 Found 247 hits with Last Name = 'zilberstein' and Initial = 'a'
Found 247 hits with Last Name = 'zilberstein' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279769
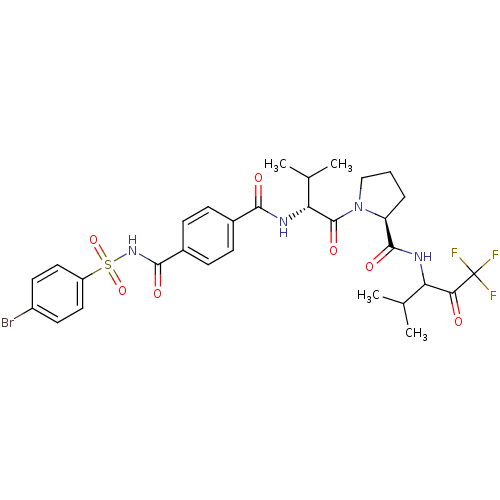
((S)-1-{(R)-2-[4-(4-Bromo-benzenesulfonylaminocarbo...)Show SMILES CC(C)[C@@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccc(Br)cc1)C(=O)N1CCC[C@H]1C(=O)NC(C(C)C)C(=O)C(F)(F)F Show InChI InChI=1S/C30H34BrF3N4O7S/c1-16(2)23(25(39)30(32,33)34)35-28(42)22-6-5-15-38(22)29(43)24(17(3)4)36-26(40)18-7-9-19(10-8-18)27(41)37-46(44,45)21-13-11-20(31)12-14-21/h7-14,16-17,22-24H,5-6,15H2,1-4H3,(H,35,42)(H,36,40)(H,37,41)/t22-,23?,24+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279767
(CHEMBL268343 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCCCCCc3ccccc3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCCCCCc5ccccc5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCCCCCc7ccccc7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C87H98O18/c1-59-40-76(95-3)64(47-75(59)100-37-23-11-20-32-60-26-14-8-15-27-60)42-71-50-79(98-6)67(54-83(71)104-57-86(90)91)44-70-49-78(97-5)66(53-82(70)102-39-25-13-22-34-62-30-18-10-19-31-62)45-72-51-80(99-7)68(55-84(72)105-58-87(92)93)43-69-48-77(96-4)65(41-63-46-73(94-2)35-36-74(63)103-56-85(88)89)52-81(69)101-38-24-12-21-33-61-28-16-9-17-29-61/h8-10,14-19,26-31,35-36,40,46-55H,11-13,20-25,32-34,37-39,41-45,56-58H2,1-7H3,(H,88,89)(H,90,91)(H,92,93) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279765
(CHEMBL215405 | {2-{4-[5-Carboxymethoxy-4-(5-cycloh...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCC3CCCCC3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCC5CCCCC5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCC7CCCCC7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C75H92O18/c1-47-25-64(83-3)52(32-63(47)88-41-48-17-11-8-12-18-48)27-59-35-67(86-6)55(39-71(59)92-45-74(78)79)29-58-34-66(85-5)54(38-70(58)90-43-50-21-15-10-16-22-50)30-60-36-68(87-7)56(40-72(60)93-46-75(80)81)28-57-33-65(84-4)53(37-69(57)89-42-49-19-13-9-14-20-49)26-51-31-61(82-2)23-24-62(51)91-44-73(76)77/h23-25,31-40,48-50H,8-22,26-30,41-46H2,1-7H3,(H,76,77)(H,78,79)(H,80,81) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50060330
(CHEMBL265335 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCCCc3ccccc3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCCCc5ccccc5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCCCc7ccccc7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C81H86O18/c1-53-34-70(89-3)58(41-69(53)94-31-17-26-54-20-11-8-12-21-54)36-65-44-73(92-6)61(48-77(65)98-51-80(84)85)38-64-43-72(91-5)60(47-76(64)96-33-19-28-56-24-15-10-16-25-56)39-66-45-74(93-7)62(49-78(66)99-52-81(86)87)37-63-42-71(90-4)59(46-75(63)95-32-18-27-55-22-13-9-14-23-55)35-57-40-67(88-2)29-30-68(57)97-50-79(82)83/h8-16,20-25,29-30,34,40-49H,17-19,26-28,31-33,35-39,50-52H2,1-7H3,(H,82,83)(H,84,85)(H,86,87) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279770
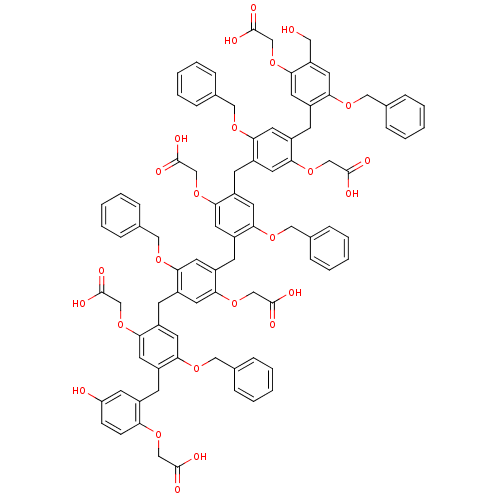
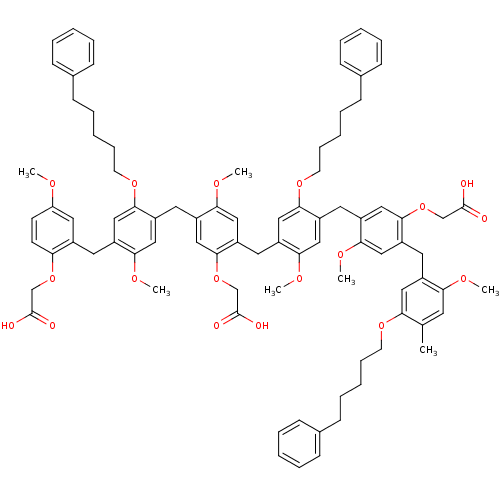
(2-{4-benzyloxy-5-[5-benzyloxy-4-(5-benzyloxy-4-{5-...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(OCc5ccccc5)c(Cc5cc(OCc6ccccc6)c(Cc6cc(O)ccc6OCC(O)=O)cc5OCC(O)=O)cc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C89H80O25/c90-44-71-43-82(108-49-60-24-14-5-15-25-60)70(42-83(71)114-55-89(102)103)32-69-37-77(107-48-59-22-12-4-13-23-59)65(41-81(69)113-54-88(100)101)31-68-36-76(106-47-58-20-10-3-11-21-58)64(40-80(68)112-53-87(98)99)30-67-35-75(105-46-57-18-8-2-9-19-57)63(39-79(67)111-52-86(96)97)29-66-34-74(104-45-56-16-6-1-7-17-56)62(38-78(66)110-51-85(94)95)28-61-33-72(91)26-27-73(61)109-50-84(92)93/h1-27,33-43,90-91H,28-32,44-55H2,(H,92,93)(H,94,95)(H,96,97)(H,98,99)(H,100,101)(H,102,103) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279771
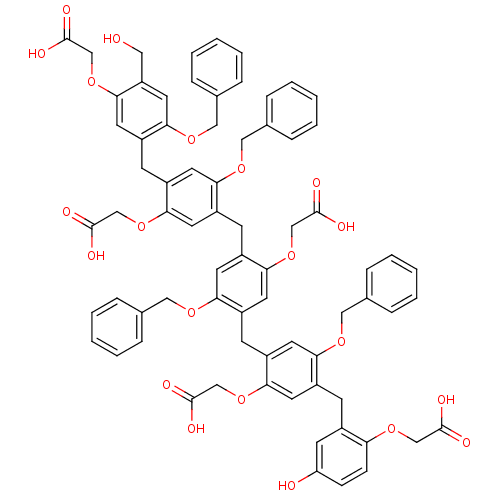
(CHEMBL217744 | [4-Benzyloxy-5-{5-benzyloxy-4-[5-be...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(OCc5ccccc5)c(Cc5cc(O)ccc5OCC(O)=O)cc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C73H66O21/c74-36-58-35-67(89-40-49-19-11-4-12-20-49)57(34-68(58)94-45-73(84)85)26-56-30-63(88-39-48-17-9-3-10-18-48)53(33-66(56)93-44-72(82)83)25-55-29-62(87-38-47-15-7-2-8-16-47)52(32-65(55)92-43-71(80)81)24-54-28-61(86-37-46-13-5-1-6-14-46)51(31-64(54)91-42-70(78)79)23-50-27-59(75)21-22-60(50)90-41-69(76)77/h1-22,27-35,74-75H,23-26,36-45H2,(H,76,77)(H,78,79)(H,80,81)(H,82,83)(H,84,85) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279770

(2-{4-benzyloxy-5-[5-benzyloxy-4-(5-benzyloxy-4-{5-...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(OCc5ccccc5)c(Cc5cc(OCc6ccccc6)c(Cc6cc(O)ccc6OCC(O)=O)cc5OCC(O)=O)cc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C89H80O25/c90-44-71-43-82(108-49-60-24-14-5-15-25-60)70(42-83(71)114-55-89(102)103)32-69-37-77(107-48-59-22-12-4-13-23-59)65(41-81(69)113-54-88(100)101)31-68-36-76(106-47-58-20-10-3-11-21-58)64(40-80(68)112-53-87(98)99)30-67-35-75(105-46-57-18-8-2-9-19-57)63(39-79(67)111-52-86(96)97)29-66-34-74(104-45-56-16-6-1-7-17-56)62(38-78(66)110-51-85(94)95)28-61-33-72(91)26-27-73(61)109-50-84(92)93/h1-27,33-43,90-91H,28-32,44-55H2,(H,92,93)(H,94,95)(H,96,97)(H,98,99)(H,100,101)(H,102,103) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Binding Affinity of the compound to inhibit HLE |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279766
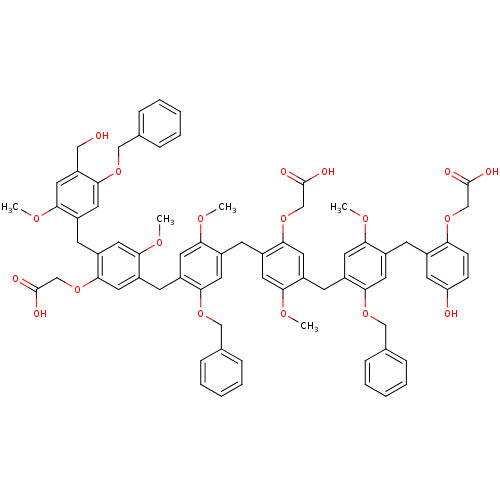
(CHEMBL213783 | {2-{5-Benzyloxy-4-[4-(5-benzyloxy-4...)Show SMILES COc1cc(CO)c(OCc2ccccc2)cc1Cc1cc(OC)c(Cc2cc(OC)c(Cc3cc(OC)c(Cc4cc(OC)c(Cc5cc(O)ccc5OCC(O)=O)cc4OCc4ccccc4)cc3OCC(O)=O)cc2OCc2ccccc2)cc1OCC(O)=O Show InChI InChI=1S/C74H72O19/c1-83-62-29-55(67(88-40-46-15-9-6-10-16-46)33-50(62)23-49-28-60(76)21-22-61(49)91-43-72(77)78)24-52-35-69(92-44-73(79)80)57(31-64(52)85-3)26-51-34-68(89-41-47-17-11-7-12-18-47)56(30-63(51)84-2)25-53-36-70(93-45-74(81)82)58(32-65(53)86-4)27-54-37-71(59(39-75)38-66(54)87-5)90-42-48-19-13-8-14-20-48/h6-22,28-38,75-76H,23-27,39-45H2,1-5H3,(H,77,78)(H,79,80)(H,81,82) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060332
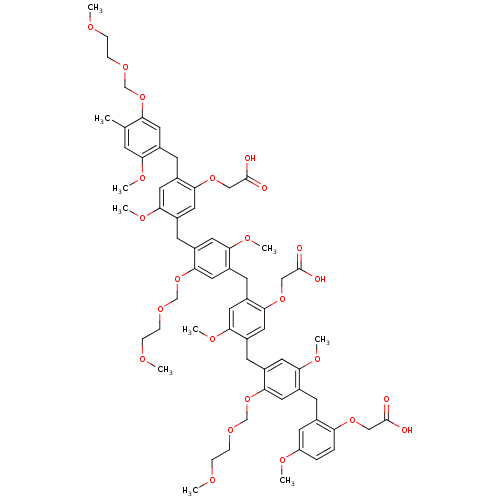
(CHEMBL215927)Show SMILES COCCOCOc1cc(Cc2cc(OC)c(Cc3cc(OC)c(Cc4cc(OC)c(Cc5cc(OC)c(Cc6cc(OC)ccc6OCC(O)=O)cc5OCOCCOC)cc4OCC(O)=O)cc3OCOCCOC)cc2OCC(O)=O)c(OC)cc1C Show InChI InChI=1S/C66H80O24/c1-41-19-55(77-6)43(26-54(41)88-38-82-16-13-73-2)21-48-27-56(78-7)45(31-60(48)86-36-65(69)70)24-51-30-59(81-10)47(34-63(51)90-40-84-18-15-75-4)22-49-28-57(79-8)46(32-61(49)87-37-66(71)72)23-50-29-58(80-9)44(33-62(50)89-39-83-17-14-74-3)20-42-25-52(76-5)11-12-53(42)85-35-64(67)68/h11-12,19,25-34H,13-18,20-24,35-40H2,1-10H3,(H,67,68)(H,69,70)(H,71,72) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50279771

(CHEMBL217744 | [4-Benzyloxy-5-{5-benzyloxy-4-[5-be...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(OCc5ccccc5)c(Cc5cc(O)ccc5OCC(O)=O)cc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C73H66O21/c74-36-58-35-67(89-40-49-19-11-4-12-20-49)57(34-68(58)94-45-73(84)85)26-56-30-63(88-39-48-17-9-3-10-18-48)53(33-66(56)93-44-72(82)83)25-55-29-62(87-38-47-15-7-2-8-16-47)52(32-65(55)92-43-71(80)81)24-54-28-61(86-37-46-13-5-1-6-14-46)51(31-64(54)91-42-70(78)79)23-50-27-59(75)21-22-60(50)90-41-69(76)77/h1-22,27-35,74-75H,23-26,36-45H2,(H,76,77)(H,78,79)(H,80,81)(H,82,83)(H,84,85) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 750 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50060332

(CHEMBL215927)Show SMILES COCCOCOc1cc(Cc2cc(OC)c(Cc3cc(OC)c(Cc4cc(OC)c(Cc5cc(OC)c(Cc6cc(OC)ccc6OCC(O)=O)cc5OCOCCOC)cc4OCC(O)=O)cc3OCOCCOC)cc2OCC(O)=O)c(OC)cc1C Show InChI InChI=1S/C66H80O24/c1-41-19-55(77-6)43(26-54(41)88-38-82-16-13-73-2)21-48-27-56(78-7)45(31-60(48)86-36-65(69)70)24-51-30-59(81-10)47(34-63(51)90-40-84-18-15-75-4)22-49-28-57(79-8)46(32-61(49)87-37-66(71)72)23-50-29-58(80-9)44(33-62(50)89-39-83-17-14-74-3)20-42-25-52(76-5)11-12-53(42)85-35-64(67)68/h11-12,19,25-34H,13-18,20-24,35-40H2,1-10H3,(H,67,68)(H,69,70)(H,71,72) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50060331

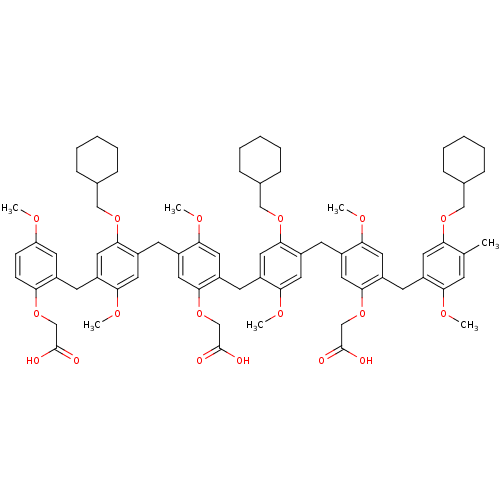
(CHEMBL412744 | bis-[4-tert-Butyl-2-[5-tert-butyl-3...)Show SMILES CC(C)(C)c1cc2Cc3cc(cc(Cc4cc(cc(Cc5cc(cc(Cc6cc(cc(Cc7cc(cc(Cc8cc(cc(Cc9cc(cc(Cc(c1)c2OCC(O)=O)c9OCC(O)=O)C(C)(C)C)c8OCC(O)=O)C(C)(C)C)c7OCC(O)=O)C(C)(C)C)c6OCC(O)=O)C(C)(C)C)c5OCC(O)=O)C(C)(C)C)c4OCC(O)=O)C(C)(C)C)c3OCC(O)=O)C(C)(C)C Show InChI InChI=1S/C104H128O24/c1-97(2,3)73-33-57-25-59-35-74(98(4,5)6)37-61(90(59)122-50-82(107)108)27-63-39-76(100(10,11)12)41-65(92(63)124-52-84(111)112)29-67-43-78(102(16,17)18)45-69(94(67)126-54-86(115)116)31-71-47-80(104(22,23)24)48-72(96(71)128-56-88(119)120)32-70-46-79(103(19,20)21)44-68(95(70)127-55-87(117)118)30-66-42-77(101(13,14)15)40-64(93(66)125-53-85(113)114)28-62-38-75(99(7,8)9)36-60(91(62)123-51-83(109)110)26-58(34-73)89(57)121-49-81(105)106/h33-48H,25-32,49-56H2,1-24H3,(H,105,106)(H,107,108)(H,109,110)(H,111,112)(H,113,114)(H,115,116)(H,117,118)(H,119,120) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50060329
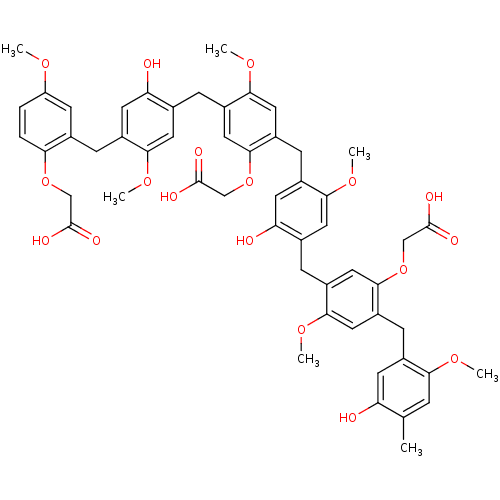
(CHEMBL385442 | {2-{4-[5-Carboxymethoxy-4-(5-hydrox...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(O)c(Cc3cc(OCC(O)=O)c(Cc4cc(O)c(Cc5cc(OCC(O)=O)c(Cc6cc(O)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C54H56O18/c1-29-10-45(65-3)33(17-41(29)55)14-38-22-48(68-6)36(24-50(38)71-27-53(60)61)12-31-21-47(67-5)35(19-43(31)57)15-39-23-49(69-7)37(25-51(39)72-28-54(62)63)11-30-20-46(66-4)34(18-42(30)56)13-32-16-40(64-2)8-9-44(32)70-26-52(58)59/h8-10,16-25,55-57H,11-15,26-28H2,1-7H3,(H,58,59)(H,60,61)(H,62,63) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50279767

(CHEMBL268343 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCCCCCc3ccccc3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCCCCCc5ccccc5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCCCCCc7ccccc7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C87H98O18/c1-59-40-76(95-3)64(47-75(59)100-37-23-11-20-32-60-26-14-8-15-27-60)42-71-50-79(98-6)67(54-83(71)104-57-86(90)91)44-70-49-78(97-5)66(53-82(70)102-39-25-13-22-34-62-30-18-10-19-31-62)45-72-51-80(99-7)68(55-84(72)105-58-87(92)93)43-69-48-77(96-4)65(41-63-46-73(94-2)35-36-74(63)103-56-85(88)89)52-81(69)101-38-24-12-21-33-61-28-16-9-17-29-61/h8-10,14-19,26-31,35-36,40,46-55H,11-13,20-25,32-34,37-39,41-45,56-58H2,1-7H3,(H,88,89)(H,90,91)(H,92,93) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50279766

(CHEMBL213783 | {2-{5-Benzyloxy-4-[4-(5-benzyloxy-4...)Show SMILES COc1cc(CO)c(OCc2ccccc2)cc1Cc1cc(OC)c(Cc2cc(OC)c(Cc3cc(OC)c(Cc4cc(OC)c(Cc5cc(O)ccc5OCC(O)=O)cc4OCc4ccccc4)cc3OCC(O)=O)cc2OCc2ccccc2)cc1OCC(O)=O Show InChI InChI=1S/C74H72O19/c1-83-62-29-55(67(88-40-46-15-9-6-10-16-46)33-50(62)23-49-28-60(76)21-22-61(49)91-43-72(77)78)24-52-35-69(92-44-73(79)80)57(31-64(52)85-3)26-51-34-68(89-41-47-17-11-7-12-18-47)56(30-63(51)84-2)25-53-36-70(93-45-74(81)82)58(32-65(53)86-4)27-54-37-71(59(39-75)38-66(54)87-5)90-42-48-19-13-8-14-20-48/h6-22,28-38,75-76H,23-27,39-45H2,1-5H3,(H,77,78)(H,79,80)(H,81,82) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060330

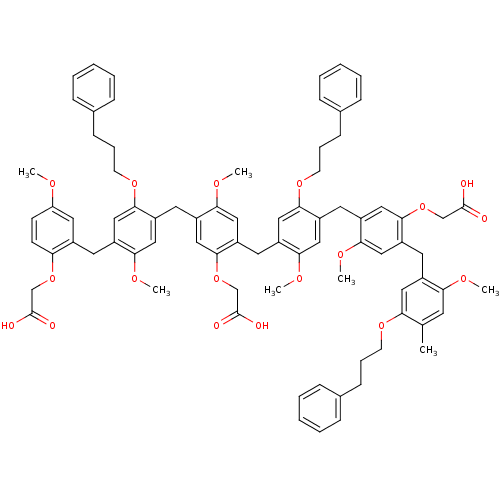
(CHEMBL265335 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCCCc3ccccc3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCCCc5ccccc5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCCCc7ccccc7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C81H86O18/c1-53-34-70(89-3)58(41-69(53)94-31-17-26-54-20-11-8-12-21-54)36-65-44-73(92-6)61(48-77(65)98-51-80(84)85)38-64-43-72(91-5)60(47-76(64)96-33-19-28-56-24-15-10-16-25-56)39-66-45-74(93-7)62(49-78(66)99-52-81(86)87)37-63-42-71(90-4)59(46-75(63)95-32-18-27-55-22-13-9-14-23-55)35-57-40-67(88-2)29-30-68(57)97-50-79(82)83/h8-16,20-25,29-30,34,40-49H,17-19,26-28,31-33,35-39,50-52H2,1-7H3,(H,82,83)(H,84,85)(H,86,87) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50279768
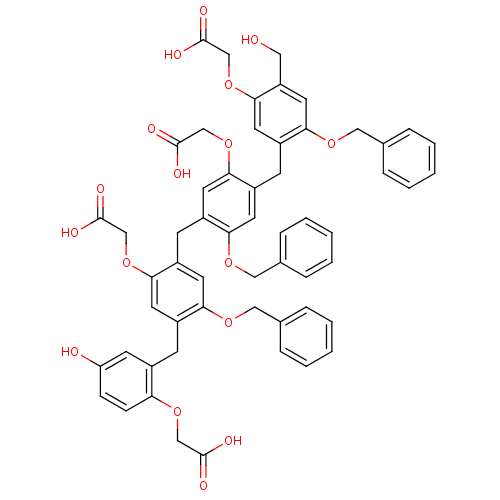
((2-{2-Benzyloxy-4-[2-benzyloxy-4-(2-benzyloxy-5-ca...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(O)ccc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C57H52O17/c58-28-45-27-52(70-31-38-14-8-3-9-15-38)44(26-53(45)74-35-57(66)67)20-43-23-49(69-30-37-12-6-2-7-13-37)41(25-51(43)73-34-56(64)65)19-42-22-48(68-29-36-10-4-1-5-11-36)40(24-50(42)72-33-55(62)63)18-39-21-46(59)16-17-47(39)71-32-54(60)61/h1-17,21-27,58-59H,18-20,28-35H2,(H,60,61)(H,62,63)(H,64,65)(H,66,67) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibitory activity against human leukocyte elastase |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50279765

(CHEMBL215405 | {2-{4-[5-Carboxymethoxy-4-(5-cycloh...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCC3CCCCC3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCC5CCCCC5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCC7CCCCC7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C75H92O18/c1-47-25-64(83-3)52(32-63(47)88-41-48-17-11-8-12-18-48)27-59-35-67(86-6)55(39-71(59)92-45-74(78)79)29-58-34-66(85-5)54(38-70(58)90-43-50-21-15-10-16-22-50)30-60-36-68(87-7)56(40-72(60)93-46-75(80)81)28-57-33-65(84-4)53(37-69(57)89-42-49-19-13-9-14-20-49)26-51-31-61(82-2)23-24-62(51)91-44-73(76)77/h23-25,31-40,48-50H,8-22,26-30,41-46H2,1-7H3,(H,76,77)(H,78,79)(H,80,81) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50279767

(CHEMBL268343 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCCCCCc3ccccc3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCCCCCc5ccccc5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCCCCCc7ccccc7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C87H98O18/c1-59-40-76(95-3)64(47-75(59)100-37-23-11-20-32-60-26-14-8-15-27-60)42-71-50-79(98-6)67(54-83(71)104-57-86(90)91)44-70-49-78(97-5)66(53-82(70)102-39-25-13-22-34-62-30-18-10-19-31-62)45-72-51-80(99-7)68(55-84(72)105-58-87(92)93)43-69-48-77(96-4)65(41-63-46-73(94-2)35-36-74(63)103-56-85(88)89)52-81(69)101-38-24-12-21-33-61-28-16-9-17-29-61/h8-10,14-19,26-31,35-36,40,46-55H,11-13,20-25,32-34,37-39,41-45,56-58H2,1-7H3,(H,88,89)(H,90,91)(H,92,93) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50279770

(2-{4-benzyloxy-5-[5-benzyloxy-4-(5-benzyloxy-4-{5-...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(OCc5ccccc5)c(Cc5cc(OCc6ccccc6)c(Cc6cc(O)ccc6OCC(O)=O)cc5OCC(O)=O)cc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C89H80O25/c90-44-71-43-82(108-49-60-24-14-5-15-25-60)70(42-83(71)114-55-89(102)103)32-69-37-77(107-48-59-22-12-4-13-23-59)65(41-81(69)113-54-88(100)101)31-68-36-76(106-47-58-20-10-3-11-21-58)64(40-80(68)112-53-87(98)99)30-67-35-75(105-46-57-18-8-2-9-19-57)63(39-79(67)111-52-86(96)97)29-66-34-74(104-45-56-16-6-1-7-17-56)62(38-78(66)110-51-85(94)95)28-61-33-72(91)26-27-73(61)109-50-84(92)93/h1-27,33-43,90-91H,28-32,44-55H2,(H,92,93)(H,94,95)(H,96,97)(H,98,99)(H,100,101)(H,102,103) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50279770

(2-{4-benzyloxy-5-[5-benzyloxy-4-(5-benzyloxy-4-{5-...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(OCc5ccccc5)c(Cc5cc(OCc6ccccc6)c(Cc6cc(O)ccc6OCC(O)=O)cc5OCC(O)=O)cc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C89H80O25/c90-44-71-43-82(108-49-60-24-14-5-15-25-60)70(42-83(71)114-55-89(102)103)32-69-37-77(107-48-59-22-12-4-13-23-59)65(41-81(69)113-54-88(100)101)31-68-36-76(106-47-58-20-10-3-11-21-58)64(40-80(68)112-53-87(98)99)30-67-35-75(105-46-57-18-8-2-9-19-57)63(39-79(67)111-52-86(96)97)29-66-34-74(104-45-56-16-6-1-7-17-56)62(38-78(66)110-51-85(94)95)28-61-33-72(91)26-27-73(61)109-50-84(92)93/h1-27,33-43,90-91H,28-32,44-55H2,(H,92,93)(H,94,95)(H,96,97)(H,98,99)(H,100,101)(H,102,103) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50279765

(CHEMBL215405 | {2-{4-[5-Carboxymethoxy-4-(5-cycloh...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCC3CCCCC3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCC5CCCCC5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCC7CCCCC7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C75H92O18/c1-47-25-64(83-3)52(32-63(47)88-41-48-17-11-8-12-18-48)27-59-35-67(86-6)55(39-71(59)92-45-74(78)79)29-58-34-66(85-5)54(38-70(58)90-43-50-21-15-10-16-22-50)30-60-36-68(87-7)56(40-72(60)93-46-75(80)81)28-57-33-65(84-4)53(37-69(57)89-42-49-19-13-9-14-20-49)26-51-31-61(82-2)23-24-62(51)91-44-73(76)77/h23-25,31-40,48-50H,8-22,26-30,41-46H2,1-7H3,(H,76,77)(H,78,79)(H,80,81) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50279771

(CHEMBL217744 | [4-Benzyloxy-5-{5-benzyloxy-4-[5-be...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(OCc5ccccc5)c(Cc5cc(O)ccc5OCC(O)=O)cc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C73H66O21/c74-36-58-35-67(89-40-49-19-11-4-12-20-49)57(34-68(58)94-45-73(84)85)26-56-30-63(88-39-48-17-9-3-10-18-48)53(33-66(56)93-44-72(82)83)25-55-29-62(87-38-47-15-7-2-8-16-47)52(32-65(55)92-43-71(80)81)24-54-28-61(86-37-46-13-5-1-6-14-46)51(31-64(54)91-42-70(78)79)23-50-27-59(75)21-22-60(50)90-41-69(76)77/h1-22,27-35,74-75H,23-26,36-45H2,(H,76,77)(H,78,79)(H,80,81)(H,82,83)(H,84,85) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50060332

(CHEMBL215927)Show SMILES COCCOCOc1cc(Cc2cc(OC)c(Cc3cc(OC)c(Cc4cc(OC)c(Cc5cc(OC)c(Cc6cc(OC)ccc6OCC(O)=O)cc5OCOCCOC)cc4OCC(O)=O)cc3OCOCCOC)cc2OCC(O)=O)c(OC)cc1C Show InChI InChI=1S/C66H80O24/c1-41-19-55(77-6)43(26-54(41)88-38-82-16-13-73-2)21-48-27-56(78-7)45(31-60(48)86-36-65(69)70)24-51-30-59(81-10)47(34-63(51)90-40-84-18-15-75-4)22-49-28-57(79-8)46(32-61(49)87-37-66(71)72)23-50-29-58(80-9)44(33-62(50)89-39-83-17-14-74-3)20-42-25-52(76-5)11-12-53(42)85-35-64(67)68/h11-12,19,25-34H,13-18,20-24,35-40H2,1-10H3,(H,67,68)(H,69,70)(H,71,72) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50060329

(CHEMBL385442 | {2-{4-[5-Carboxymethoxy-4-(5-hydrox...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(O)c(Cc3cc(OCC(O)=O)c(Cc4cc(O)c(Cc5cc(OCC(O)=O)c(Cc6cc(O)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C54H56O18/c1-29-10-45(65-3)33(17-41(29)55)14-38-22-48(68-6)36(24-50(38)71-27-53(60)61)12-31-21-47(67-5)35(19-43(31)57)15-39-23-49(69-7)37(25-51(39)72-28-54(62)63)11-30-20-46(66-4)34(18-42(30)56)13-32-16-40(64-2)8-9-44(32)70-26-52(58)59/h8-10,16-25,55-57H,11-15,26-28H2,1-7H3,(H,58,59)(H,60,61)(H,62,63) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50060329

(CHEMBL385442 | {2-{4-[5-Carboxymethoxy-4-(5-hydrox...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(O)c(Cc3cc(OCC(O)=O)c(Cc4cc(O)c(Cc5cc(OCC(O)=O)c(Cc6cc(O)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C54H56O18/c1-29-10-45(65-3)33(17-41(29)55)14-38-22-48(68-6)36(24-50(38)71-27-53(60)61)12-31-21-47(67-5)35(19-43(31)57)15-39-23-49(69-7)37(25-51(39)72-28-54(62)63)11-30-20-46(66-4)34(18-42(30)56)13-32-16-40(64-2)8-9-44(32)70-26-52(58)59/h8-10,16-25,55-57H,11-15,26-28H2,1-7H3,(H,58,59)(H,60,61)(H,62,63) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50060330

(CHEMBL265335 | {5-[4-(2-Carboxymethoxy-5-methoxy-b...)Show SMILES COc1ccc(OCC(O)=O)c(Cc2cc(OCCCc3ccccc3)c(Cc3cc(OCC(O)=O)c(Cc4cc(OCCCc5ccccc5)c(Cc5cc(OCC(O)=O)c(Cc6cc(OCCCc7ccccc7)c(C)cc6OC)cc5OC)cc4OC)cc3OC)cc2OC)c1 Show InChI InChI=1S/C81H86O18/c1-53-34-70(89-3)58(41-69(53)94-31-17-26-54-20-11-8-12-21-54)36-65-44-73(92-6)61(48-77(65)98-51-80(84)85)38-64-43-72(91-5)60(47-76(64)96-33-19-28-56-24-15-10-16-25-56)39-66-45-74(93-7)62(49-78(66)99-52-81(86)87)37-63-42-71(90-4)59(46-75(63)95-32-18-27-55-22-13-9-14-23-55)35-57-40-67(88-2)29-30-68(57)97-50-79(82)83/h8-16,20-25,29-30,34,40-49H,17-19,26-28,31-33,35-39,50-52H2,1-7H3,(H,82,83)(H,84,85)(H,86,87) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50279766

(CHEMBL213783 | {2-{5-Benzyloxy-4-[4-(5-benzyloxy-4...)Show SMILES COc1cc(CO)c(OCc2ccccc2)cc1Cc1cc(OC)c(Cc2cc(OC)c(Cc3cc(OC)c(Cc4cc(OC)c(Cc5cc(O)ccc5OCC(O)=O)cc4OCc4ccccc4)cc3OCC(O)=O)cc2OCc2ccccc2)cc1OCC(O)=O Show InChI InChI=1S/C74H72O19/c1-83-62-29-55(67(88-40-46-15-9-6-10-16-46)33-50(62)23-49-28-60(76)21-22-61(49)91-43-72(77)78)24-52-35-69(92-44-73(79)80)57(31-64(52)85-3)26-51-34-68(89-41-47-17-11-7-12-18-47)56(30-63(51)84-2)25-53-36-70(93-45-74(81)82)58(32-65(53)86-4)27-54-37-71(59(39-75)38-66(54)87-5)90-42-48-19-13-8-14-20-48/h6-22,28-38,75-76H,23-27,39-45H2,1-5H3,(H,77,78)(H,79,80)(H,81,82) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Cathepsin G
(Homo sapiens (Human)) | BDBM50279768

((2-{2-Benzyloxy-4-[2-benzyloxy-4-(2-benzyloxy-5-ca...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(O)ccc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C57H52O17/c58-28-45-27-52(70-31-38-14-8-3-9-15-38)44(26-53(45)74-35-57(66)67)20-43-23-49(69-30-37-12-6-2-7-13-37)41(25-51(43)73-34-56(64)65)19-42-22-48(68-29-36-10-4-1-5-11-36)40(24-50(42)72-33-55(62)63)18-39-21-46(59)16-17-47(39)71-32-54(60)61/h1-17,21-27,58-59H,18-20,28-35H2,(H,60,61)(H,62,63)(H,64,65)(H,66,67) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin G at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Suc-Ala-Ala-Pro-Phe-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50279768

((2-{2-Benzyloxy-4-[2-benzyloxy-4-(2-benzyloxy-5-ca...)Show SMILES OCc1cc(OCc2ccccc2)c(Cc2cc(OCc3ccccc3)c(Cc3cc(OCc4ccccc4)c(Cc4cc(O)ccc4OCC(O)=O)cc3OCC(O)=O)cc2OCC(O)=O)cc1OCC(O)=O Show InChI InChI=1S/C57H52O17/c58-28-45-27-52(70-31-38-14-8-3-9-15-38)44(26-53(45)74-35-57(66)67)20-43-23-49(69-30-37-12-6-2-7-13-37)41(25-51(43)73-34-56(64)65)19-42-22-48(68-29-36-10-4-1-5-11-36)40(24-50(42)72-33-55(62)63)18-39-21-46(59)16-17-47(39)71-32-54(60)61/h1-17,21-27,58-59H,18-20,28-35H2,(H,60,61)(H,62,63)(H,64,65)(H,66,67) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of thrombin at pH 7.5 in HEPES buffer containing 200 mM NaCl with substrate Bz-Phe-Val-Arg-pNA. |
J Med Chem 40: 3408-22 (1997)
Article DOI: 10.1021/jm970251r
BindingDB Entry DOI: 10.7270/Q26D5S4T |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3289
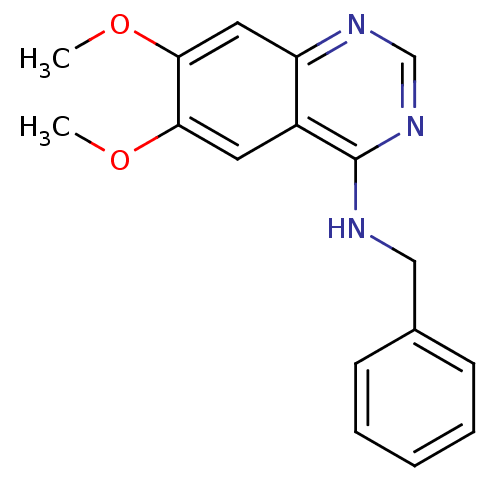
(4-(Benzylamino)quinazoline deriv. 40 | CHEMBL54554...)Show InChI InChI=1S/C17H17N3O2/c1-21-15-8-13-14(9-16(15)22-2)19-11-20-17(13)18-10-12-6-4-3-5-7-12/h3-9,11H,10H2,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor autophosphorylation. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50142887
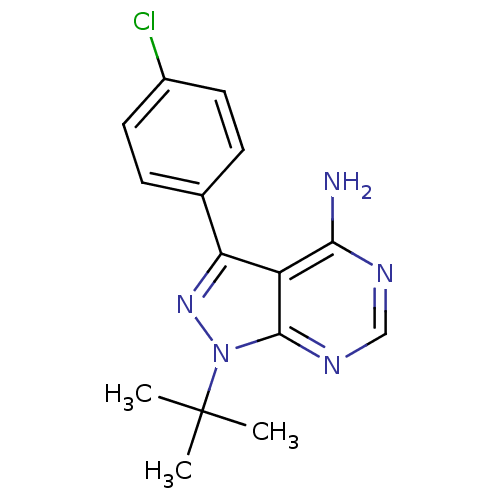
(1-(tert-butyl)-3-(4-chlorophenyl)-4-aminopyrazolo[...)Show InChI InChI=1S/C15H16ClN5/c1-15(2,3)21-14-11(13(17)18-8-19-14)12(20-21)9-4-6-10(16)7-5-9/h4-8H,1-3H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase in Jurkat cells where p56lck autophosphorylation is inhibited. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039087
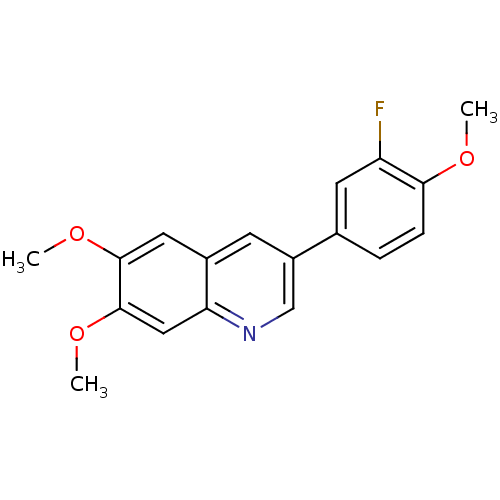
(3-(3-Fluoro-4-methoxy-phenyl)-6,7-dimethoxy-quinol...)Show InChI InChI=1S/C18H16FNO3/c1-21-16-5-4-11(7-14(16)19)13-6-12-8-17(22-2)18(23-3)9-15(12)20-10-13/h4-10H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.003-0.005 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
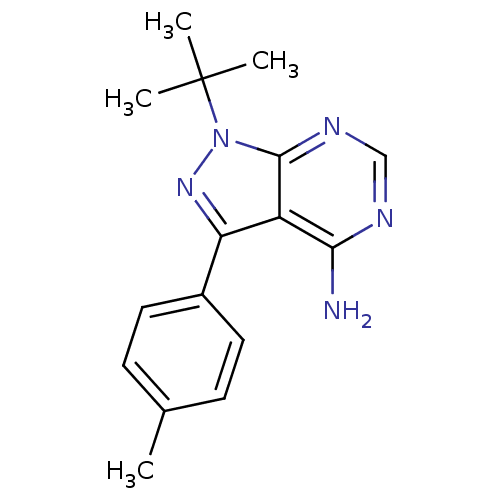
(Homo sapiens (Human)) | BDBM25116
(1-tert-butyl-3-(4-methylphenyl)-1H-pyrazolo[3,4-d]...)Show InChI InChI=1S/C16H19N5/c1-10-5-7-11(8-6-10)13-12-14(17)18-9-19-15(12)21(20-13)16(2,3)4/h5-9H,1-4H3,(H2,17,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase in Jurkat cells where p56lck autophosphorylation is inhibited. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039073

(5,7-Dimethyl-3-thiophen-3-yl-quinoline | CHEMBL304...)Show InChI InChI=1S/C15H13NS/c1-10-5-11(2)14-7-13(8-16-15(14)6-10)12-3-4-17-9-12/h3-9H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.001-0.005 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
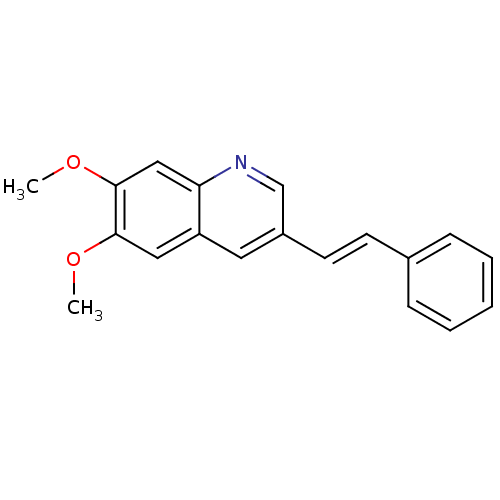
(Homo sapiens (Human)) | BDBM50039041
(6,7-Dimethoxy-3-((E)-styryl)-quinoline | CHEMBL701...)Show InChI InChI=1S/C19H17NO2/c1-21-18-11-16-10-15(9-8-14-6-4-3-5-7-14)13-20-17(16)12-19(18)22-2/h3-13H,1-2H3/b9-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.001-0.005 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
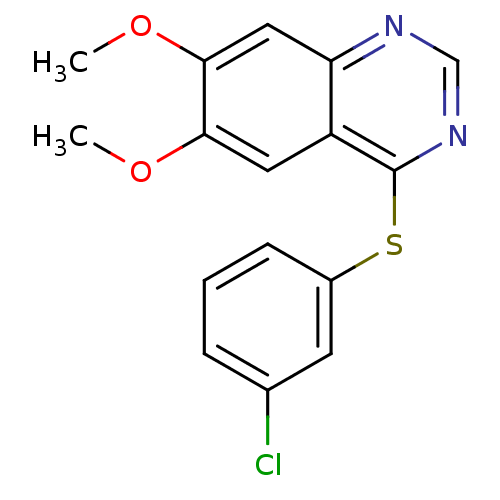
(Homo sapiens (Human)) | BDBM50291091
(4-(3-Chloro-phenylsulfanyl)-6,7-dimethoxy-quinazol...)Show InChI InChI=1S/C16H13ClN2O2S/c1-20-14-7-12-13(8-15(14)21-2)18-9-19-16(12)22-11-5-3-4-10(17)6-11/h3-9H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor autophosphorylation. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039071

(6,7-Dimethoxy-3-(4-methoxy-phenyl)-quinoline | CHE...)Show InChI InChI=1S/C18H17NO3/c1-20-15-6-4-12(5-7-15)14-8-13-9-17(21-2)18(22-3)10-16(13)19-11-14/h4-11H,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.001-0.015 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50366492
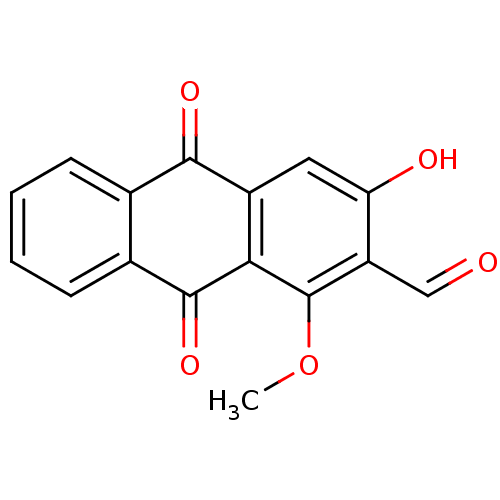
(DAMNACANTHAL)Show InChI InChI=1S/C16H10O5/c1-21-16-11(7-17)12(18)6-10-13(16)15(20)9-5-3-2-4-8(9)14(10)19/h2-7,18H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of p56 Lck tyrosine kinase in Jurkat cells where p56lck autophosphorylation is inhibited. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50291080
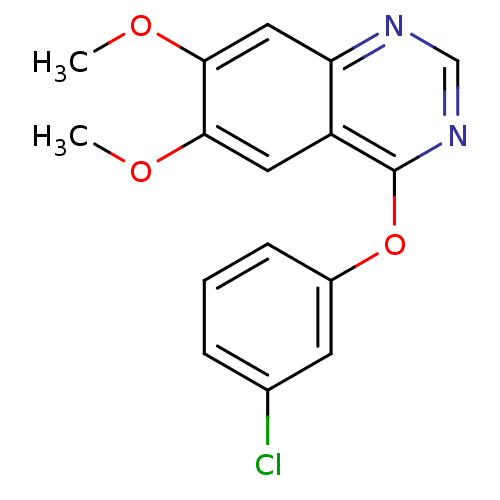
(4-(3-Chloro-phenoxy)-6,7-dimethoxy-quinazoline | C...)Show InChI InChI=1S/C16H13ClN2O3/c1-20-14-7-12-13(8-15(14)21-2)18-9-19-16(12)22-11-5-3-4-10(17)6-11/h3-9H,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor autophosphorylation. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039082
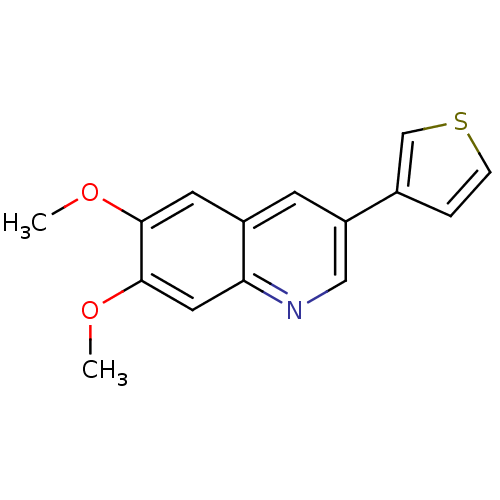
(6,7-Dimethoxy-3-thiophen-3-yl-quinoline | CHEMBL66...)Show InChI InChI=1S/C15H13NO2S/c1-17-14-6-11-5-12(10-3-4-19-9-10)8-16-13(11)7-15(14)18-2/h3-9H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.01-0.02 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039047

(5-Fluoro-3-thiophen-3-yl-quinoline | CHEMBL305056)Show InChI InChI=1S/C13H8FNS/c14-12-2-1-3-13-11(12)6-10(7-15-13)9-4-5-16-8-9/h1-8H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of isolated human PDGF receptor phosphorylation |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3531

(CHEMBL541586 | CHEMBL94431 | N-(3-fluorophenyl)-6,...)Show InChI InChI=1S/C16H14FN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor autophosphorylation. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039069

(7-Fluoro-3-thiophen-3-yl-quinoline | CHEMBL307093)Show InChI InChI=1S/C13H8FNS/c14-12-2-1-9-5-11(7-15-13(9)6-12)10-3-4-16-8-10/h1-8H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.004-0.025 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039084
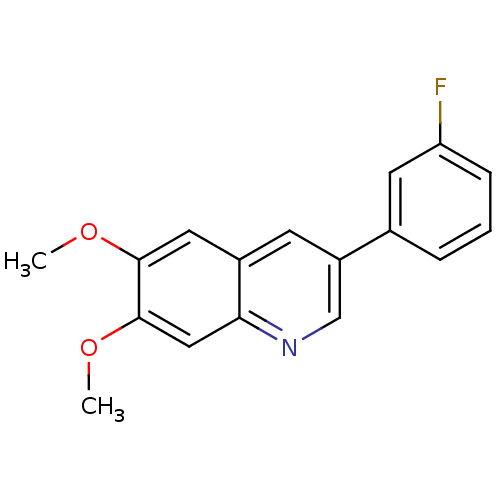
(3-(3-Fluoro-phenyl)-6,7-dimethoxy-quinoline | CHEM...)Show InChI InChI=1S/C17H14FNO2/c1-20-16-8-12-6-13(11-4-3-5-14(18)7-11)10-19-15(12)9-17(16)21-2/h3-10H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.015-0.025 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039067

(5-(6,7-Dimethoxy-quinolin-3-yl)-1H-pyridin-2-one |...)Show InChI InChI=1S/C16H14N2O3/c1-20-14-6-11-5-12(10-3-4-16(19)18-8-10)9-17-13(11)7-15(14)21-2/h3-9H,1-2H3,(H,18,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.015-0.03 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039037
(4-(6,7-Dimethoxy-quinolin-3-yl)-phenol | CHEMBL303...)Show InChI InChI=1S/C17H15NO3/c1-20-16-8-12-7-13(11-3-5-14(19)6-4-11)10-18-15(12)9-17(16)21-2/h3-10,19H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.02-0.03 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM3532

(CHEMBL540068 | CHEMBL7917 | N-(3-chlorophenyl)-6,7...)Show InChI InChI=1S/C16H14ClN3O2/c1-21-14-7-12-13(8-15(14)22-2)18-9-19-16(12)20-11-5-3-4-10(17)6-11/h3-9H,1-2H3,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Epidermal growth factor receptor autophosphorylation. |
Bioorg Med Chem Lett 7: 417-420 (1997)
Article DOI: 10.1016/S0960-894X(97)00034-6
BindingDB Entry DOI: 10.7270/Q2J966VN |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039042

(5,7-Dimethoxy-3-thiophen-3-yl-quinoline | CHEMBL66...)Show InChI InChI=1S/C15H13NO2S/c1-17-12-6-14-13(15(7-12)18-2)5-11(8-16-14)10-3-4-19-9-10/h3-9H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.025-0.03 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha/beta
(Homo sapiens (Human)) | BDBM50039072
(3-(5-Chloro-thiophen-2-yl)-6,7-dimethoxy-quinoline...)Show InChI InChI=1S/C15H12ClNO2S/c1-18-12-6-9-5-10(14-3-4-15(16)20-14)8-17-11(9)7-13(12)19-2/h3-8H,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research
Curated by ChEMBL
| Assay Description
Inhibition of human PDGF receptor phosphorylation; 0.007-0.03 |
J Med Chem 37: 2129-37 (1994)
BindingDB Entry DOI: 10.7270/Q29Z93Z8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data