

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM7842 (BAY 59-7939 Analog 17 | US8822458, 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7840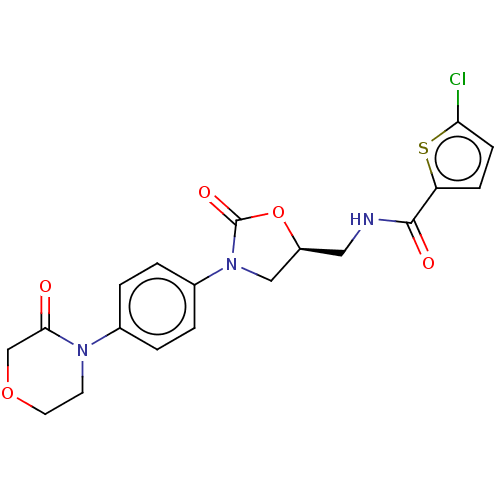 (RIVAROXABAN | US8822458, 44 | US8822458, 97) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12998 (5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12997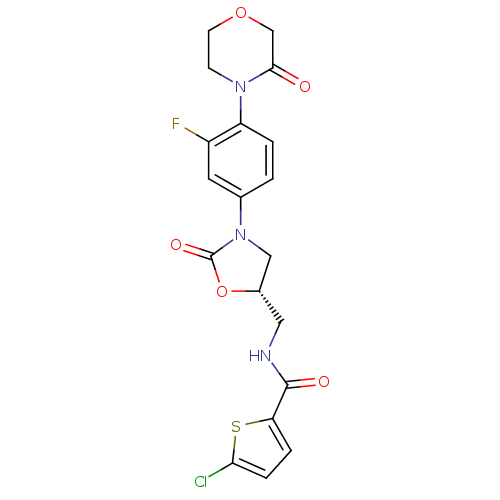 (5-Chloro-N-({(5S)-3-[3-fluoro-4-(3-oxomorpholin-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12999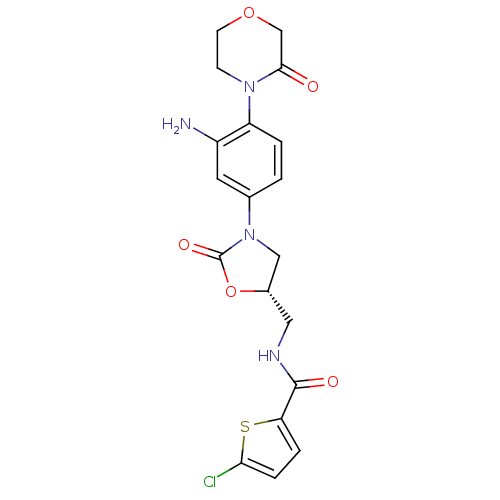 (BAY 59-7939 Analog 14 | N-({(5S)-3-[3-Amino-4-(3-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396564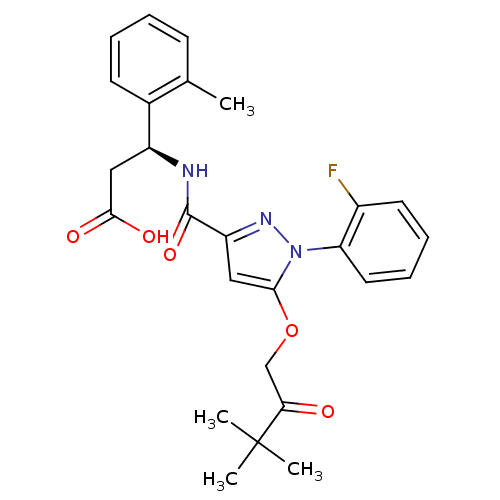 (CHEMBL2171390) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7552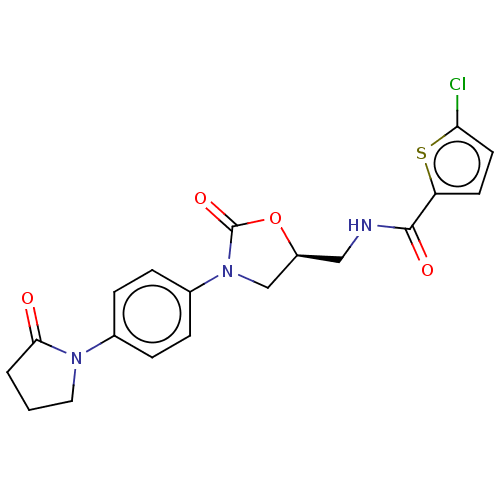 (BAY 59-7939 Analog 11 | US8822458, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7841 (BAY 59-7939 Analog 18 | US8822458, 45 | US9359341,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396562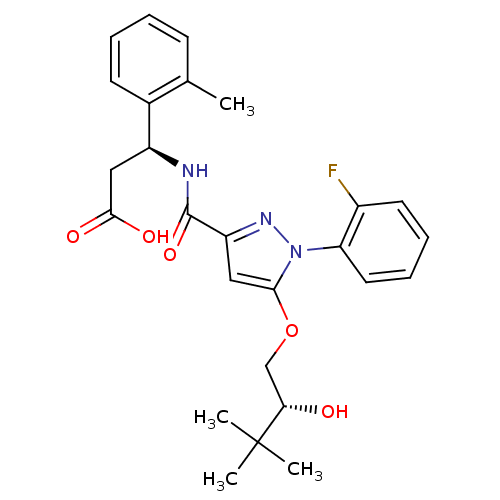 (CHEMBL2171392) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13008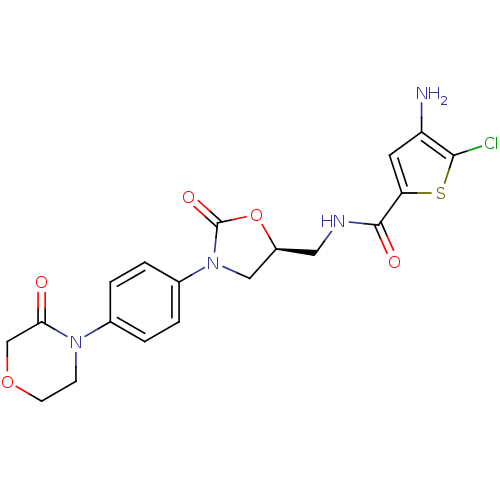 (4-Amino-5-chloro-N-({(5S)-2-oxo-3-[4-(3-oxomorphol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396560 (CHEMBL2171394) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396556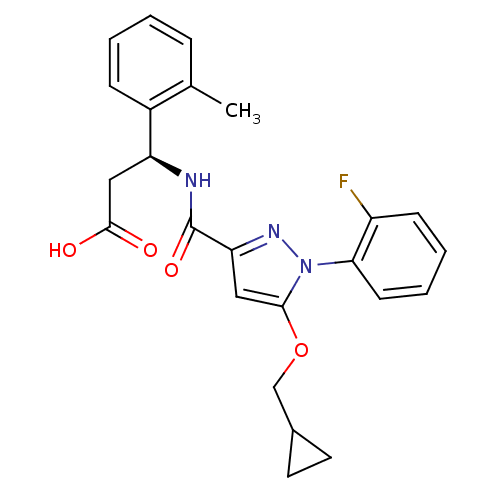 (CHEMBL2171398) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396557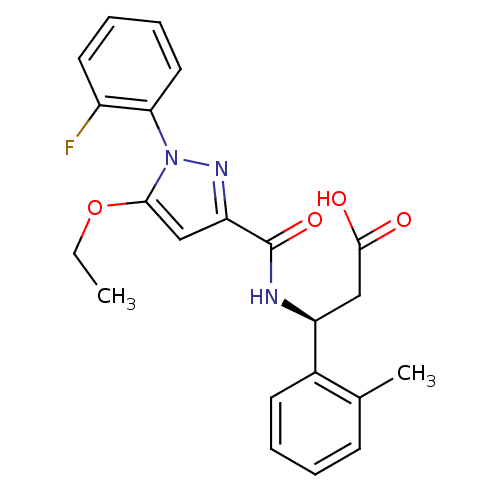 (CHEMBL2171397) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50396556 (CHEMBL2171398) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50396557 (CHEMBL2171397) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179693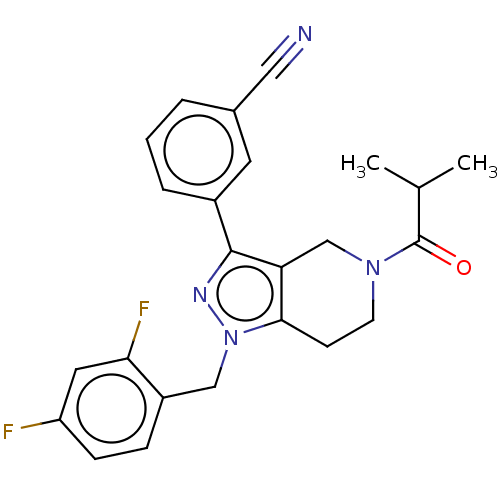 (US9127001, 8b | US9598410, Compound 8b) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliculated. Subsequently,... | US Patent US9127001 (2015) BindingDB Entry DOI: 10.7270/Q2HM576Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179693 (US9127001, 8b | US9598410, Compound 8b) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliated. Subsequently, TA... | US Patent US9598410 (2017) BindingDB Entry DOI: 10.7270/Q2377BRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13005 (4-chloro-N-{[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396583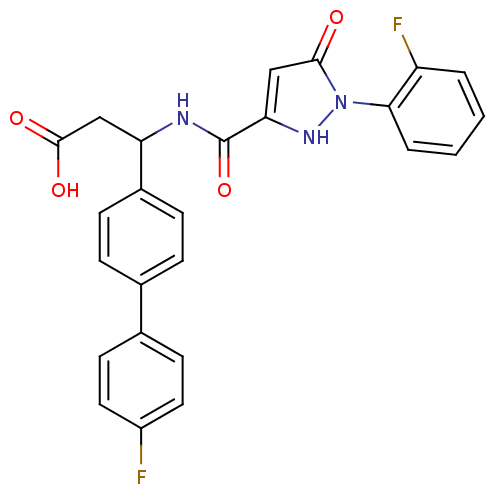 (CHEMBL2171404) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13004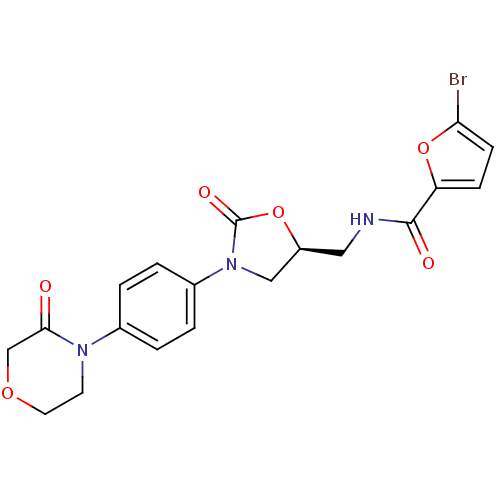 (5-Bromo-N-({(5S)-2-oxo-3-[4-(3-oxo-4-morpholinyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50396555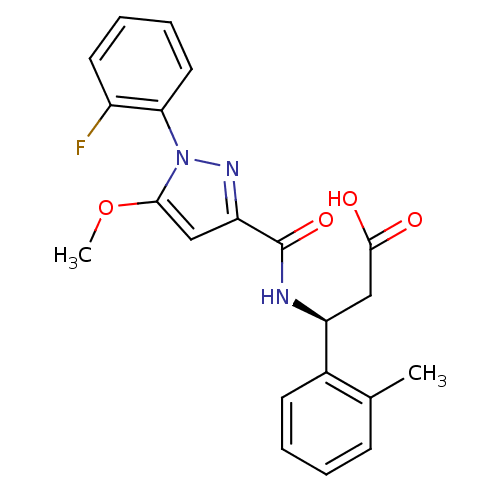 (CHEMBL2171396) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396555 (CHEMBL2171396) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13010 (5-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396587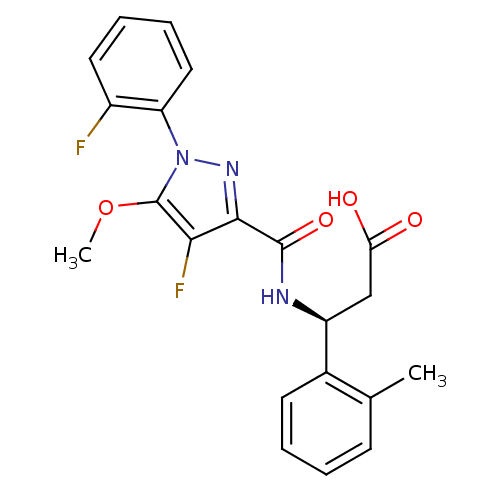 (CHEMBL2171399) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12990 (5-Chloro-N-{[(5S)-3-(3-fluoro-4-morpholin-4-ylphen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50396558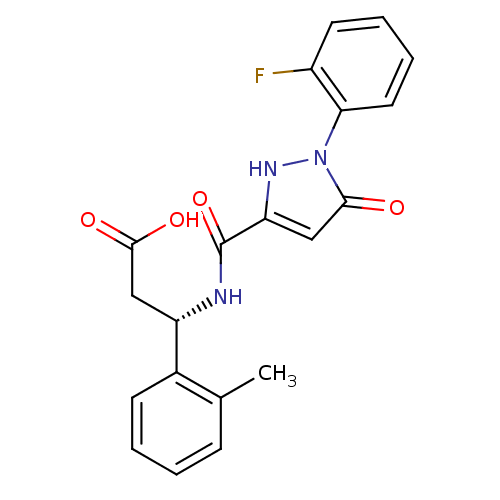 (CHEMBL2171403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of CYP3A4 | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396558 (CHEMBL2171403) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12992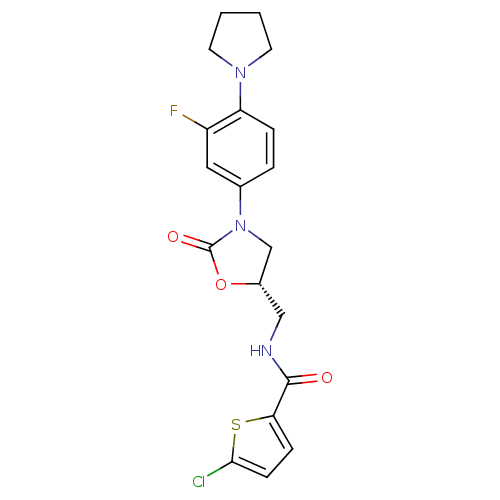 (5-Chloro-N-{[(5S)-3-(3-fluoro-4-pyrrolidin-1-ylphe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM7495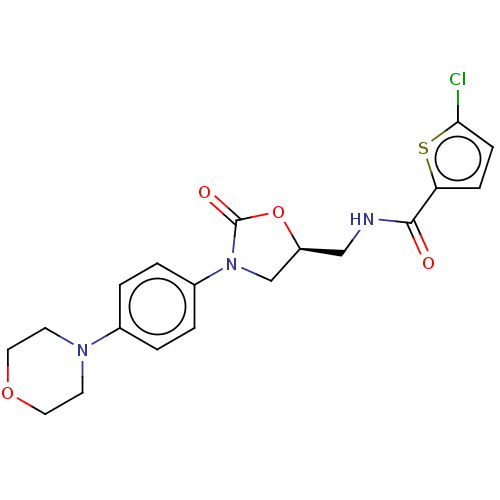 (BAY 59-7939 Analog 7 | US8822458, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179694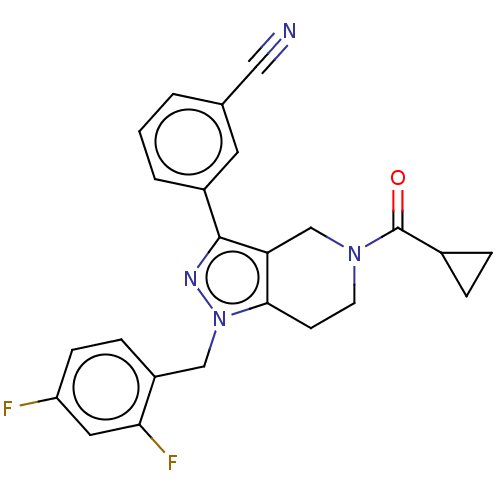 (US9127001, 8c | US9598410, Compound 8c) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliculated. Subsequently,... | US Patent US9127001 (2015) BindingDB Entry DOI: 10.7270/Q2HM576Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179694 (US9127001, 8c | US9598410, Compound 8c) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliated. Subsequently, TA... | US Patent US9598410 (2017) BindingDB Entry DOI: 10.7270/Q2377BRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396561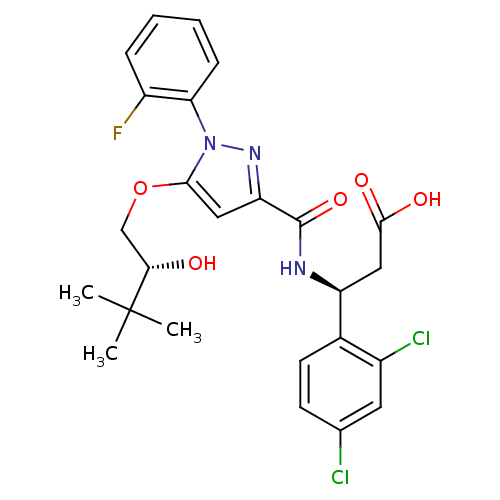 (CHEMBL2171393) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396582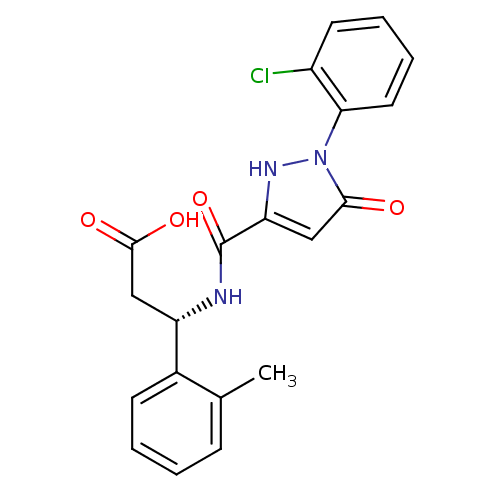 (CHEMBL2171405) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396581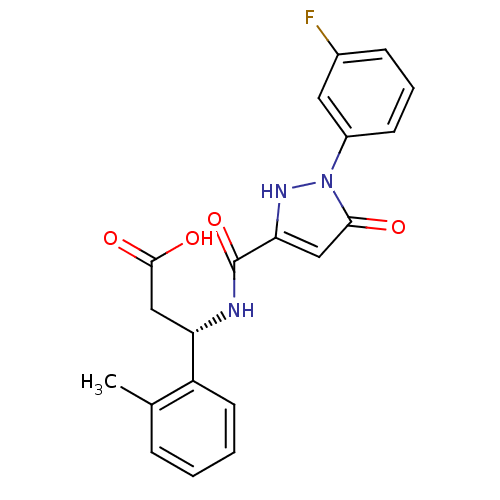 (CHEMBL2171406) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396563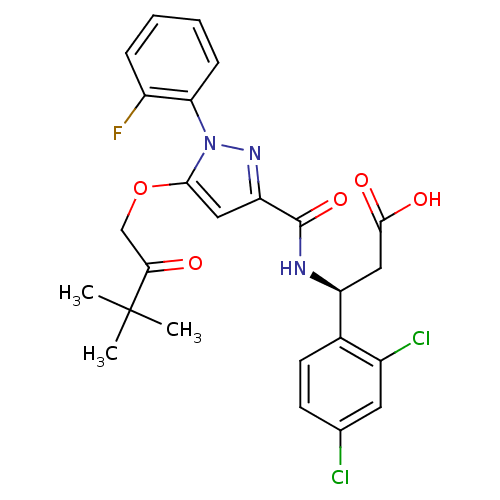 (CHEMBL2171391) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179702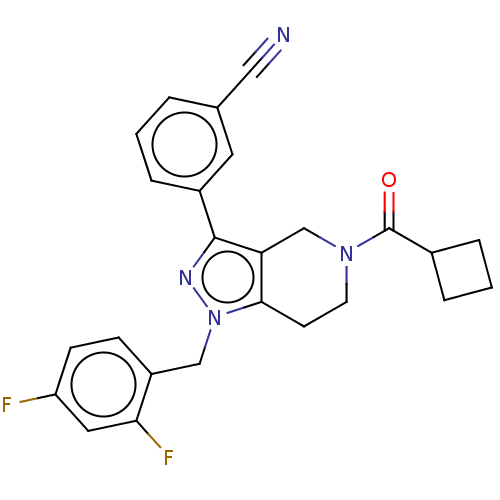 (US9127001, 8i | US9598410, Compound 8i) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliated. Subsequently, TA... | US Patent US9598410 (2017) BindingDB Entry DOI: 10.7270/Q2377BRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179702 (US9127001, 8i | US9598410, Compound 8i) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliculated. Subsequently,... | US Patent US9127001 (2015) BindingDB Entry DOI: 10.7270/Q2HM576Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM159297 (US9034897, 14a) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliculated. Subsequently,... | US Patent US9034897 (2015) BindingDB Entry DOI: 10.7270/Q23R0RNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396566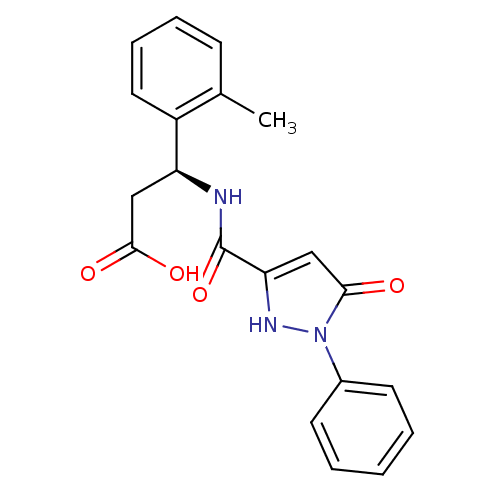 (CHEMBL2171388) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396559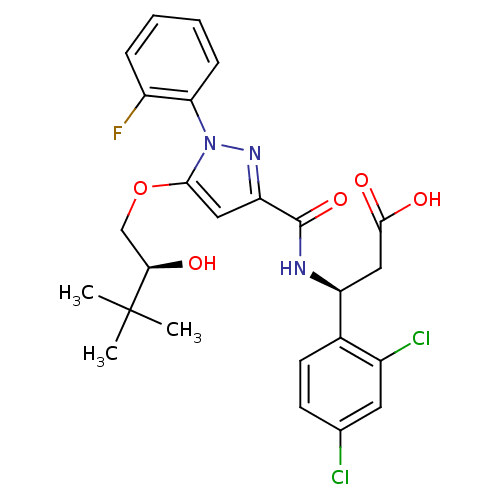 (CHEMBL2171395) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12993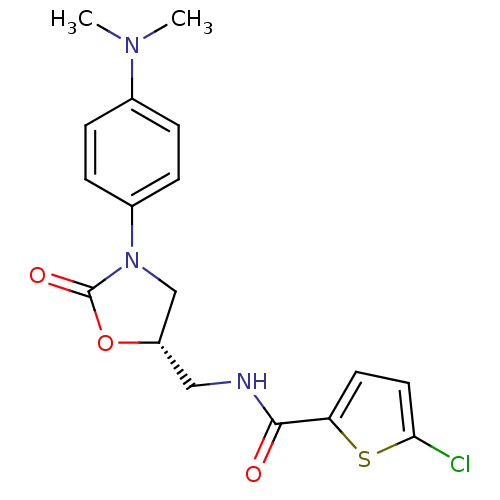 (5-Chloro-N-({(5S)-3-[4-(dimethylamino)phenyl]-2-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396580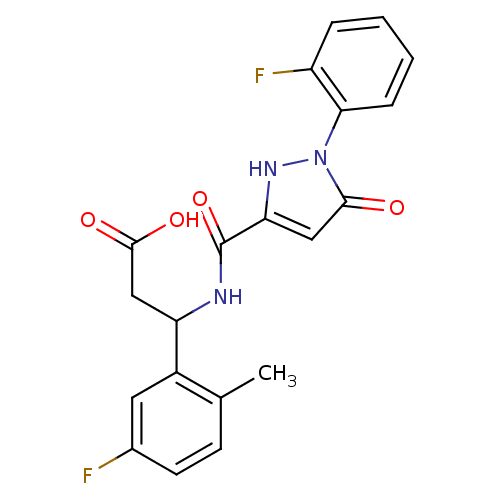 (CHEMBL2169902) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM159298 (US9034897, 14b) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 83.1 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliculated. Subsequently,... | US Patent US9034897 (2015) BindingDB Entry DOI: 10.7270/Q23R0RNR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysosomal protective protein (Homo sapiens (Human)) | BDBM50396578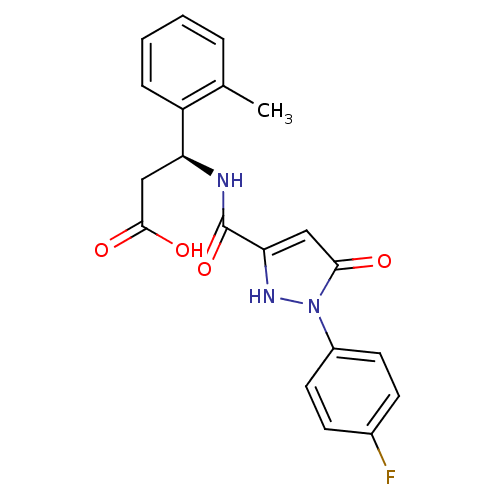 (CHEMBL2171604) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Aventis Deutschland GmbH Curated by ChEMBL | Assay Description Inhibition of human recombinant Myc-His10-tagged cathepsin A expressed in baculovirus infected Sf9 cells using BodipyFL labeled bradykinin as substra... | J Med Chem 55: 7636-49 (2012) Article DOI: 10.1021/jm300663n BindingDB Entry DOI: 10.7270/Q2SJ1MRZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12988 (5-Chloro-N-{[(5S)-3-(3-fluoro-4-thiomorpholin-4-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | 8.3 | 25 |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179635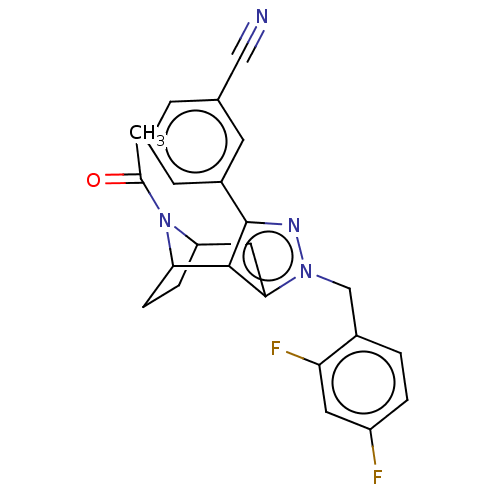 (US9127001, 6n | US9598410, Compound 6n) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliated. Subsequently, TA... | US Patent US9598410 (2017) BindingDB Entry DOI: 10.7270/Q2377BRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179635 (US9127001, 6n | US9598410, Compound 6n) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliculated. Subsequently,... | US Patent US9127001 (2015) BindingDB Entry DOI: 10.7270/Q2HM576Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13011 (6-Chloro-N-({(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer HealthCare AG | Assay Description The enzymatic activity was measured using chromogenic or fluorogenic substrates in 96-well microtiter plates.Color change was monitored continuously ... | J Med Chem 48: 5900-8 (2005) Article DOI: 10.1021/jm050101d BindingDB Entry DOI: 10.7270/Q2J38QR6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179623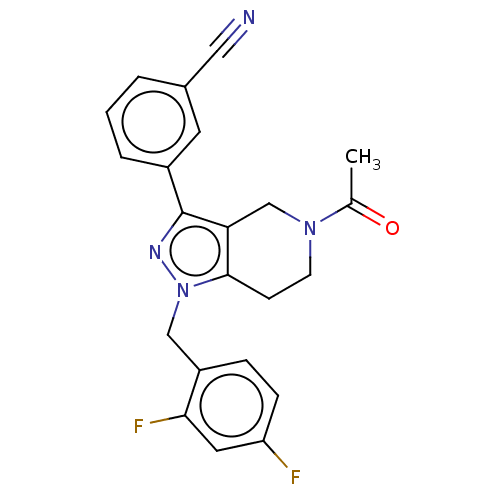 (US9127001, 6a | US9598410, Compound 6a) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | 25 |
Sanofi US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliculated. Subsequently,... | US Patent US9127001 (2015) BindingDB Entry DOI: 10.7270/Q2HM576Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium channel subfamily K member 3 (Homo sapiens (Human)) | BDBM179623 (US9127001, 6a | US9598410, Compound 6a) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
SANOFI US Patent | Assay Description Human TASK-1 channels were expressed in Xenopus oocytes. For this purpose, oocytes were isolated from Xenopus laevis and defoliated. Subsequently, TA... | US Patent US9598410 (2017) BindingDB Entry DOI: 10.7270/Q2377BRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 300 total ) | Next | Last >> |