

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50381654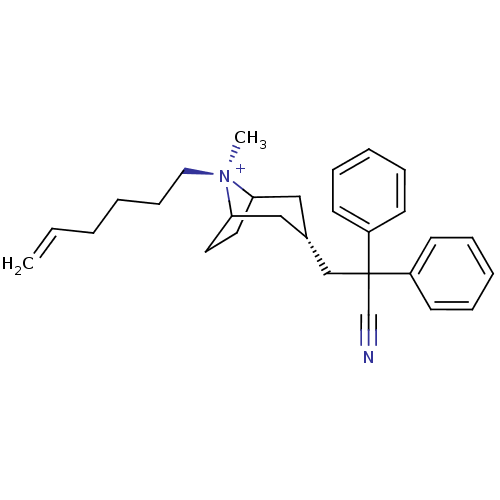 (CHEMBL2023764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M2 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50381654 (CHEMBL2023764) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M1 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381654 (CHEMBL2023764) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of [3H]-N-methyl scopolamine from muscarinic acetylcholine M3 receptor expressed in CHO cell membrane | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213750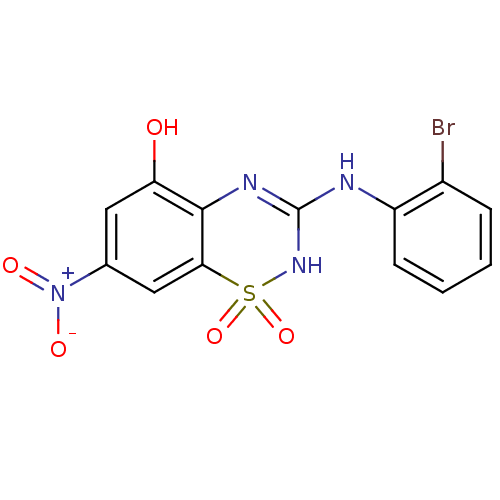 (3-(2-bromo-phenylamino)-7-nitro-1,1-dioxo-1,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381644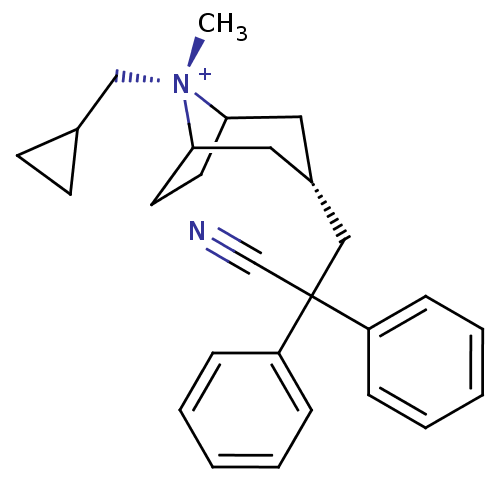 (CHEMBL2023761) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26.3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of Ach-induced calcium mobilization b... | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412556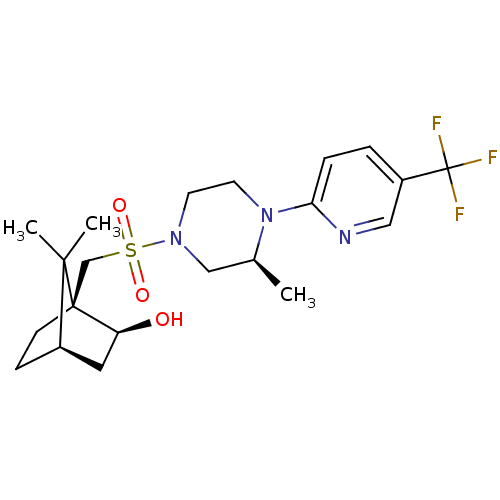 (CHEMBL481198) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50254758 ((1S,2S,4R)-1-(((S)-4-(3-fluoro-5-(trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381645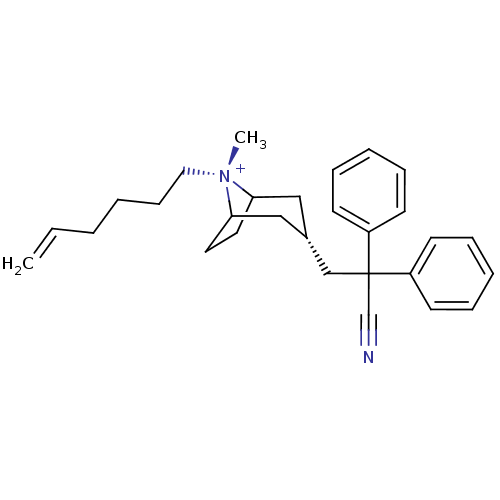 (CHEMBL2023763) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of Ach-induced calcium mobilization b... | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412557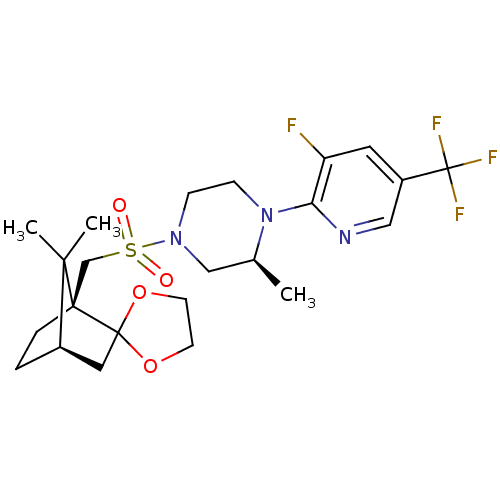 (CHEMBL464244) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39.8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50254758 ((1S,2S,4R)-1-(((S)-4-(3-fluoro-5-(trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213737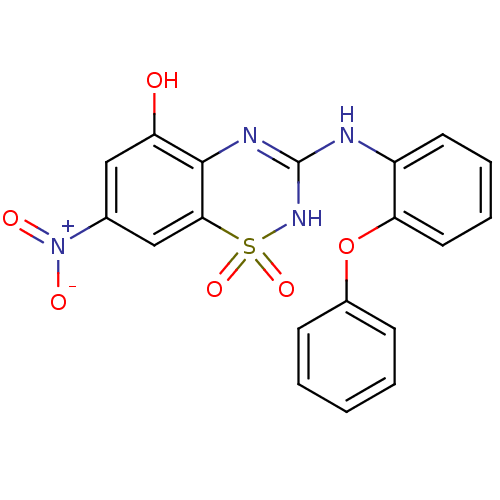 (7-nitro-1,1-dioxo-3-(2-phenoxy-phenylamino)-1,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of human recombinant [125I]IL8 from CXCR2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213736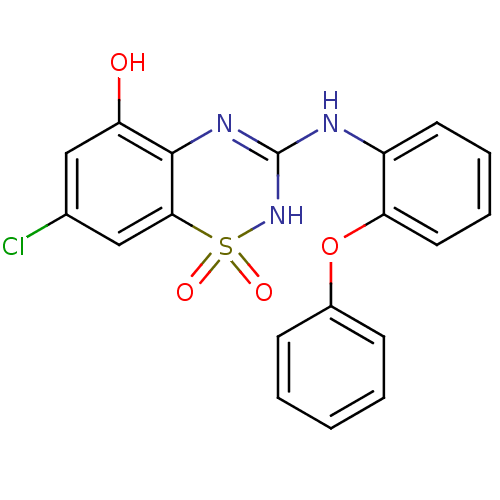 (7-chloro-1,1-dioxo-3-(2-phenoxy-phenylamino)-1,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412560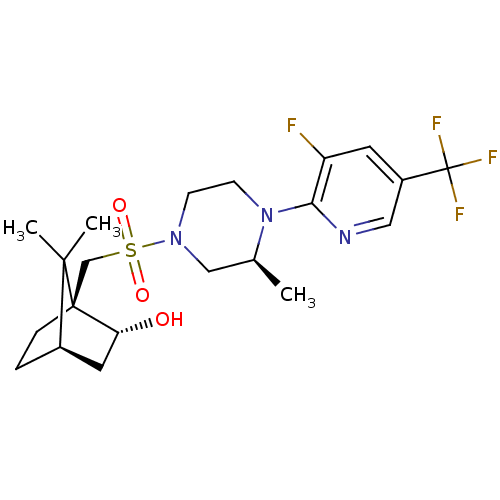 (CHEMBL464065) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.1 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213746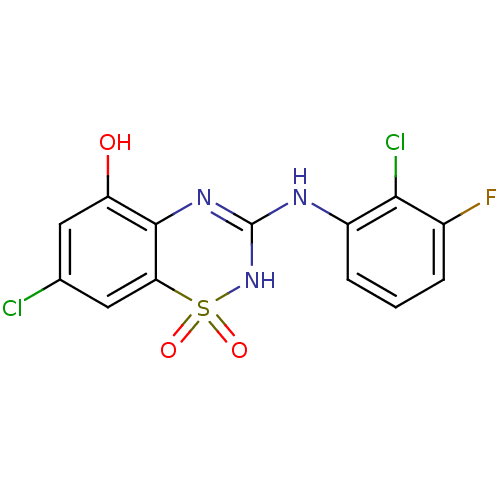 (7-chloro-3-(2-chloro-3-fluoro-phenylamino)-1,1-dio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213741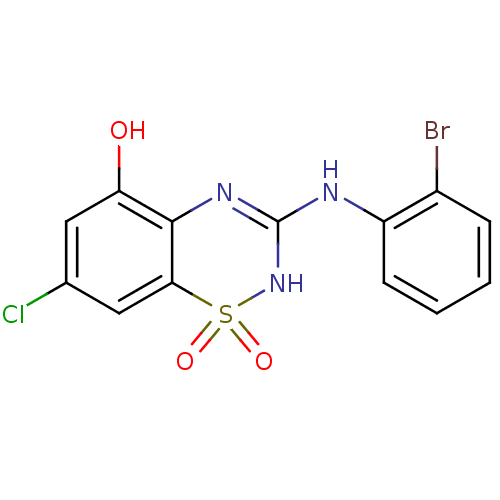 (3-(2-bromo-phenylamino)-7-chloro-1,1-dioxo-1,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381643 (CHEMBL2023759) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of Ach-induced calcium mobilization b... | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213758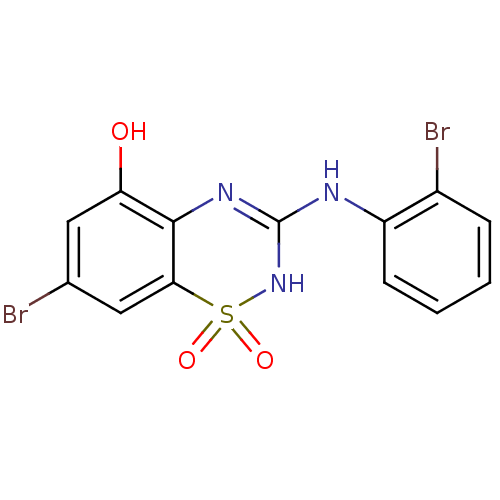 (7-bromo-3-(2-bromo-phenylamino)-1,1-dioxo-1,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50254678 ((1S,4R)-1-(((S)-4-(3-fluoro-5-(trifluoromethyl)pyr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213744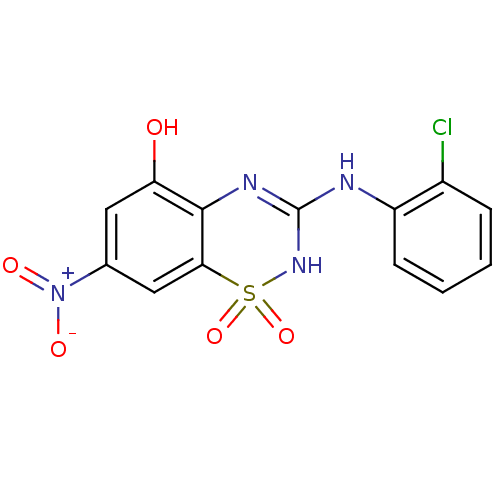 (3-(2-chloro-phenylamino)-7-nitro-1,1-dioxo-1,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Displacement of human recombinant [125I]IL8 from CXCR2 receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381642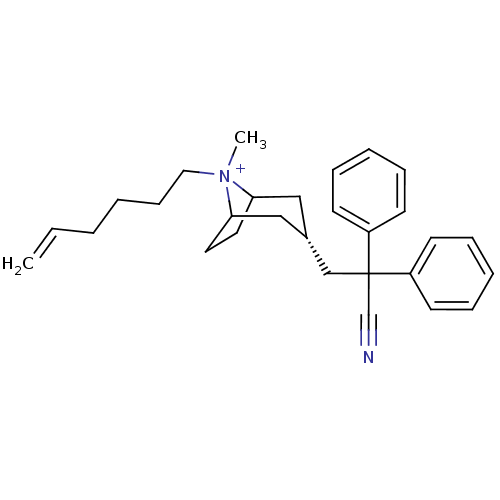 (CHEMBL2023757) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85.2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of Ach-induced calcium mobilization b... | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381637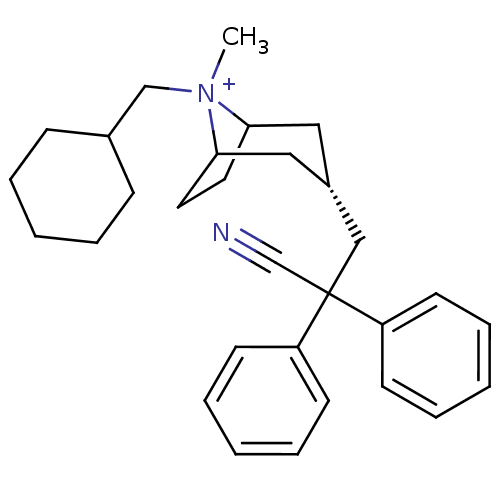 (CHEMBL2023754) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 89.6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of Ach-induced calcium mobilization b... | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213747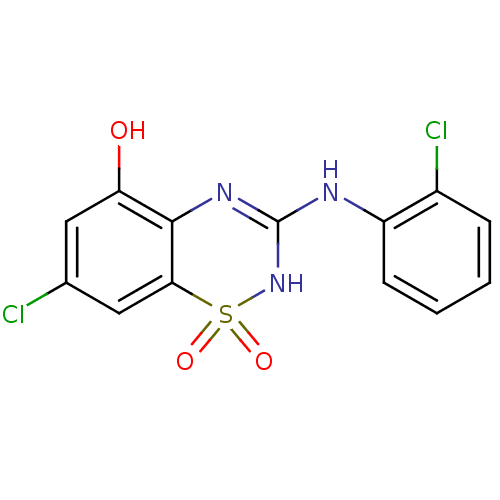 (7-chloro-3-(2-chloro-phenylamino)-1,1-dioxo-1,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381639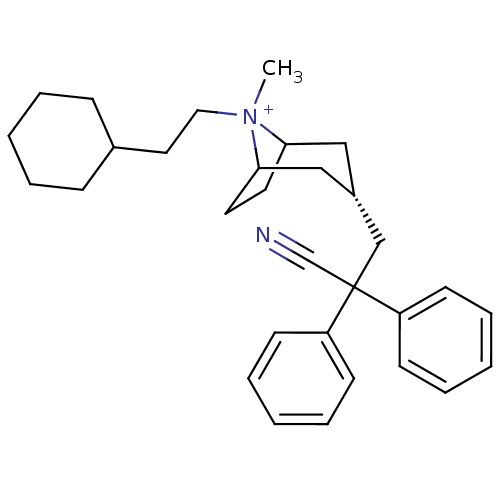 (CHEMBL2023756) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 109 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of Ach-induced calcium mobilization b... | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213755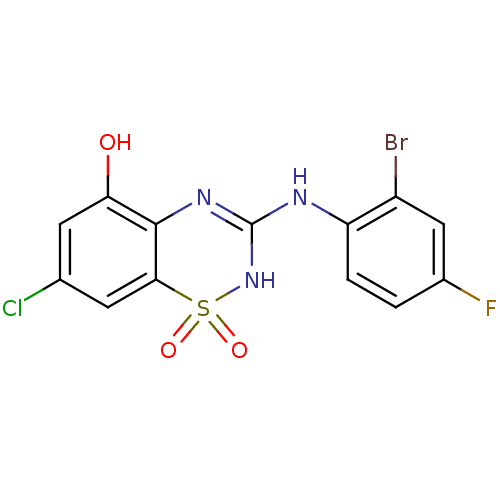 (3-(2-bromo-4-fluoro-phenylamino)-7-chloro-1,1-diox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412559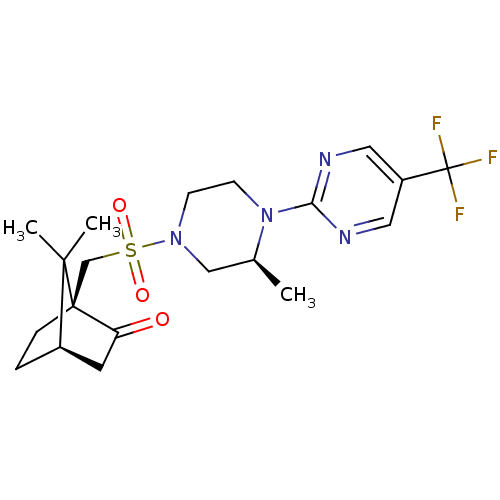 (CHEMBL464083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412564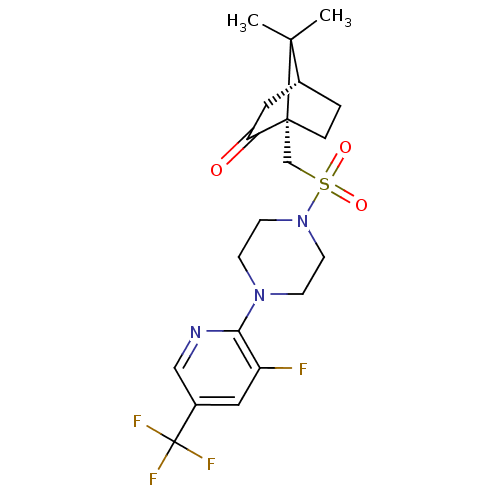 (CHEMBL479426) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412599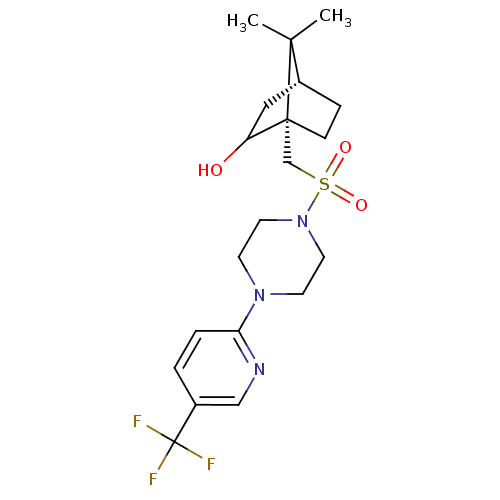 (CHEMBL459950) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50254758 ((1S,2S,4R)-1-(((S)-4-(3-fluoro-5-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at mouse recombinant CXCR3 expressed in human U2OS cells | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412563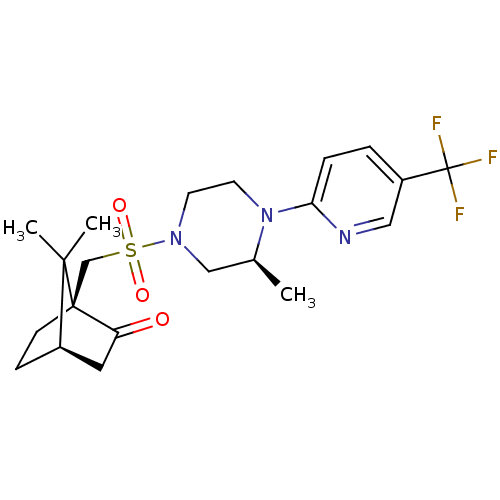 (CHEMBL465283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412555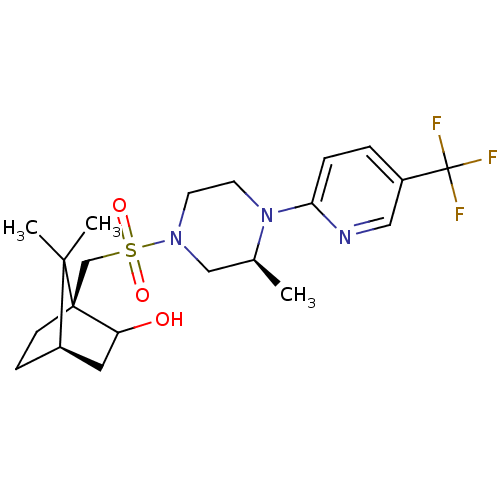 (CHEMBL481197) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50254678 ((1S,4R)-1-(((S)-4-(3-fluoro-5-(trifluoromethyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP1A2 (unknown origin) | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412600 (CHEMBL468468) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412597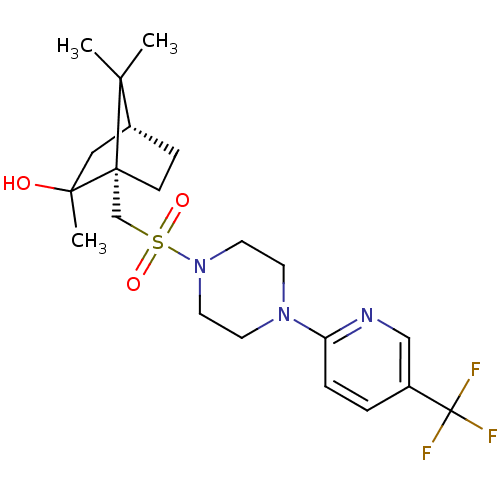 (CHEMBL459951) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50412556 (CHEMBL481198) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at mouse recombinant CXCR3 expressed in human U2OS cells | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Mus musculus) | BDBM50254758 ((1S,2S,4R)-1-(((S)-4-(3-fluoro-5-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at mouse recombinant CXCR3 expressed in human U2OS cells | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213763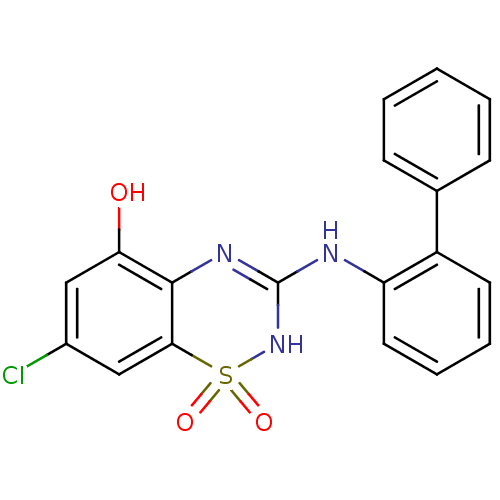 (3-(biphenyl-2-ylamino)-7-chloro-1,1-dioxo-1,4-dihy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213762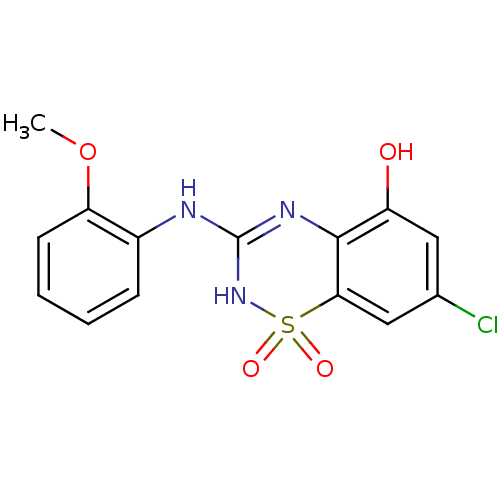 (7-chloro-3-(2-methoxy-phenylamino)-1,1-dioxo-1,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412558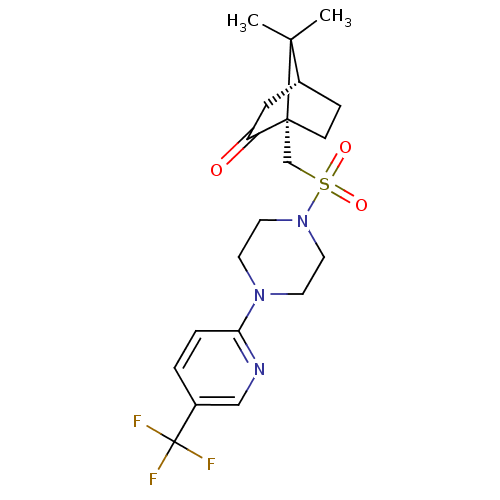 (CHEMBL464081) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412575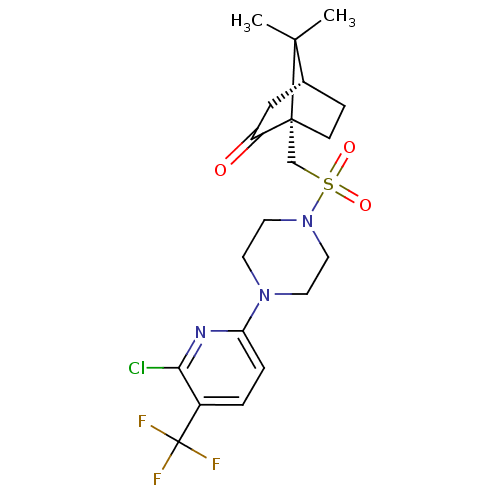 (CHEMBL481780) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213756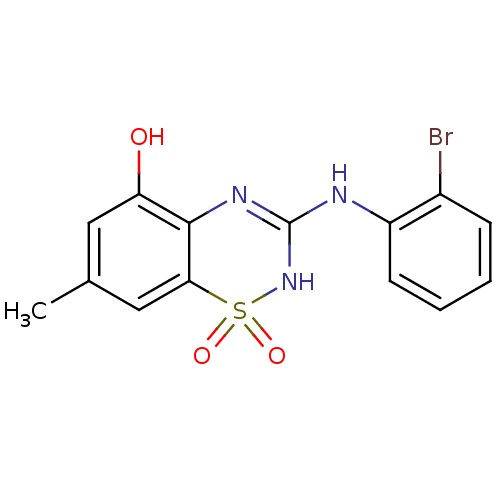 (3-(2-bromo-phenylamino)-7-methyl-1,1-dioxo-1,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412576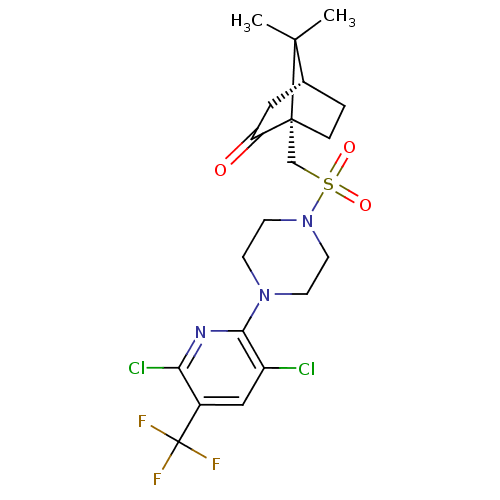 (CHEMBL480996) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412579 (CHEMBL479828) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412563 (CHEMBL465283) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412562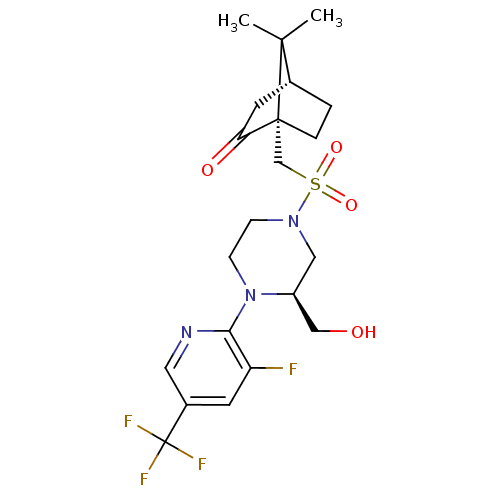 (CHEMBL481196) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 3 (Homo sapiens (Human)) | BDBM50412561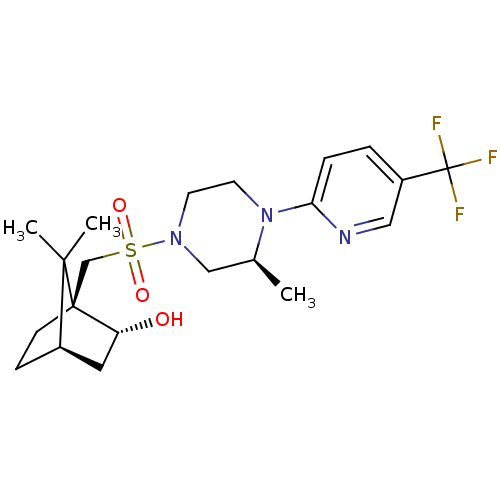 (CHEMBL465918) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human recombinant CXCR3 receptor expressed in CHO-K1 cells assessed as human IP10-induced calcium flux by FLIPR assay | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50381638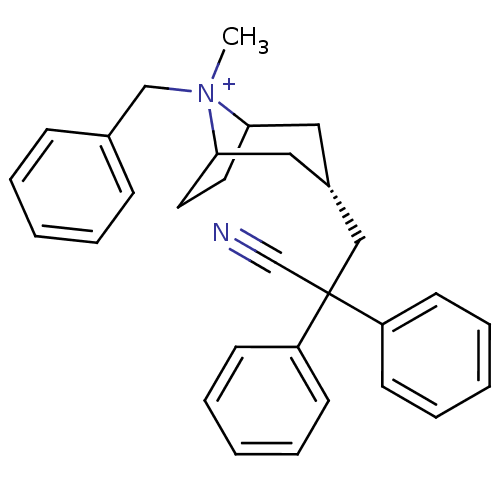 (CHEMBL2023755) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 318 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human muscarinic acetylcholine M3 receptor expressed in CHO cells assessed as inhibition of Ach-induced calcium mobilization b... | Bioorg Med Chem Lett 22: 3366-9 (2012) Article DOI: 10.1016/j.bmcl.2012.02.015 BindingDB Entry DOI: 10.7270/Q2C82B9Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213736 (7-chloro-1,1-dioxo-3-(2-phenoxy-phenylamino)-1,4-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50254678 ((1S,4R)-1-(((S)-4-(3-fluoro-5-(trifluoromethyl)pyr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 red (unknown origin) | Bioorg Med Chem Lett 19: 114-8 (2008) Article DOI: 10.1016/j.bmcl.2008.11.008 BindingDB Entry DOI: 10.7270/Q29Z94R0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213761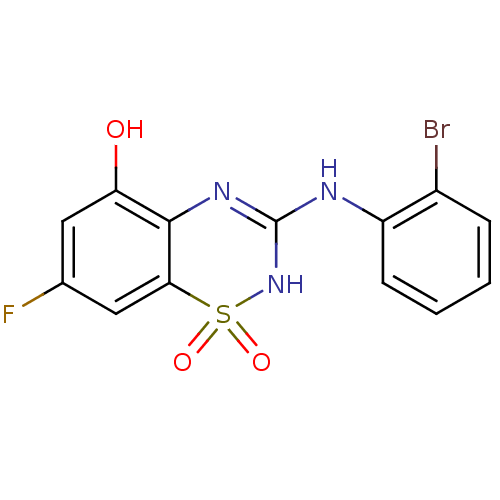 (3-(2-bromo-phenylamino)-7-fluoro-1,1-dioxo-1,4-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50213759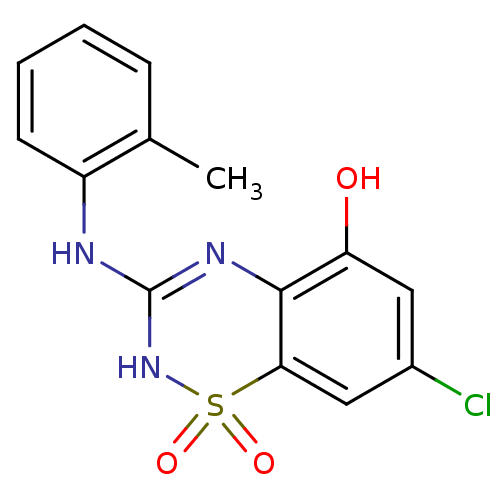 (7-chloro-1,1-dioxo-3-o-tolylamino-1,4-dihydro-1lam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at CXCR2 receptor expressed in CHOK1 cells assessed as human IL8-induced calcium mobilization by FLIPR | Bioorg Med Chem Lett 17: 3864-7 (2007) Article DOI: 10.1016/j.bmcl.2007.05.011 BindingDB Entry DOI: 10.7270/Q21C1WKG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 170 total ) | Next | Last >> |