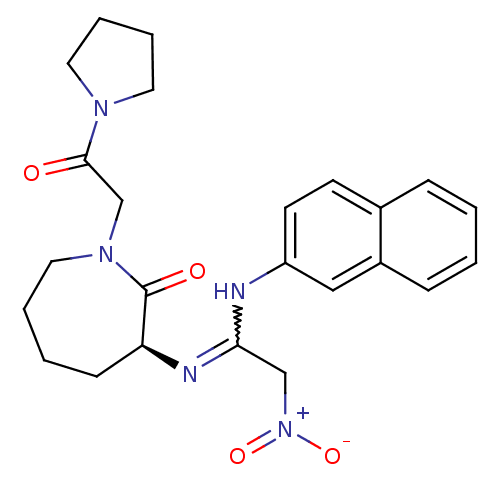
(Homo sapiens (Human)) | BDBM50175086
((S,Z)-3-(1-(naphthalen-2-ylamino)-2-nitrovinylamin...)Show SMILES [O-][N+](=O)CC(Nc1ccc2ccccc2c1)=N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |w:4.4| Show InChI InChI=1S/C24H29N5O4/c30-23(27-12-5-6-13-27)17-28-14-4-3-9-21(24(28)31)26-22(16-29(32)33)25-20-11-10-18-7-1-2-8-19(18)15-20/h1-2,7-8,10-11,15,21H,3-6,9,12-14,16-17H2,(H,25,26)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data