

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120502 (2-Amino-N-[(R)-2-(3a-benzyl-2-tert-butyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120504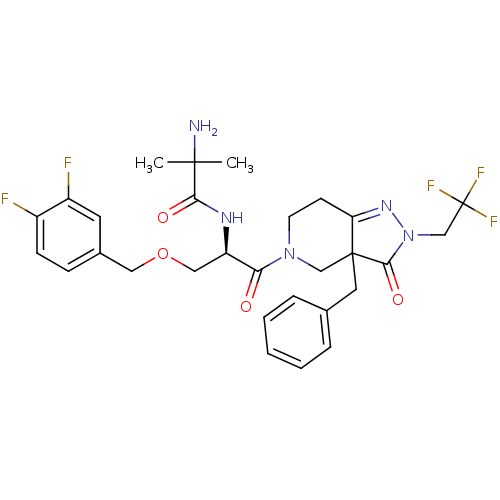 (2-Amino-N-[(R)-2-[3a-benzyl-3-oxo-2-(2,2,2-trifluo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120505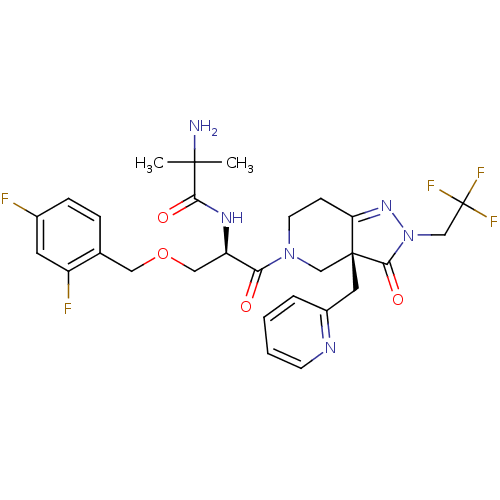 (2-Amino-N-{(R)-1-(2,4-difluoro-benzyloxymethyl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50083974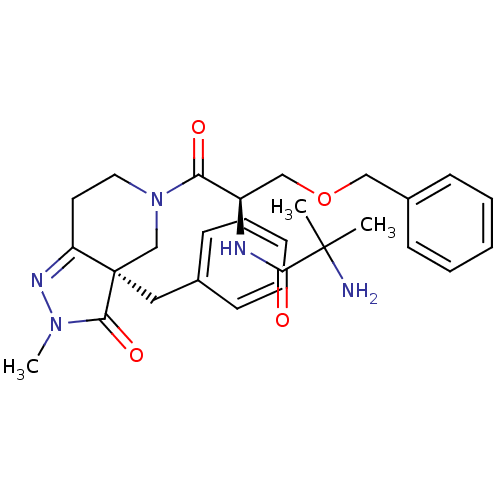 (2-Amino-N-[(R)-2-((R)-3a-benzyl-2-methyl-3-oxo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50120503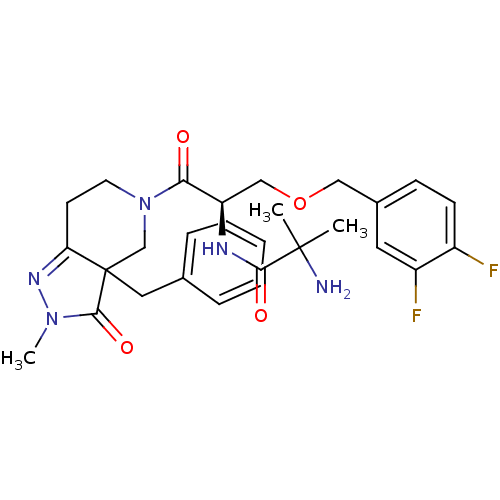 (2-Amino-N-[(R)-2-(3a-benzyl-2-methyl-3-oxo-2,3,3a,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research & Development Curated by ChEMBL | Assay Description In vitro binding affinity of the compound was determined against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]-ghrelin ... | Bioorg Med Chem Lett 12: 3279-82 (2002) BindingDB Entry DOI: 10.7270/Q23F4NZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246239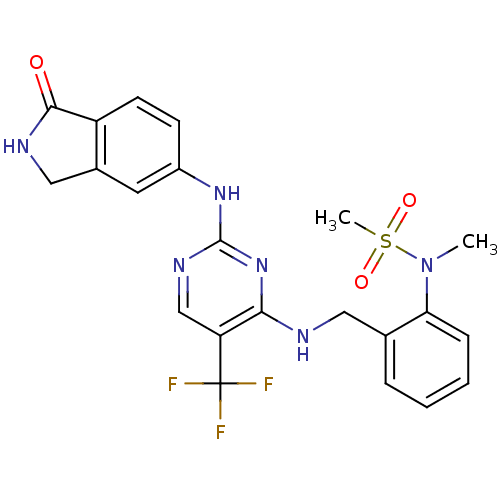 (CHEMBL487229 | N-methyl-N-(2-((2-(1-oxoisoindolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146201 (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246286 (CHEMBL488247 | N-methyl-N-(2-((2-(4-(methylsulfony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246378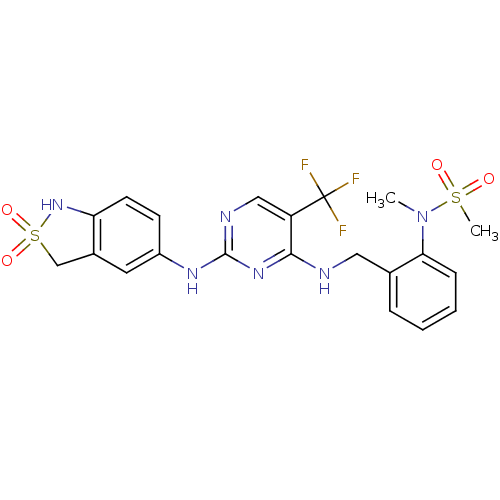 (CHEMBL452341 | N-(2-{[2-(2,2-Dioxo-2,3-dihydro-1H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246060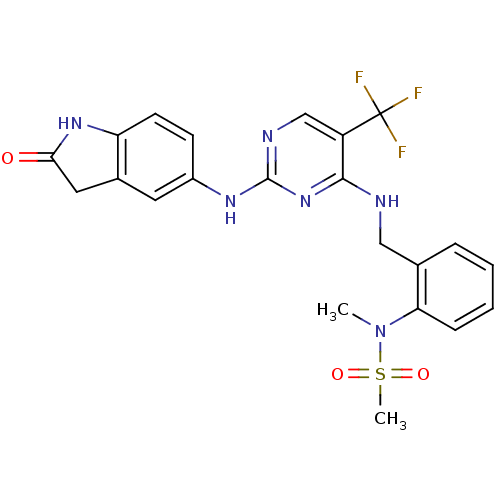 (CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of purified activated FAK kinase domain (410-689) using ATP and Glu and Tyr random peptide polymer substrate by fluorescence polarization ... | Bioorg Med Chem Lett 19: 3253-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.093 BindingDB Entry DOI: 10.7270/Q2GH9J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50181274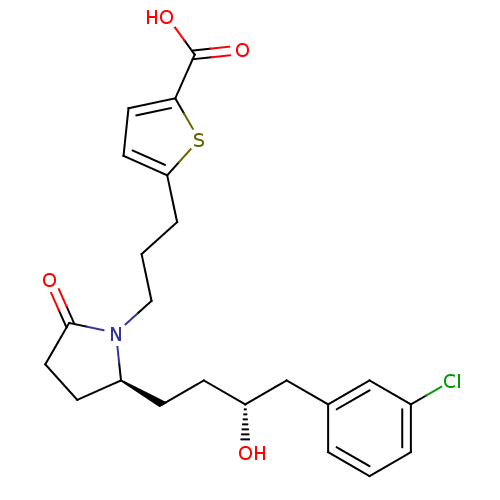 (5-(3-((S)-2-((R)-4-(3-chlorophenyl)-3-hydroxybutyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to rat EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 1799-802 (2006) Article DOI: 10.1016/j.bmcl.2006.01.018 BindingDB Entry DOI: 10.7270/Q2SQ8ZZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146201 (6-Phenyl-5-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-5...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246060 (CHEMBL472212 | CHEMBL541649 | D3RKN_6 | N-methyl-N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246140 (CHEMBL513744 | D3RKN_5 | N-methyl-N-(3-((2-(2-oxoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50246378 (CHEMBL452341 | N-(2-{[2-(2,2-Dioxo-2,3-dihydro-1H-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 2075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.059 BindingDB Entry DOI: 10.7270/Q2PR7W18 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146206 (2-Benzyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-phenyl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146200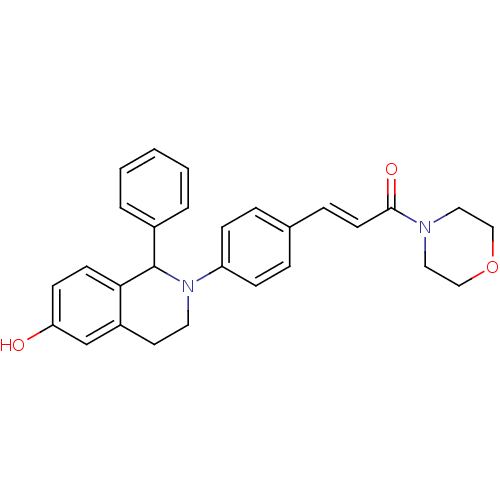 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50246239 (CHEMBL487229 | N-methyl-N-(2-((2-(1-oxoisoindolin-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Rattus norvegicus) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of rat EP2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 19: 2075-8 (2009) Article DOI: 10.1016/j.bmcl.2009.01.059 BindingDB Entry DOI: 10.7270/Q2PR7W18 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146223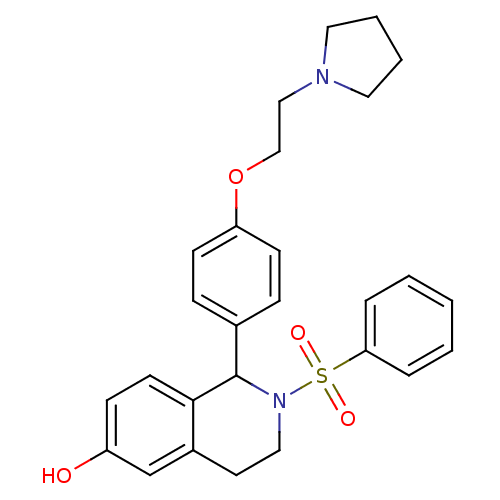 (2-Benzenesulfonyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146216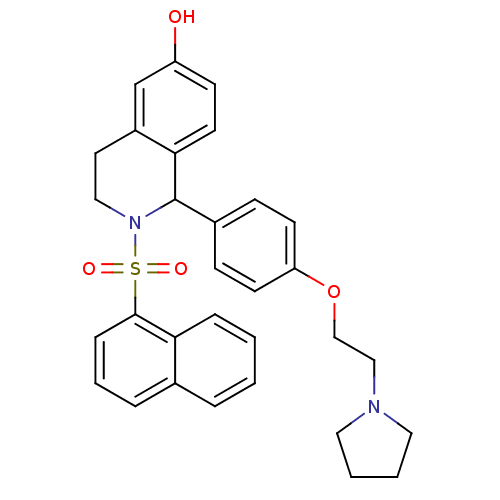 (2-(Naphthalene-1-sulfonyl)-1-[4-(2-pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146200 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50181298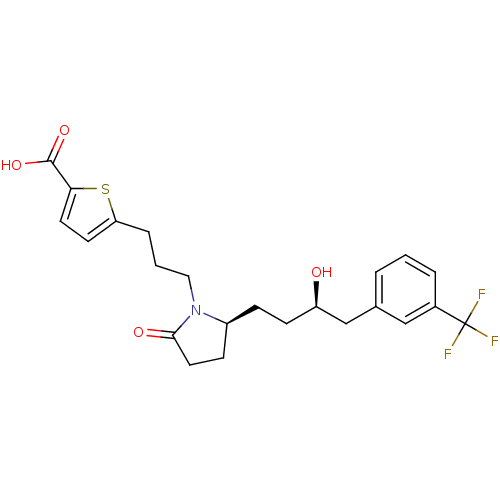 (5-(3-((S)-2-((R)-3-hydroxy-4-(3-(trifluoromethyl)p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to rat EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 1799-802 (2006) Article DOI: 10.1016/j.bmcl.2006.01.018 BindingDB Entry DOI: 10.7270/Q2SQ8ZZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50246330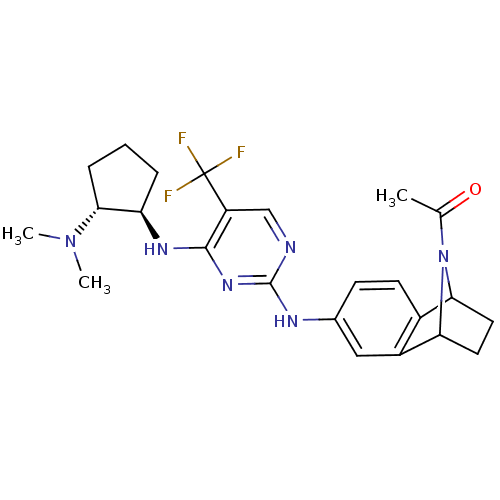 (1-{6-[4-((1R,2R)-2-Dimethylamino-cyclopentylamino)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50402996 (CHEMBL2207440) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His-tagged PYK2 using ATP as substrate incubated for 1 hr prior to substrate addition | Bioorg Med Chem Lett 22: 7523-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.039 BindingDB Entry DOI: 10.7270/Q2319X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146222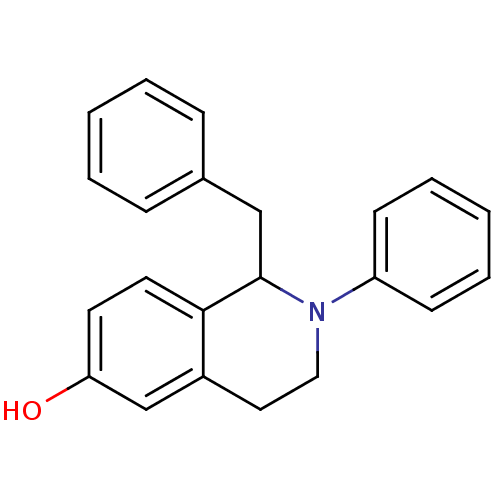 (1-Benzyl-2-phenyl-1,2,3,4-tetrahydro-isoquinolin-6...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146199 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146199 ((E)-3-[4-(6-Hydroxy-1-phenyl-3,4-dihydro-1H-isoqui...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146220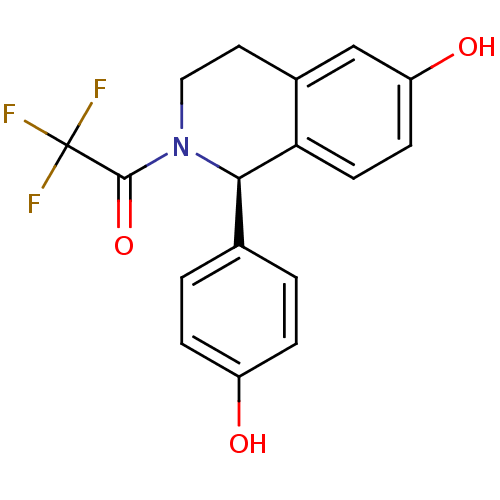 (2,2,2-Trifluoro-1-[(R)-6-hydroxy-1-(4-hydroxy-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246187 (5-(4-(3-(methylsulfonyl)benzylamino)-5-(trifluorom...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146205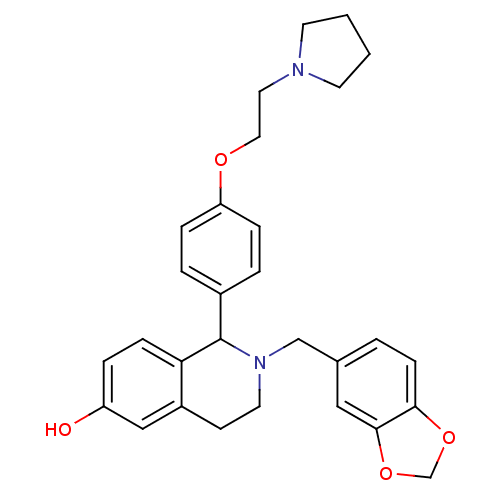 (2-Benzo[1,3]dioxol-5-ylmethyl-1-[4-(2-pyrrolidin-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50418579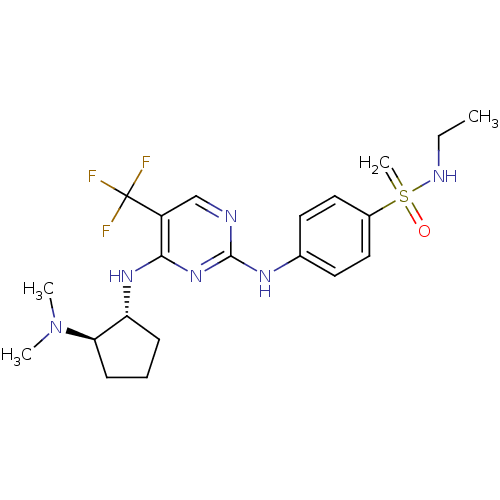 (CHEMBL2029181) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PYK2 by PYK2-LI-COR cellular assay | Bioorg Med Chem Lett 19: 3253-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.093 BindingDB Entry DOI: 10.7270/Q2GH9J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146207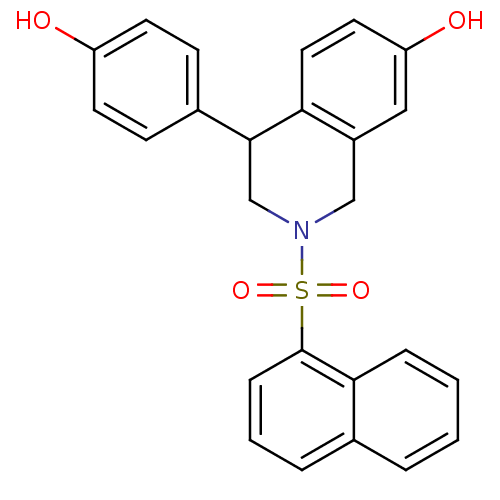 (4-(4-Hydroxy-phenyl)-2-(naphthalene-1-sulfonyl)-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146211 (2,2,2-Trifluoro-1-[7-hydroxy-4-(4-hydroxy-phenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM20585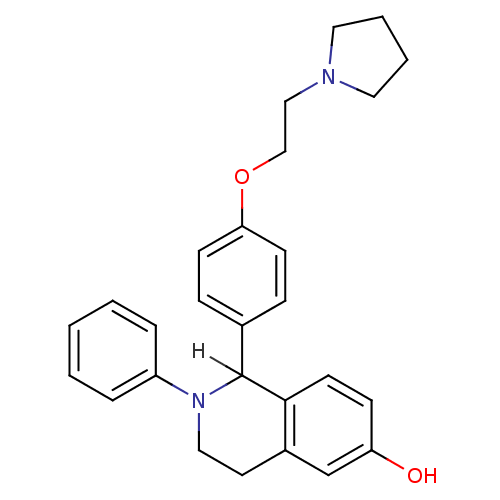 (2-phenyl-1-{4-[2-(pyrrolidin-1-yl)ethoxy]phenyl}-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50402999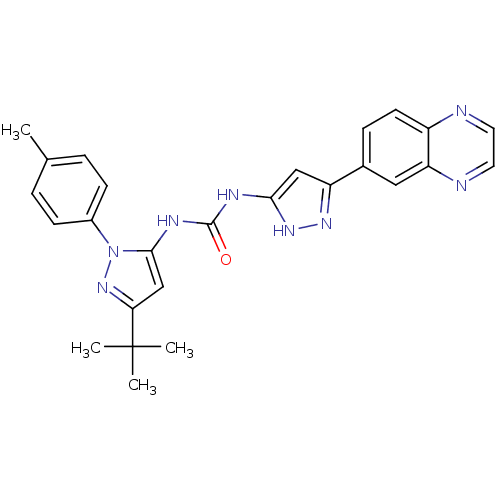 (CHEMBL2207441) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant C-terminal His-tagged PYK2 using ATP as substrate incubated for 1 hr prior to substrate addition | Bioorg Med Chem Lett 22: 7523-9 (2012) Article DOI: 10.1016/j.bmcl.2012.10.039 BindingDB Entry DOI: 10.7270/Q2319X2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146198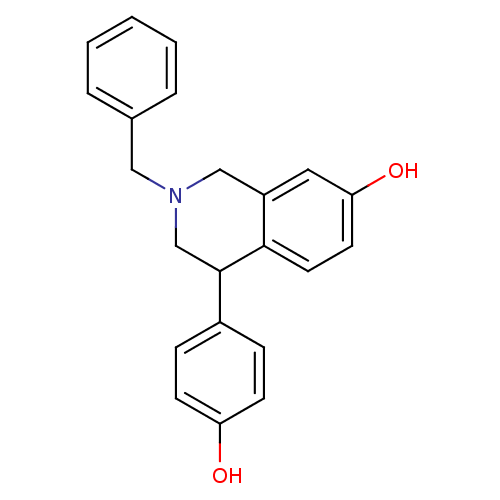 (2-Benzyl-4-(4-hydroxy-phenyl)-1,2,3,4-tetrahydro-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50246188 (5-(4-(2-(methylsulfonyl)benzylamino)-5-(trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246096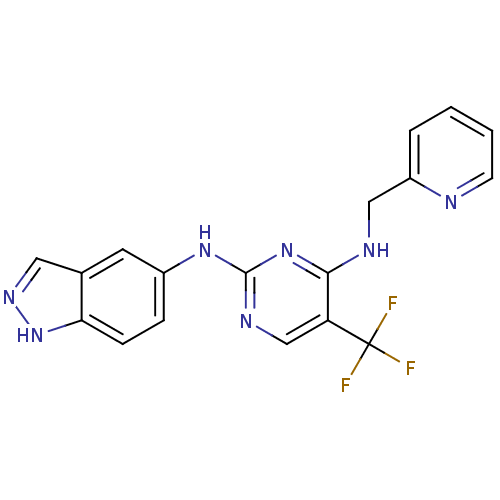 (CHEMBL471526 | N2-(1H-indazol-5-yl)-N4-(pyridin-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50418579 (CHEMBL2029181) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PYK2 assessed as reduction in peptide substrate phosphoryltion by fluorimetric method | Bioorg Med Chem Lett 19: 3253-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.093 BindingDB Entry DOI: 10.7270/Q2GH9J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50246189 (5-(4-(2-(pyrrolidin-1-ylsulfonyl)benzylamino)-5-(t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged pyk2 (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50181289 (5-(3-((S)-2-(4-(3-fluorophenyl)-3-hydroxybutyl)-5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to rat EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 1799-802 (2006) Article DOI: 10.1016/j.bmcl.2006.01.018 BindingDB Entry DOI: 10.7270/Q2SQ8ZZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146223 (2-Benzenesulfonyl-1-[4-(2-pyrrolidin-1-yl-ethoxy)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246139 (5-(4-(pyridin-2-ylmethylamino)-5-(trifluoromethyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50146198 (2-Benzyl-4-(4-hydroxy-phenyl)-1,2,3,4-tetrahydro-i...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor alpha expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50146216 (2-(Naphthalene-1-sulfonyl)-1-[4-(2-pyrrolidin-1-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description In vitro concentration required to inhibit [3H]-estradiol binding to human estrogen receptor beta expressed in 293T cells | Bioorg Med Chem Lett 14: 2729-33 (2004) Article DOI: 10.1016/j.bmcl.2004.03.077 BindingDB Entry DOI: 10.7270/Q2J67GBK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50142488 (7-((S)-2-((R)-3-hydroxy-4-(3-(trifluoromethyl)phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to rat EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 1799-802 (2006) Article DOI: 10.1016/j.bmcl.2006.01.018 BindingDB Entry DOI: 10.7270/Q2SQ8ZZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50181296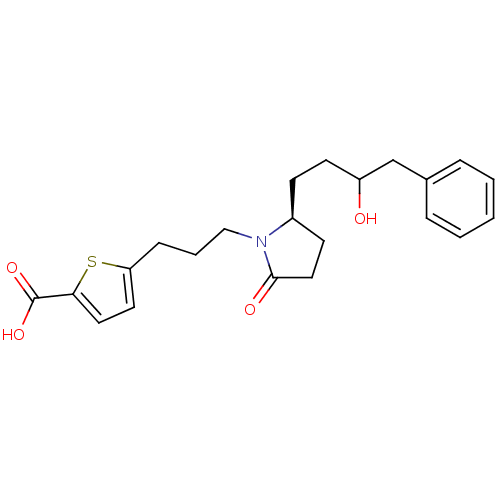 (5-(3-((S)-2-(3-hydroxy-4-phenylbutyl)-5-oxopyrroli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Binding affinity to rat EP4 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 1799-802 (2006) Article DOI: 10.1016/j.bmcl.2006.01.018 BindingDB Entry DOI: 10.7270/Q2SQ8ZZ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-tyrosine kinase 2-beta (Homo sapiens (Human)) | BDBM50418584 (CHEMBL2029177) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PYK2 assessed as reduction in peptide substrate phosphoryltion by fluorimetric method | Bioorg Med Chem Lett 19: 3253-8 (2009) Article DOI: 10.1016/j.bmcl.2009.04.093 BindingDB Entry DOI: 10.7270/Q2GH9J96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 377 total ) | Next | Last >> |