

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagenase 3 (Homo sapiens (Human)) | BDBM30369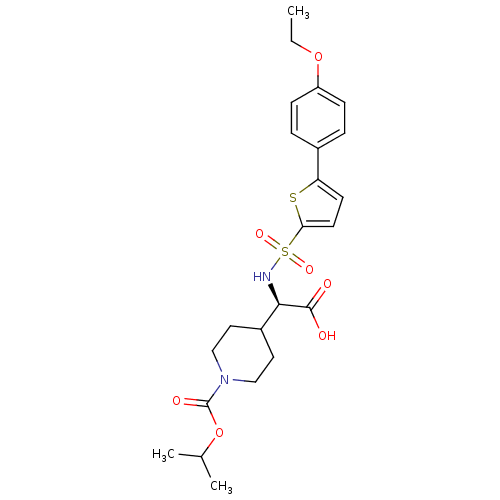 (piperidinyl glycine derivative, 24f) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -54.9 | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM11863 (4-({[4-(4-chlorophenoxy)benzene]sulfonyl}methyl)-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.280 | -54.0 | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30344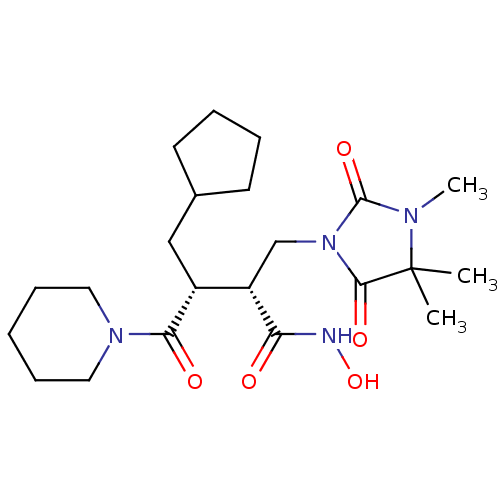 (Cipemastat | Trocade) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | -52.4 | 3.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM8465 ((2R)-N-hydroxy-2-[(4-methoxybenzene)(pyridin-3-ylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.27 | -50.3 | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30345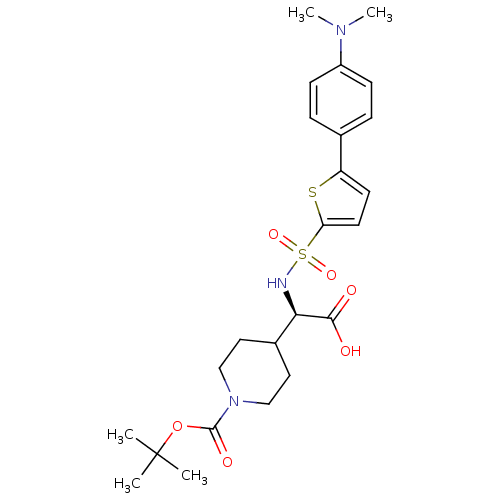 (BOC-piperidinyl glycine derivative, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30356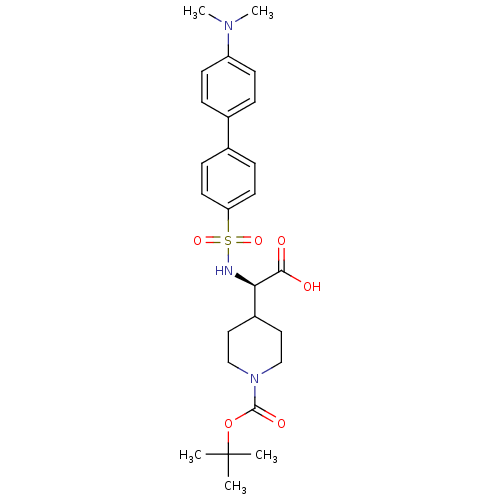 (BOC-piperidinyl glycine derivative, 31) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30380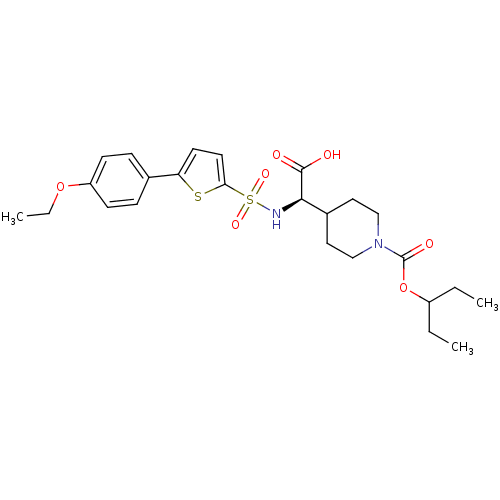 (piperidinyl glycine derivative, 24q) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324297 (4-(1-Piperazin-1-yl[2,6]naphthyridin-3-yl)pyridin-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30384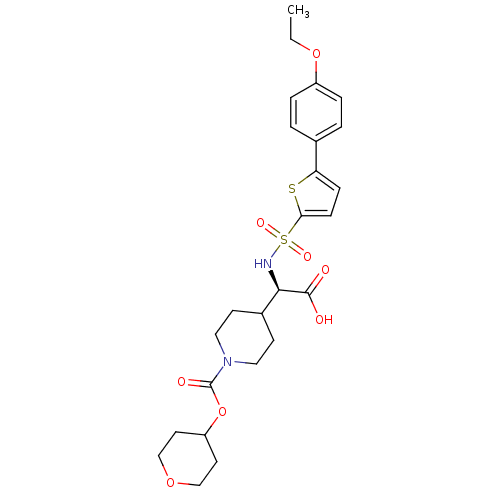 (piperidinyl glycine derivative, 24u) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30383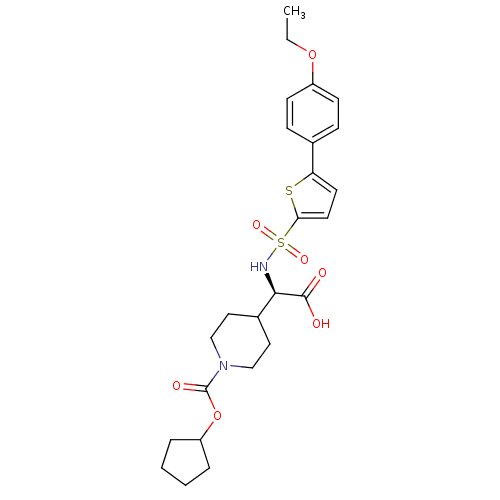 (piperidinyl glycine derivative, 24t) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30359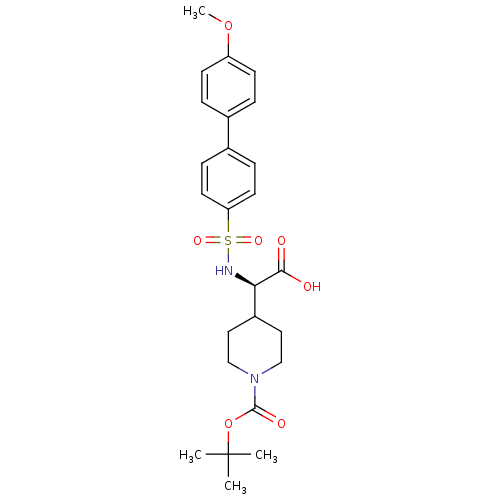 (BOC-piperidinyl glycine derivative, 34) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30360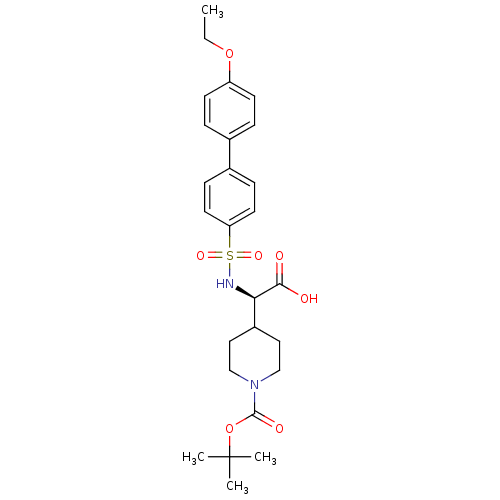 (BOC-piperidinyl glycine derivative, 35) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30367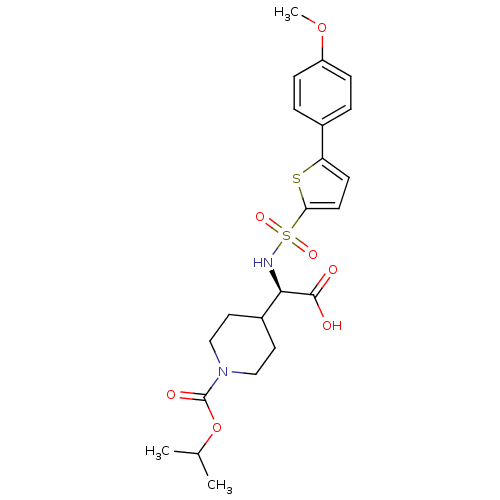 (piperidinyl glycine derivative, 24d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30382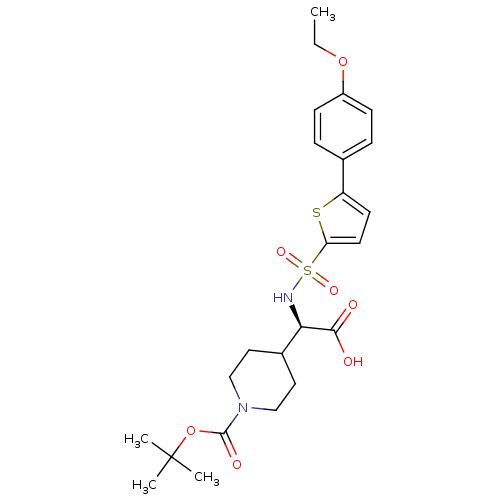 (piperidinyl glycine derivative, 24s) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324315 (CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30379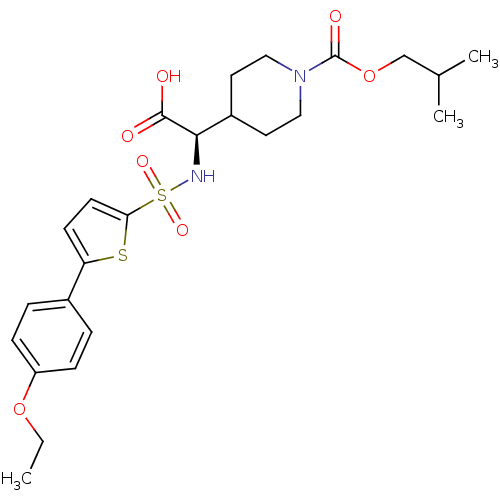 (piperidinyl glycine derivative, 24p) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324315 (CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30357 (BOC-piperidinyl glycine derivative, 32) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444549 (CHEMBL3099695) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30396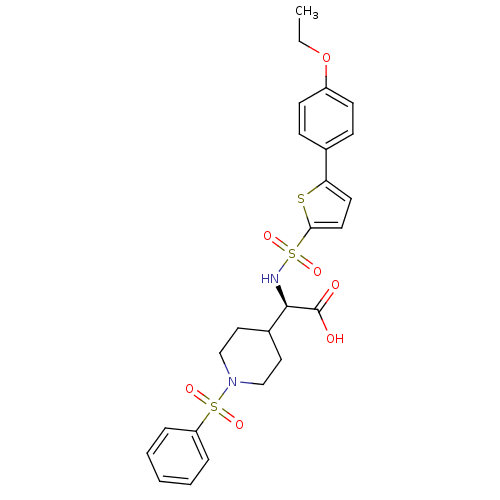 (piperidinyl glycine derivative, 27c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30397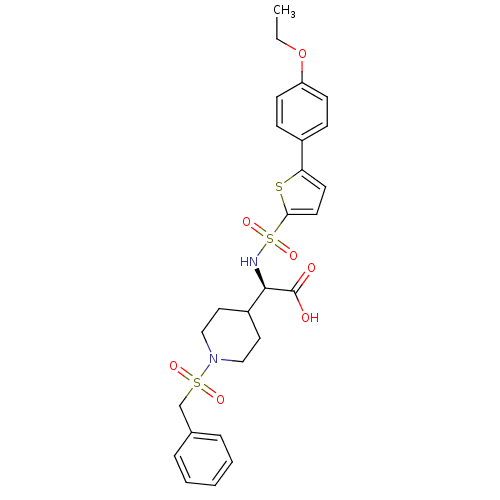 (piperidinyl glycine derivative, 27d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30347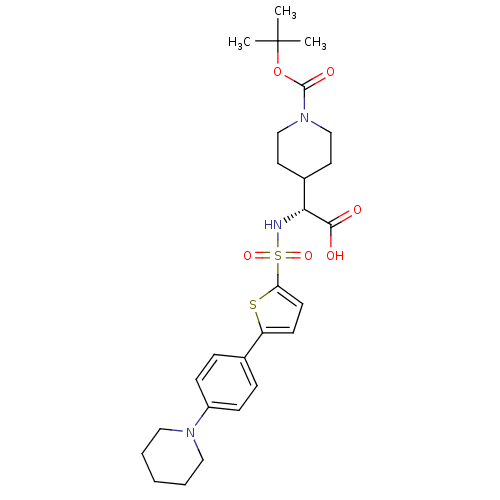 (BOC-piperidinyl glycine derivative, 22a) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30370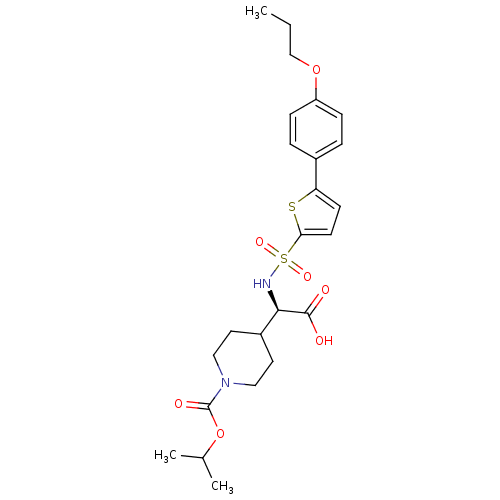 (piperidinyl glycine derivative, 24g) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30387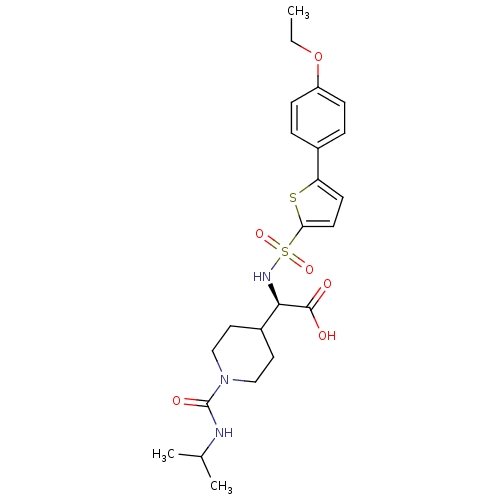 (piperidinyl glycine derivative, 25c) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30378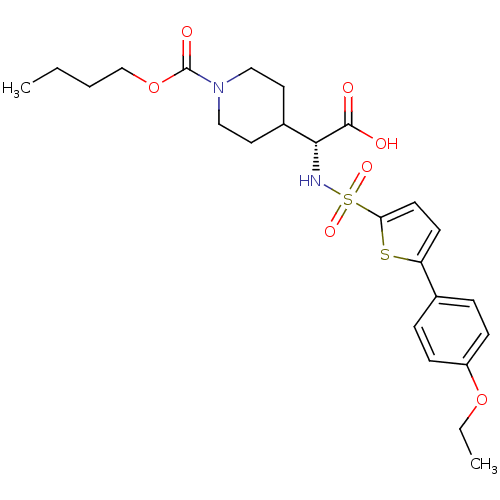 (piperidinyl glycine derivative, 24o) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30381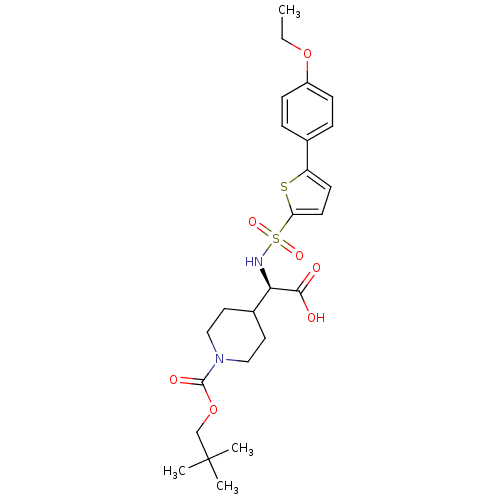 (piperidinyl glycine derivative, 24r) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444548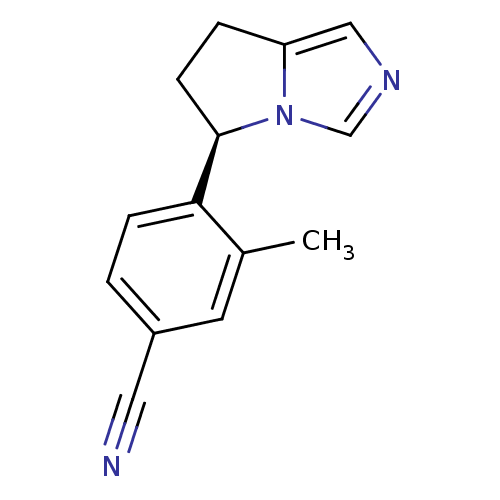 (CHEMBL3099696) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30392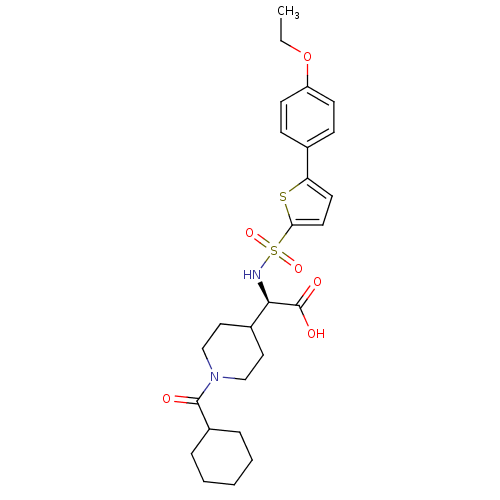 (piperidinyl glycine derivative, 26d) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30372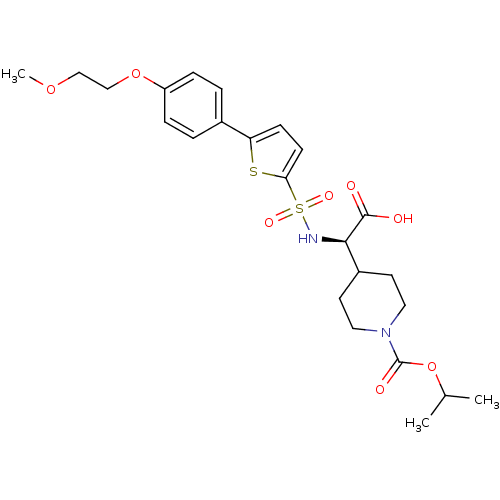 (piperidinyl glycine derivative, 24i) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B2 using 11-deoxycorticosterone as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324347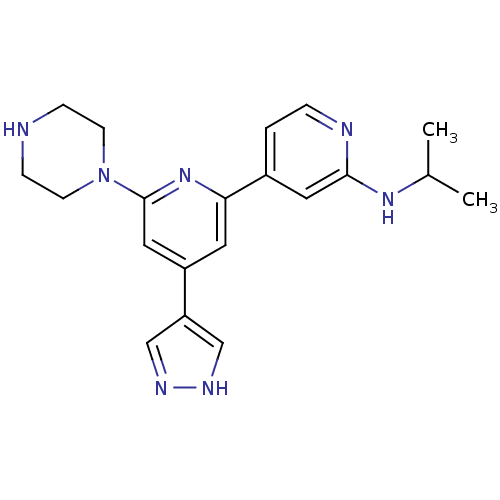 (CHEMBL1215153 | Isopropyl-[6-piperazin-1-yl-4-(1H-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324314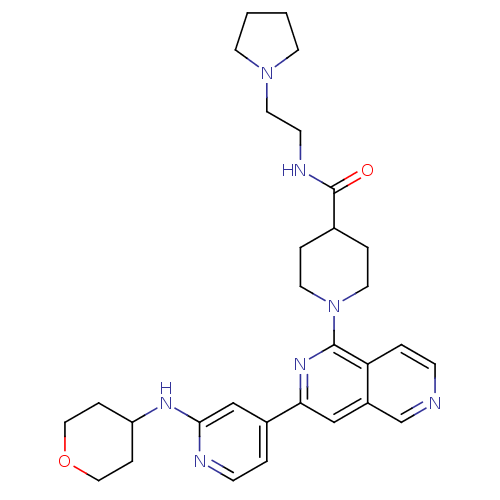 (1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324298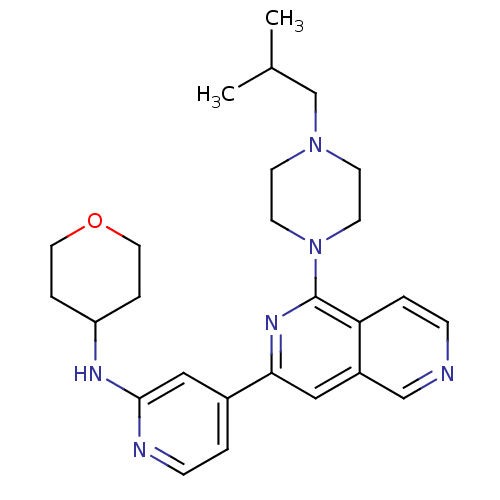 (CHEMBL1215712 | {4-[1-(4-Isobutylpiperazin-1-yl)[2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM30377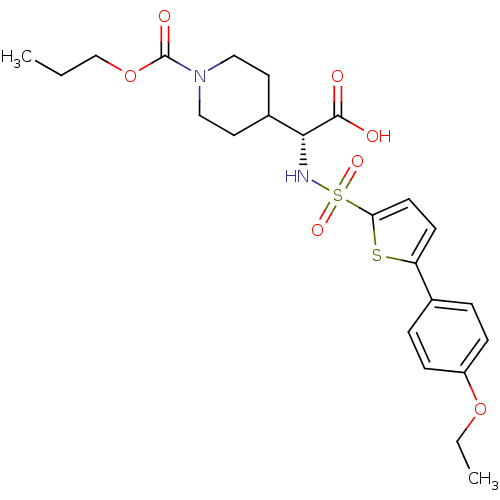 (piperidinyl glycine derivative, 24n) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 25 |
Novartis | Assay Description Test compounds were serially diluted in the assay buffer. In each well of a 96-well microtiter plate (Immunofluor B, Dynatech), the inhibitor solutio... | J Med Chem 52: 3523-38 (2009) Article DOI: 10.1021/jm801394m BindingDB Entry DOI: 10.7270/Q2B27SN3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B2, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of CYP11B2 in human NCI-H295R cells assessed as inhibition of angiotensin-2-induced aldosterone production after 24 hrs by RIA | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324325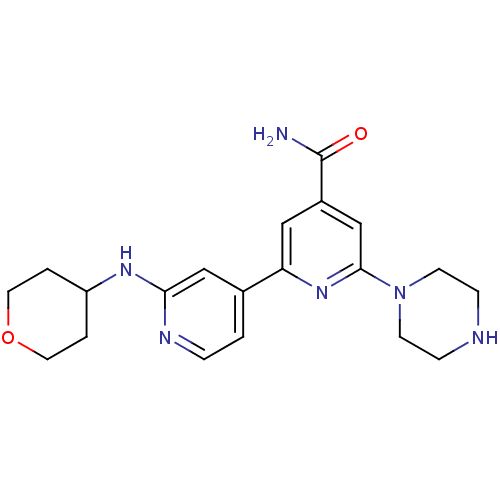 (6-Piperazin-1-yl-2'-(tetrahydropyran-4-ylamino)[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324326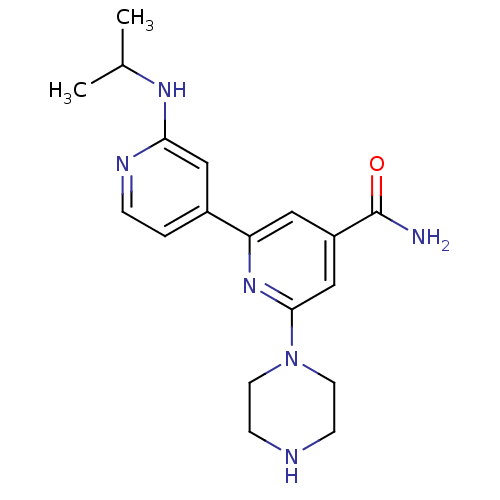 (4'-tert-Butylcarbamoyl-2''-isopropylamino-3,4,5,6-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324324 (2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324327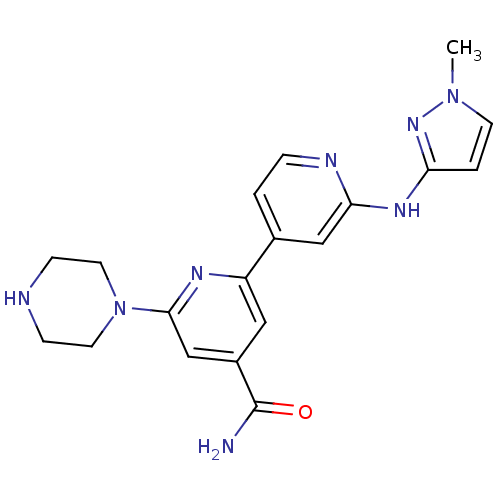 (2'-(1-Methyl-1H-pyrazol-3-ylamino)-6-piperazin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324328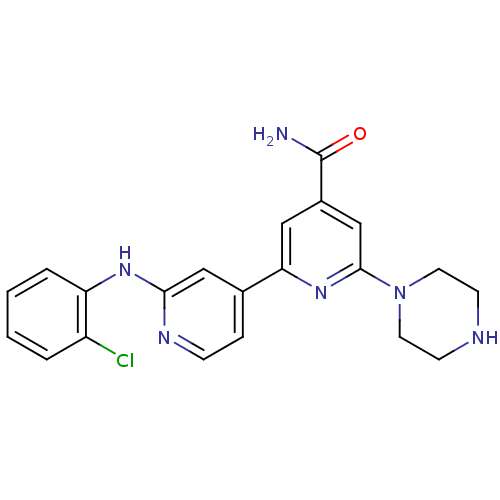 (2'-(2-Chlorophenylamino)-6-piperazin-1-yl[2,4']bip...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324323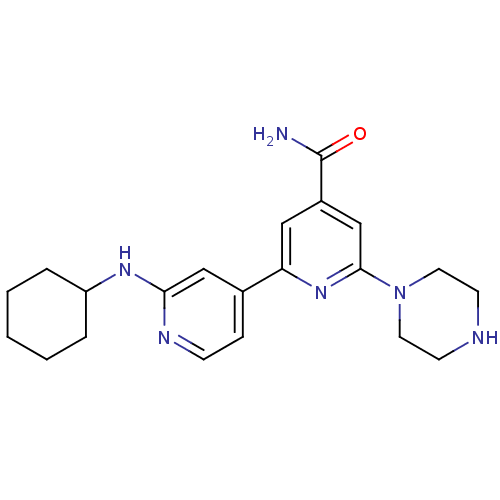 (2'-Cyclohexylamino-6-piperazin-1-yl[2,4']bipyridin...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324348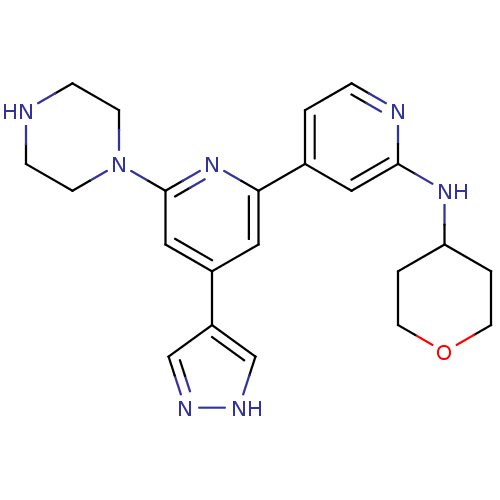 (6-(piperazin-1-yl)-4-(4H-pyrazol-4-yl)-N-(tetrahyd...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324346 (CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324322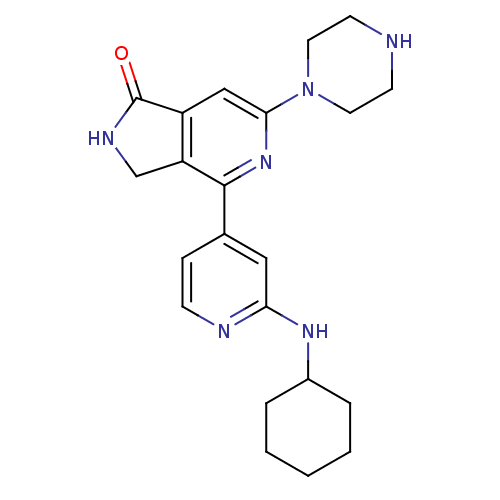 (4-(2-cyclohexylaminopyridin-4-yl)-6-(piperazin-1-y...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycystin-2 (Homo sapiens (Human)) | BDBM50324324 (2'-Phenylamino-6-piperazin-1-yl[2,4']bipyridinyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD2 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycystin-2 (Homo sapiens (Human)) | BDBM50324346 (CHEMBL1215151 | Cyclohexyl-[6-piperazin-1-yl-4-(1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD2 by TR-FRET assay | J Med Chem 53: 5422-38 (2010) Article DOI: 10.1021/jm100076w BindingDB Entry DOI: 10.7270/Q2P84C2J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324306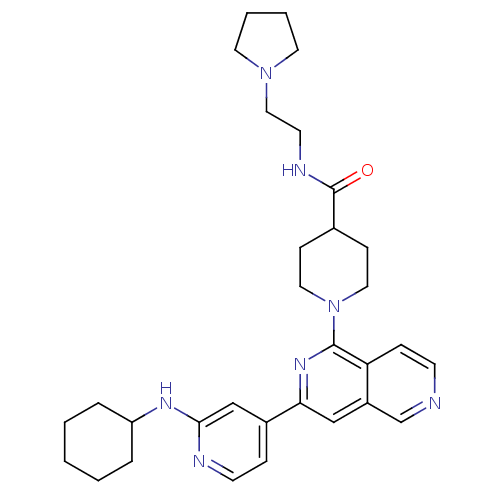 (1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 345 total ) | Next | Last >> |