

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317628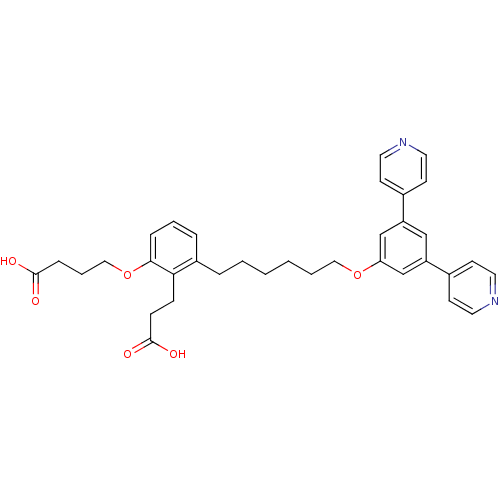 (4-{2-(2-Carboxy-ethyl)-3-[6-(3,5-di-pyridin-4-yl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50213060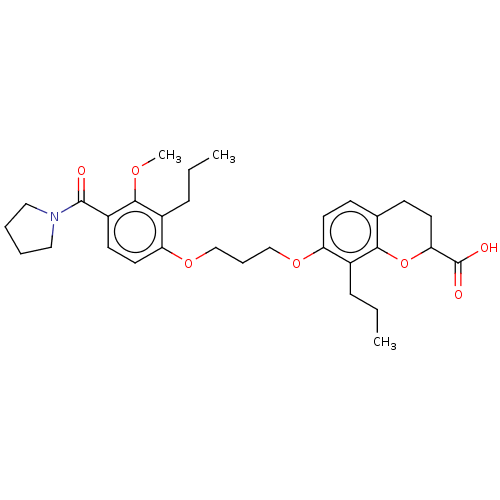 (CHEMBL355401 | SC-50135) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against leukotriene B4 receptor | Bioorg Med Chem Lett 4: 811-816 (1994) Article DOI: 10.1016/S0960-894X(01)80853-2 BindingDB Entry DOI: 10.7270/Q2DN451X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50284125 (7-[3-(4-Isopropylcarbamoyl-3-methoxy-2-propyl-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against leukotriene B4 receptor | Bioorg Med Chem Lett 4: 811-816 (1994) Article DOI: 10.1016/S0960-894X(01)80853-2 BindingDB Entry DOI: 10.7270/Q2DN451X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50284122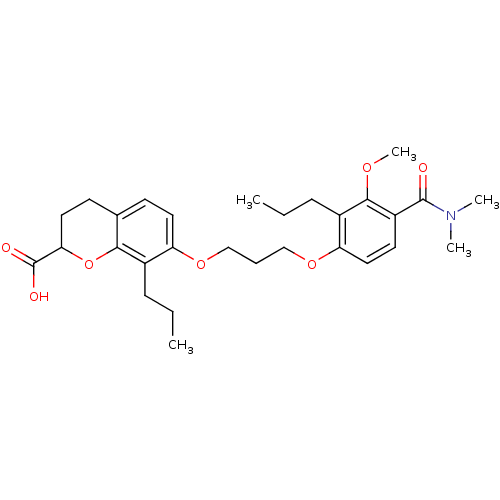 (7-[3-(4-Dimethylcarbamoyl-3-methoxy-2-propyl-pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against leukotriene B4 receptor | Bioorg Med Chem Lett 4: 811-816 (1994) Article DOI: 10.1016/S0960-894X(01)80853-2 BindingDB Entry DOI: 10.7270/Q2DN451X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317631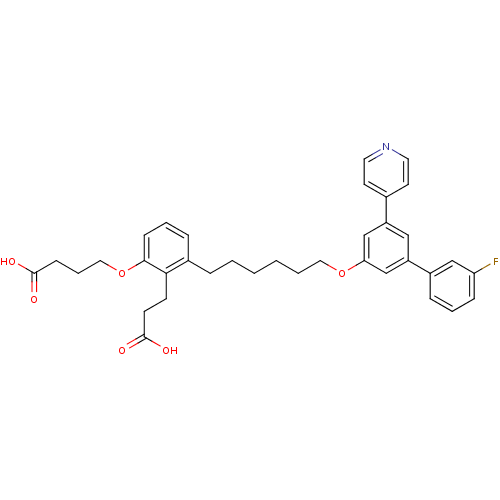 (4-{2-(2-Carboxy-ethyl)-3-[6-(3'-fluoro-5-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317632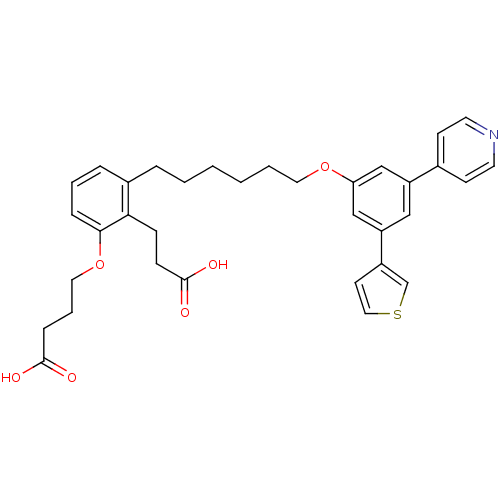 (4-{2-(2-Carboxy-ethyl)-3-[6-(3-pyridin-4-yl-5-thio...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317625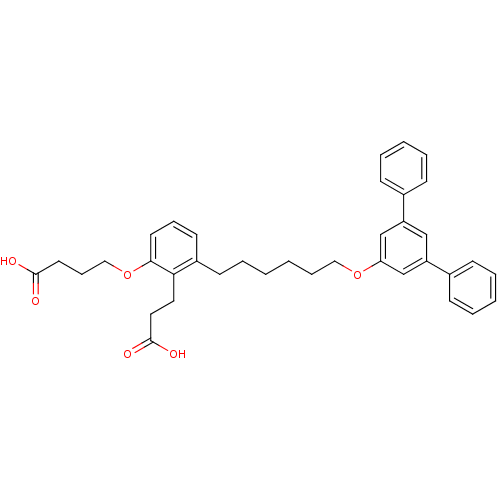 (4-{2-(2-Carboxyethyl)-3-[6-([1,1',3,1'']terphenyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317633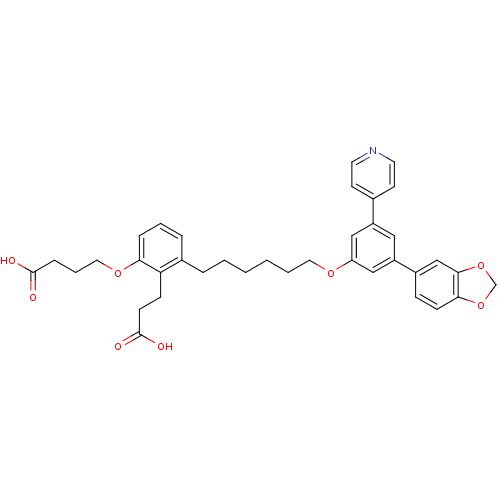 (4-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-pyridin-4-yl-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317634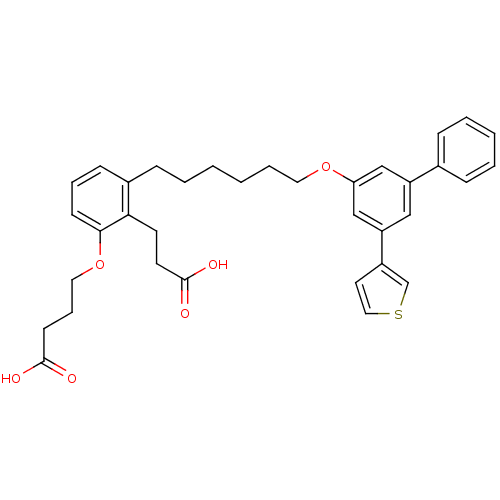 (4-{2-(2-Carboxyethyl)-3-[6-(5-thiophen-3-ylbipheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609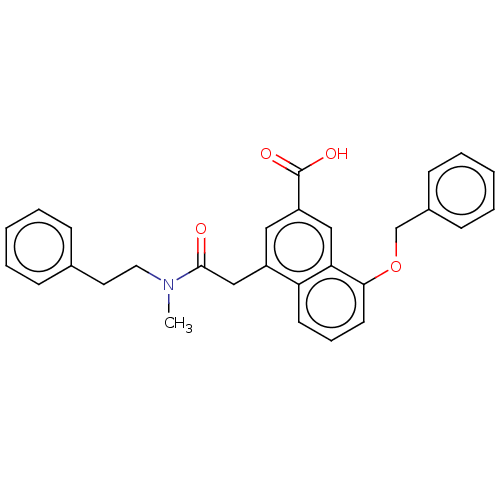 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Affinity of the compound for human PMN LTB-4 receptors. | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317635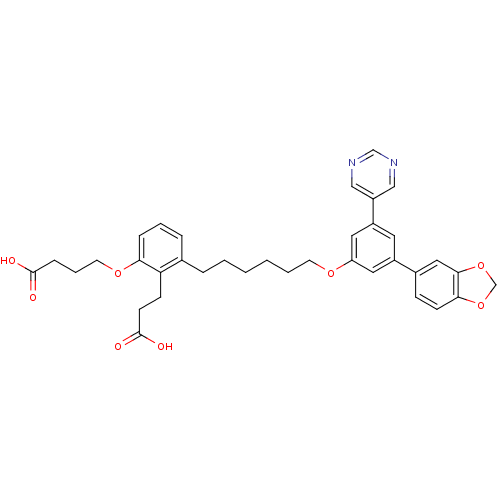 (4-{3-[6-(3-Benzo[1,3]dioxol-5-yl-5-pyrimidin-5-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317636 (4-{2-(2-Carboxy-ethyl)-3-[6-(3-pyrimidin-5-yl-5-th...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317626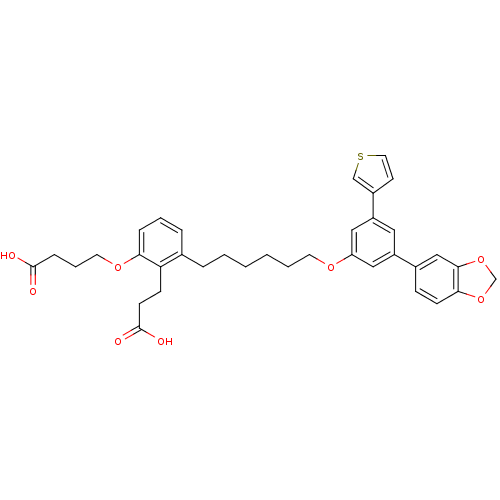 (4-{3-[6-(3-5-Benzo[1,3]dioxolyl-5-thiophen-3-ylphe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317624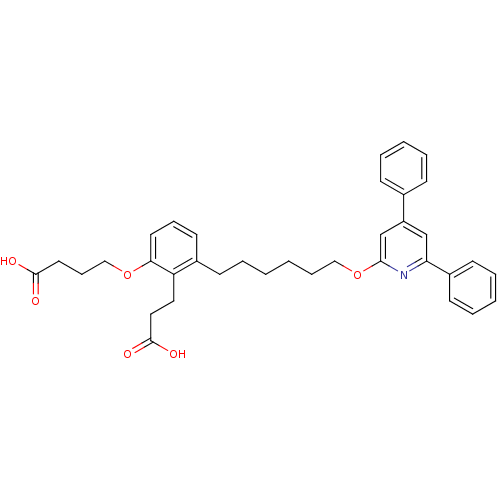 (4-(2-(2-carboxyethyl)-3-(6-(4,6-diphenylpyridin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317637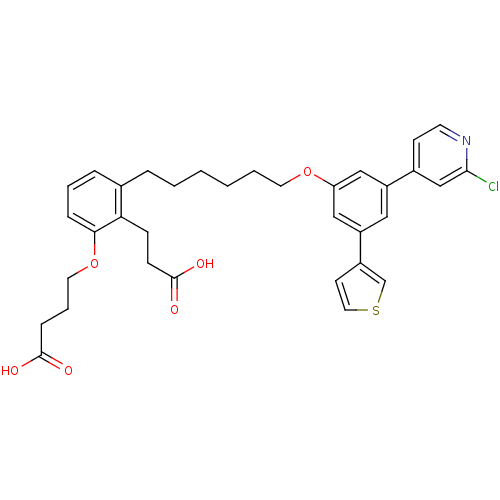 (4-(2-(2-Carboxy-ethyl)-3-{6-[3-(2-chloro-pyridin-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317638 (4-{2-(2-Carboxy-ethyl)-3-[6-(3,5-di-thiophen-3-yl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317639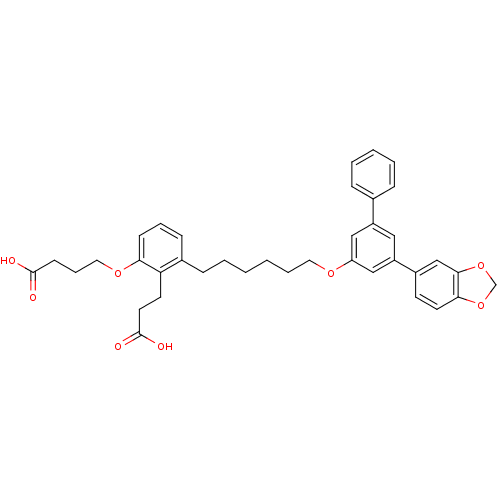 (4-[3-[6-(5-Benzo[1,3]dioxol-5-yl-biphenyl-3-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317630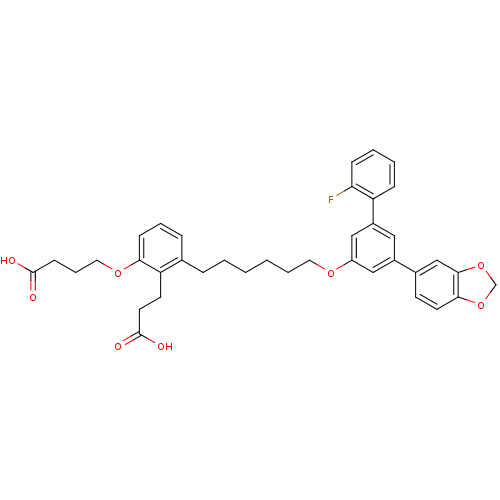 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-2'-fluorobip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001609 (8-Benzyloxy-4-[(methyl-phenethyl-carbamoyl)-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhone-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Concentration of the compound inhibiting 1 nM LTB4-induced aggregation in GP polymorphonuclear (PMN) leukocytes. | J Med Chem 35: 4253-5 (1992) BindingDB Entry DOI: 10.7270/Q208648W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50284123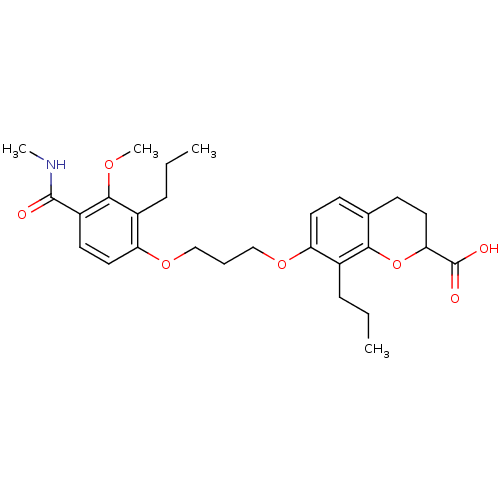 (7-[3-(3-Methoxy-4-methylcarbamoyl-2-propyl-phenoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against leukotriene B4 receptor | Bioorg Med Chem Lett 4: 811-816 (1994) Article DOI: 10.1016/S0960-894X(01)80853-2 BindingDB Entry DOI: 10.7270/Q2DN451X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001610 (7-[3-(4-Acetyl-3-methoxy-2-propyl-phenoxy)-propoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against leukotriene B4 receptor | Bioorg Med Chem Lett 4: 811-816 (1994) Article DOI: 10.1016/S0960-894X(01)80853-2 BindingDB Entry DOI: 10.7270/Q2DN451X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001668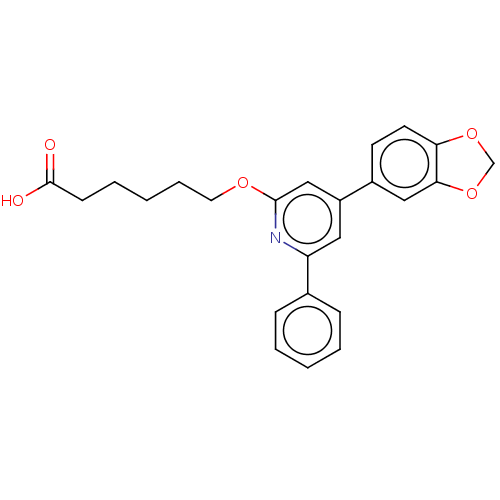 (6-(4-Benzo[1,3]dioxol-5-yl-6-phenyl-pyridin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317623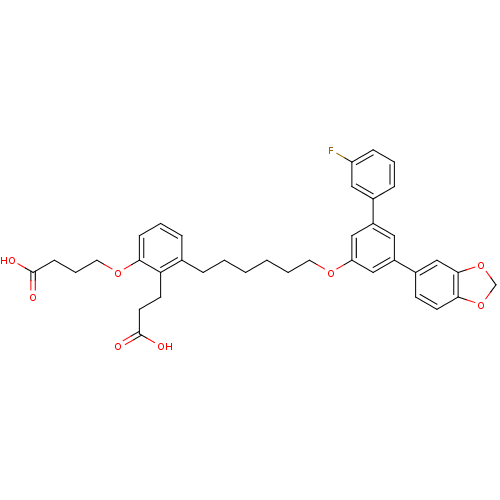 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-3'-fluorobip...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50029464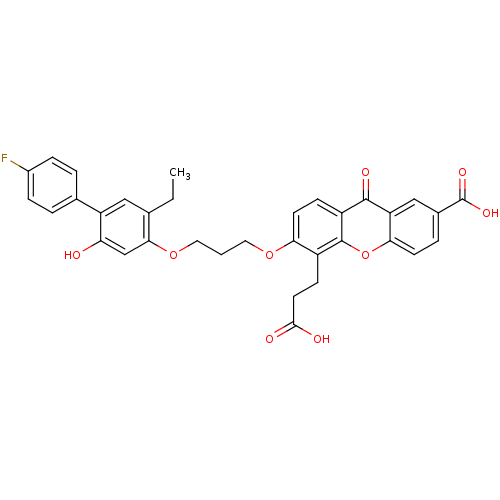 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of LTB4-induced up-regulation of human neutrophil CD11b/CD18 integrin | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037390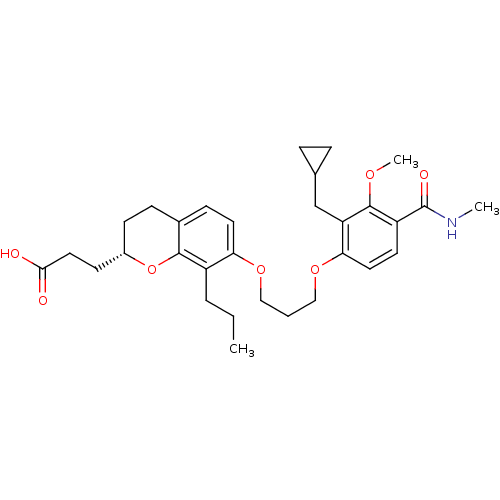 (3-{(S)-2-(2-Carboxy-ethyl)-7-[3-(2-cyclopropylmeth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052017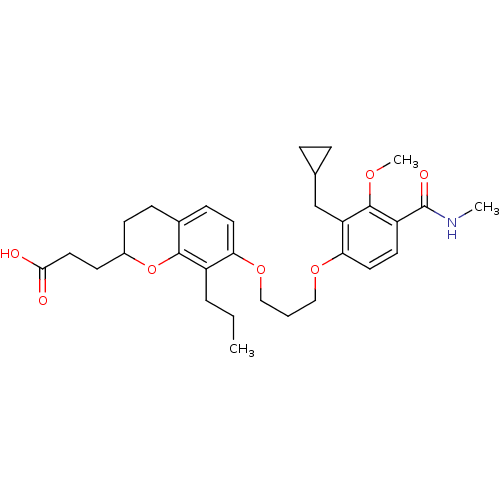 (3-{7-[3-(2-Cyclopropylmethyl-3-methoxy-4-methylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50029460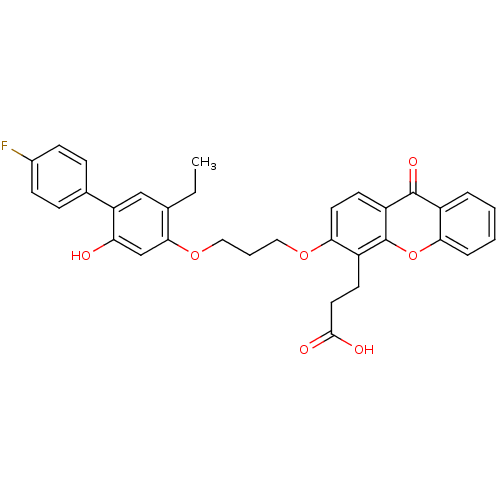 (3-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibition of specific binding of [3H]-LTB4 to guinea pig lung membranes expressing LTB4 receptor | J Med Chem 36: 3982-4 (1994) BindingDB Entry DOI: 10.7270/Q2H1313H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50013889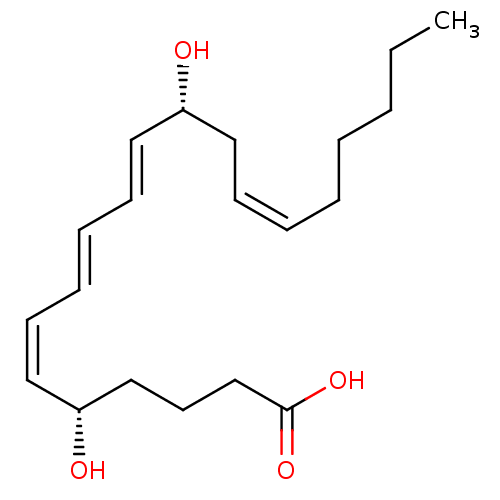 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of specific binding of [3H]LTB4 to LTB4 receptor in human neutrophils | Citation and Details BindingDB Entry DOI: 10.7270/Q23N25HN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro antagonistic activity towards LTB4 receptor was evaluated by inhibition of binding of [3H]LTB4 to human neutrophils | Bioorg Med Chem Lett 3: 1981-1984 (1993) Article DOI: 10.1016/S0960-894X(01)80999-9 BindingDB Entry DOI: 10.7270/Q2VH5P9R | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50013889 ((5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxyicosa-6,8,10,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-LTB4 to Leukotriene B4 receptor in human neutrophils | J Med Chem 33: 2807-13 (1990) BindingDB Entry DOI: 10.7270/Q2F18XQM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611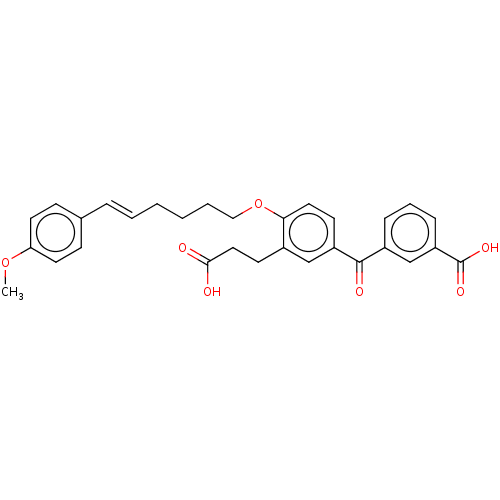 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-LTB4 to receptor on nonradioactive LTB4 | J Med Chem 36: 1726-34 (1993) BindingDB Entry DOI: 10.7270/Q2JQ1023 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001637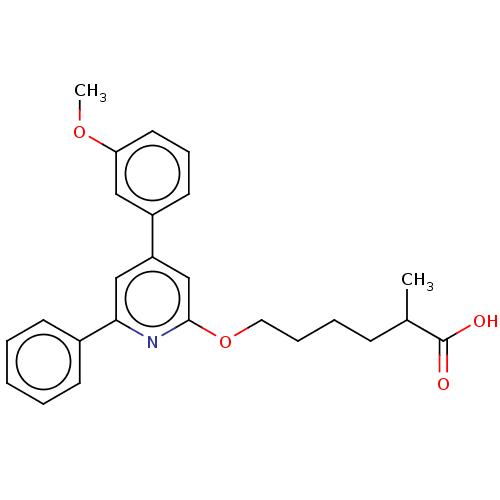 (6-[4-(3-Methoxy-phenyl)-6-phenyl-pyridin-2-yloxy]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50215854 (CHEMBL301829) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. | Bioorg Med Chem Lett 8: 1781-6 (1998) BindingDB Entry DOI: 10.7270/Q2RR21F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes | J Med Chem 35: 4306-14 (1992) BindingDB Entry DOI: 10.7270/Q2VH5MS6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50215854 (CHEMBL301829) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. | Bioorg Med Chem Lett 8: 1781-6 (1998) BindingDB Entry DOI: 10.7270/Q2RR21F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001657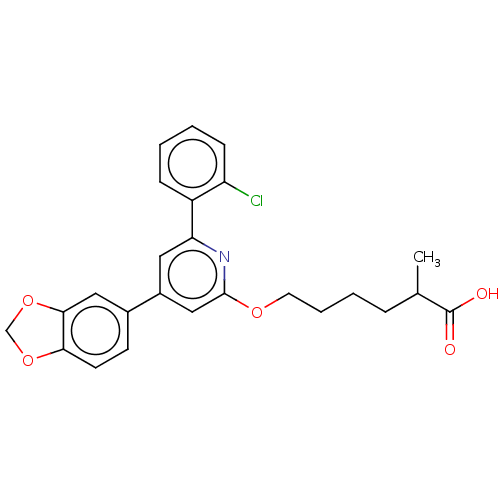 (6-[4-Benzo[1,3]dioxol-5-yl-6-(2-chloro-phenyl)-pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of LTB4-induced elastase release in human polymorphonuclear leukocytes | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001667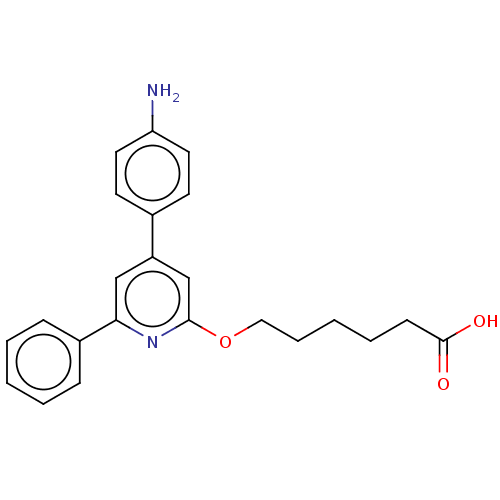 (6-[4-(4-Amino-phenyl)-6-phenyl-pyridin-2-yloxy]-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the human polymorphonuclear leukocytes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50215854 (CHEMBL301829) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Ability to inhibit LTB4-induced chemotaxis of isolated human neutrophils. | Bioorg Med Chem Lett 8: 1781-6 (1998) BindingDB Entry DOI: 10.7270/Q2RR21F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50033746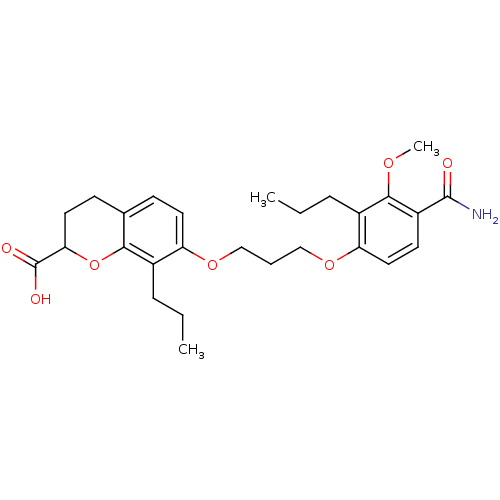 (7-[3-(4-Carbamoyl-3-methoxy-2-propyl-phenoxy)-prop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against leukotriene B4 receptor | Bioorg Med Chem Lett 4: 811-816 (1994) Article DOI: 10.1016/S0960-894X(01)80853-2 BindingDB Entry DOI: 10.7270/Q2DN451X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50231624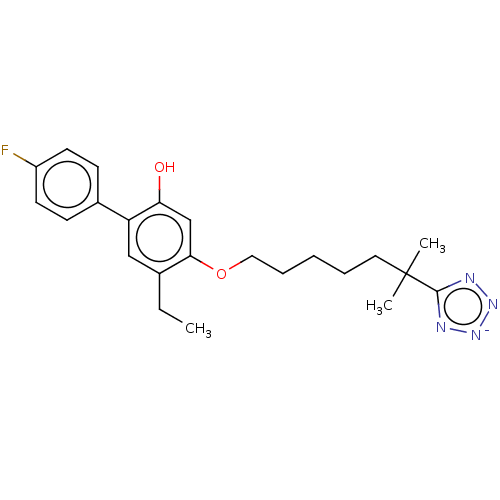 (CHEMBL129703) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]LTB4 receptor binding in human neutrophils | J Med Chem 36: 3978-81 (1993) Article DOI: 10.1021/jm00076a029 BindingDB Entry DOI: 10.7270/Q20V8G1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50280292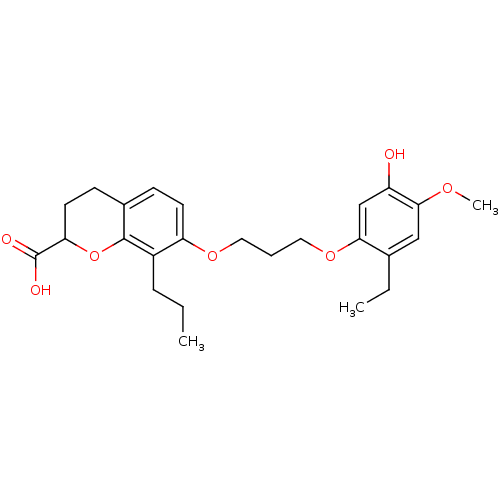 (7-[3-(2-Ethyl-5-hydroxy-4-methoxy-phenoxy)-propoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro binding affinity using [3H]-LTB4 radioligand binding to leukotriene B4 receptor in human neutrophil binding assay | Bioorg Med Chem Lett 2: 1675-1680 (1992) Article DOI: 10.1016/S0960-894X(00)80454-0 BindingDB Entry DOI: 10.7270/Q2JW8DT9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50231606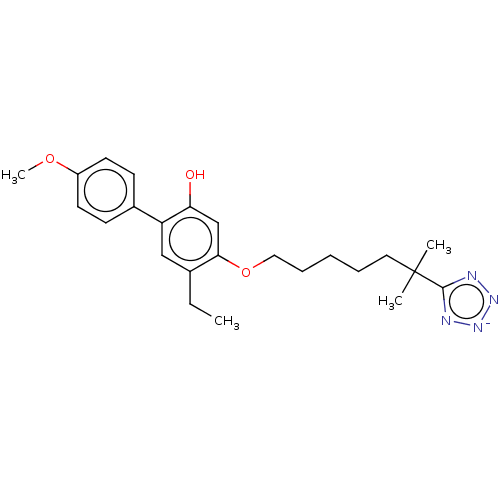 (CHEMBL128755) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]LTB4 receptor binding in human neutrophils | J Med Chem 36: 3978-81 (1993) Article DOI: 10.1021/jm00076a029 BindingDB Entry DOI: 10.7270/Q20V8G1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50231625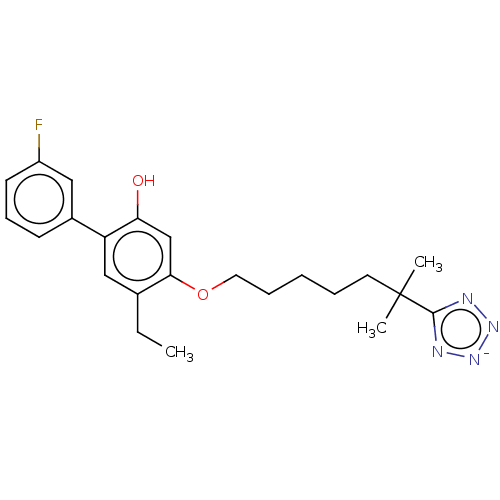 (CHEMBL128067) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]LTB4 receptor binding in human neutrophils | J Med Chem 36: 3978-81 (1993) Article DOI: 10.1021/jm00076a029 BindingDB Entry DOI: 10.7270/Q20V8G1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001658 (6-(4,6-Diphenyl-pyridin-2-yloxy)-hexanoic acid | C...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherche de Vitry-Alfortville Curated by ChEMBL | Assay Description Inhibition of [3H]-LTB4 binding to Leukotriene B4 receptor in the guinea pig spleen membranes. | J Med Chem 35: 4315-24 (1992) BindingDB Entry DOI: 10.7270/Q2QR4W2W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50215738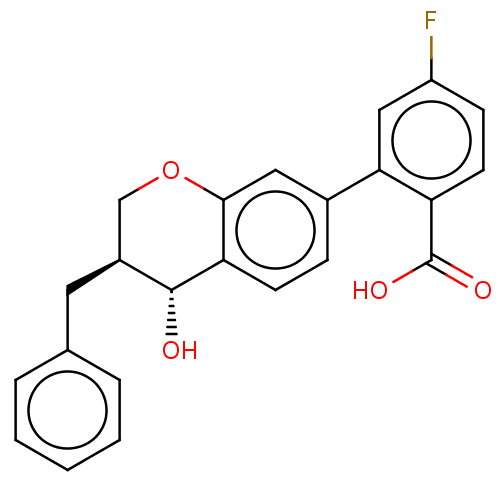 (CHEMBL52675) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Ability to inhibit LTB4 binding to LTB receptors on isolated human neutrophils. | Bioorg Med Chem Lett 8: 1781-6 (1998) BindingDB Entry DOI: 10.7270/Q2RR21F1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50231626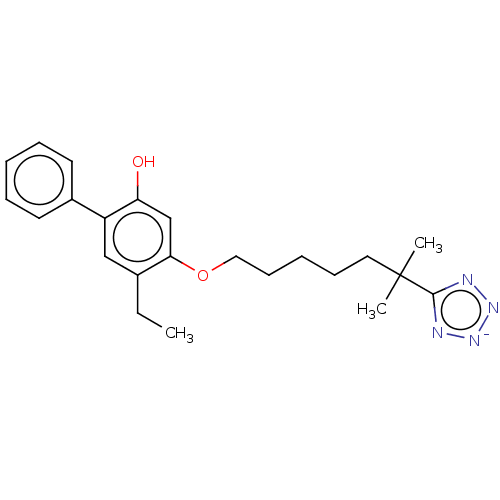 (CHEMBL128013) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]LTB4 receptor binding in human neutrophils | J Med Chem 36: 3978-81 (1993) Article DOI: 10.1021/jm00076a029 BindingDB Entry DOI: 10.7270/Q20V8G1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1/2 (Homo sapiens (Human)) | BDBM50037218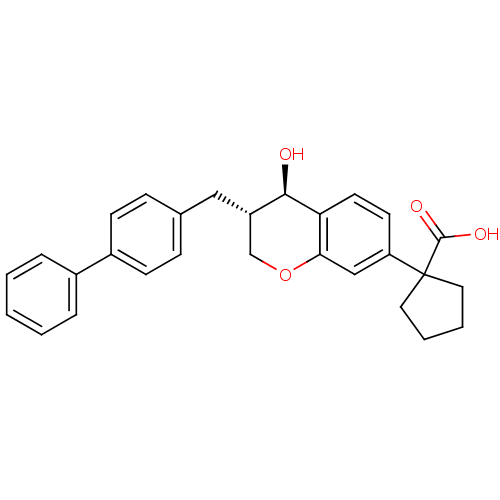 (1-((3S,4R)-3-Biphenyl-4-ylmethyl-4-hydroxy-chroman...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Inhibition of leukotriene B4 (LTB4) binding to guinea pig spleen | Citation and Details BindingDB Entry DOI: 10.7270/Q2ZG6VFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50033743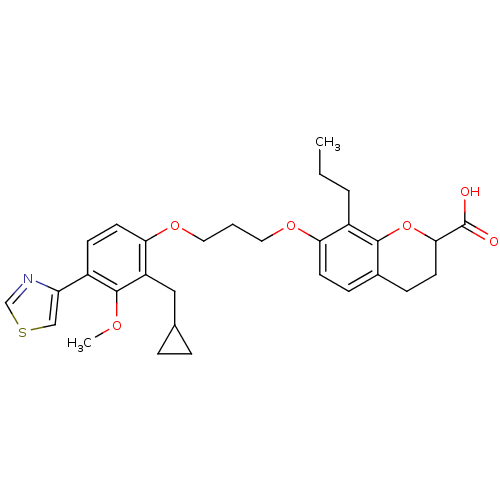 (7-[3-(2-Cyclopropylmethyl-3-methoxy-4-thiazol-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Research and Development Curated by ChEMBL | Assay Description Binding affinity of the compound towards Leukotriene B4 (LTB4) Receptor. Experiment conducted in the absence of NDGA. | J Med Chem 38: 858-68 (1995) BindingDB Entry DOI: 10.7270/Q22806N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50317641 (4-(3-(6-(5-(benzo[d][1,3]dioxol-5-yl)-4'-methoxybi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center Curated by ChEMBL | Assay Description Antagonist activity at BLT1 receptor expressed in human HL60 cells assessed as inhibition of LTB4-stimulated calcium flux after 30 mins | J Med Chem 53: 3502-16 (2010) Article DOI: 10.1021/jm1001919 BindingDB Entry DOI: 10.7270/Q2KW5G67 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 622 total ) | Next | Last >> |