

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121409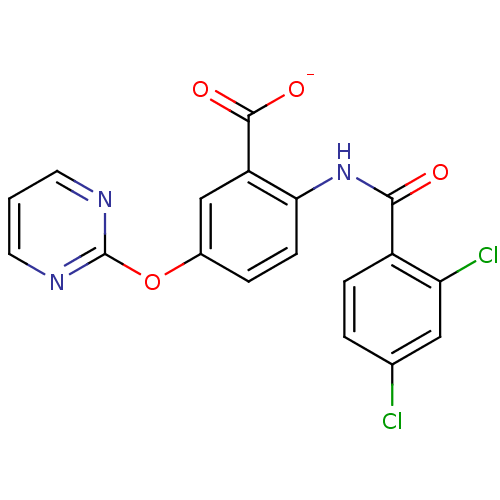 (CHEMBL118206 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121424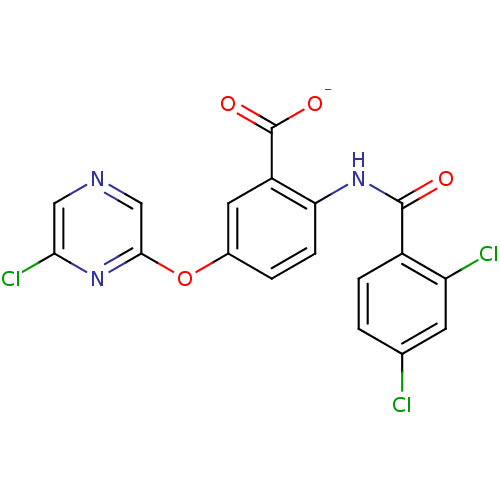 (CHEMBL119111 | Lithium; 5-(6-chloro-pyrazin-2-ylox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121420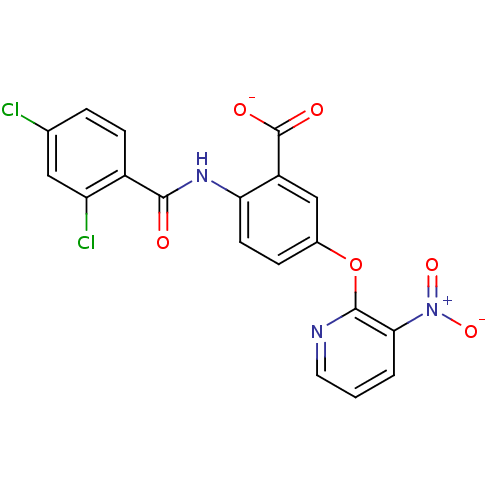 (CHEMBL330938 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121421 (CHEMBL118546 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121422 (CHEMBL119869 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121415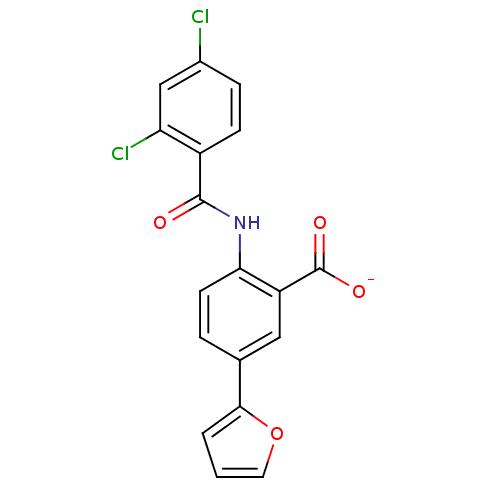 (CHEMBL119030 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121414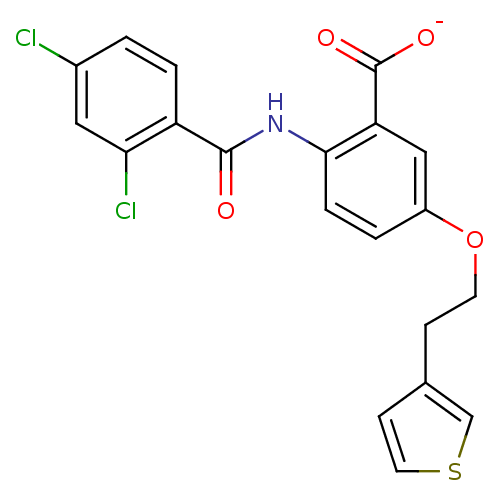 (CHEMBL333890 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121418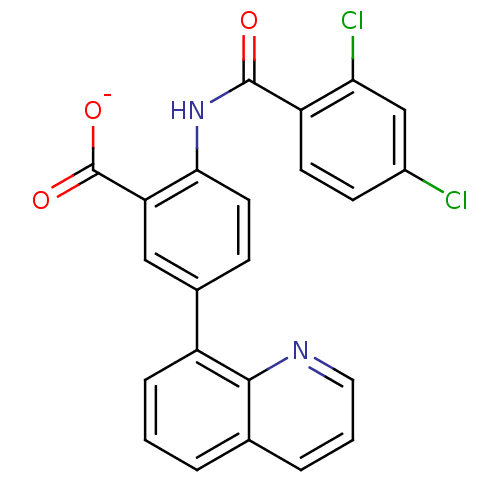 (CHEMBL119078 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121411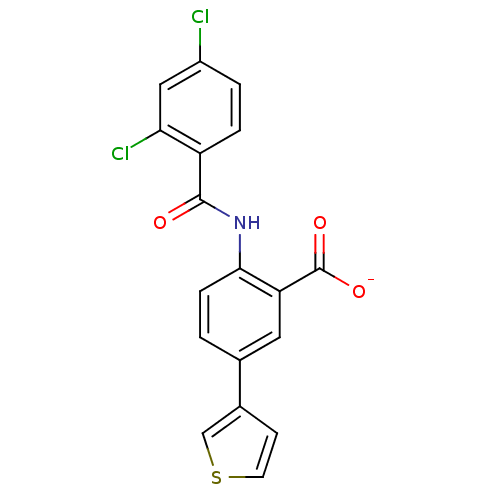 (CHEMBL118617 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121423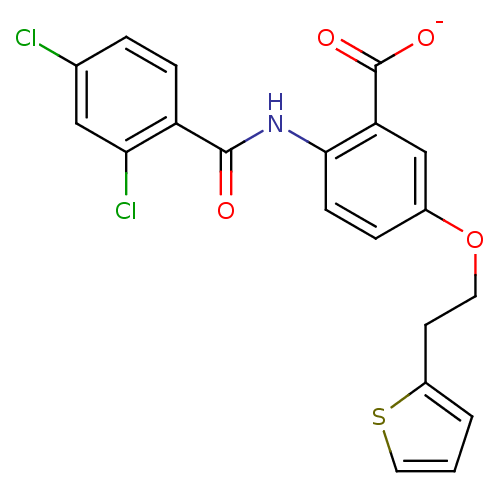 (CHEMBL119278 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121413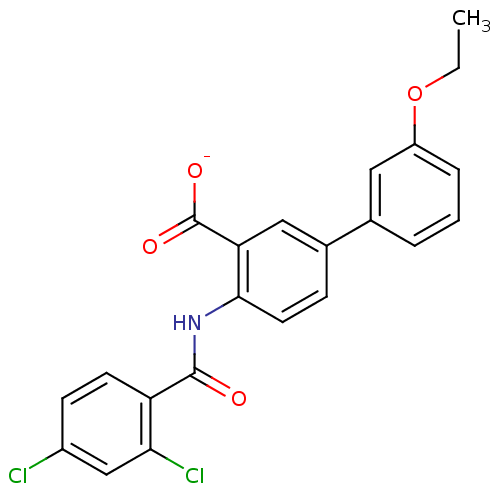 (CHEMBL118852 | Lithium; 4-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121416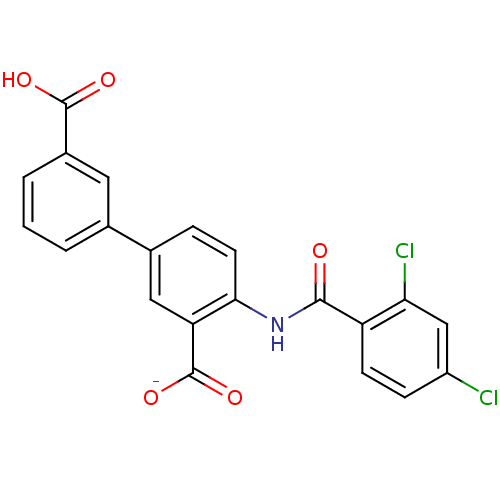 (CHEMBL325172 | Lithium; 3'-carboxy-4-(2,4-dichloro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121410 (CHEMBL420525 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121417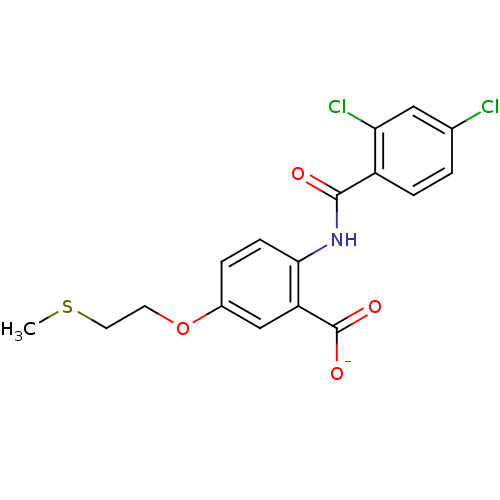 (CHEMBL119646 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121412 (2-(2,4-Dichloro-benzoylamino)-5-methyl-benzoic aci...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50121419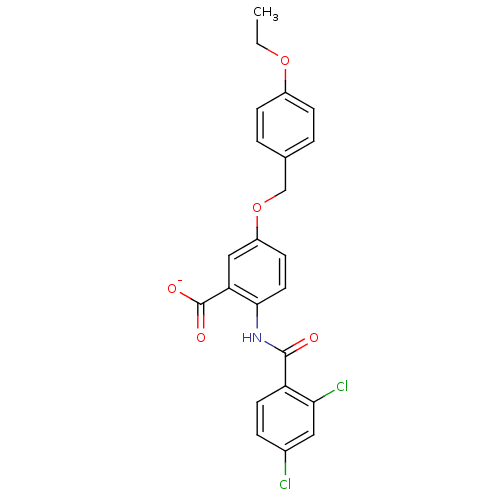 (CHEMBL119798 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121417 (CHEMBL119646 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121414 (CHEMBL333890 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121424 (CHEMBL119111 | Lithium; 5-(6-chloro-pyrazin-2-ylox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121415 (CHEMBL119030 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121422 (CHEMBL119869 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121411 (CHEMBL118617 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121412 (2-(2,4-Dichloro-benzoylamino)-5-methyl-benzoic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121416 (CHEMBL325172 | Lithium; 3'-carboxy-4-(2,4-dichloro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121418 (CHEMBL119078 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR alpha | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121420 (CHEMBL330938 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR gamma | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121413 (CHEMBL118852 | Lithium; 4-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121421 (CHEMBL118546 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Transactivation potency was measured by luciferase activity in Caco- 2/TC7 cells transiently co-transfected for the fusion-protein Gal4-PPAR gamma | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121419 (CHEMBL119798 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121410 (CHEMBL420525 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121423 (CHEMBL119278 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50121409 (CHEMBL118206 | Lithium; 2-(2,4-dichloro-benzoylami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Biovitrum AB Curated by ChEMBL | Assay Description Ligand binding affinity was determined by displacement of a tritiated tracer by the unlabeled compound to a GST-PPAR fusion protein for Peroxisome pr... | Bioorg Med Chem Lett 12: 3565-7 (2002) BindingDB Entry DOI: 10.7270/Q2Q23ZK5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307546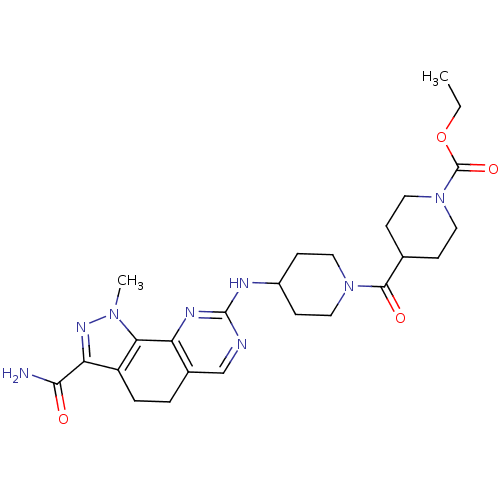 (CHEMBL598401 | Ethyl 4-[(3-Carbamoyl-1-methyl-4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307543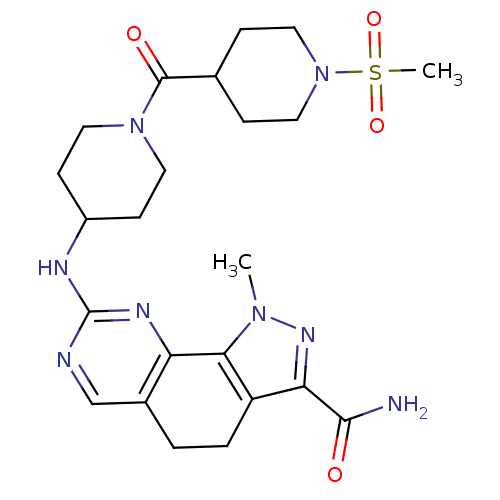 (1-Methyl-8-{[1-(methylsulfonyl)piperidin-4-yl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307522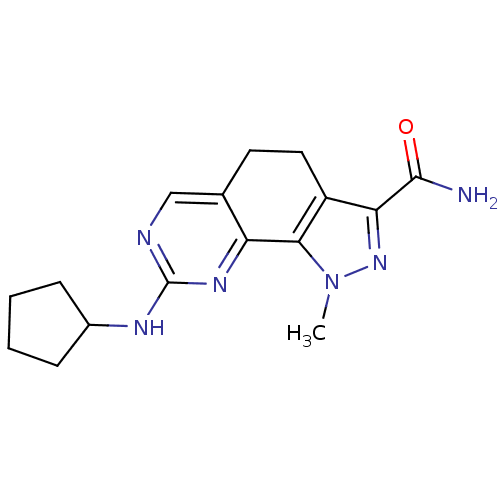 (8-(Cyclopentylamino)-1-methyl-4,5-dihydro-1H-pyraz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307535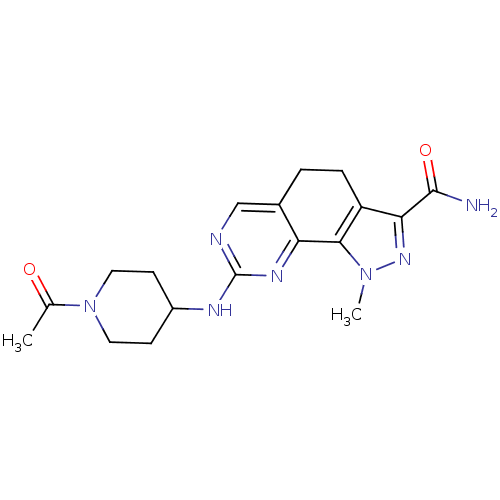 (8-[(1-Acetylpiperidin-4-yl)amino]-1-methyl-4,5-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307507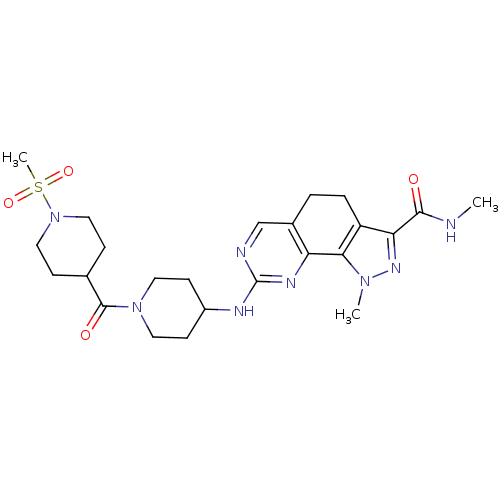 (CHEMBL597754 | N-1-Dimethyl-8-{[1-(methylsulfonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307506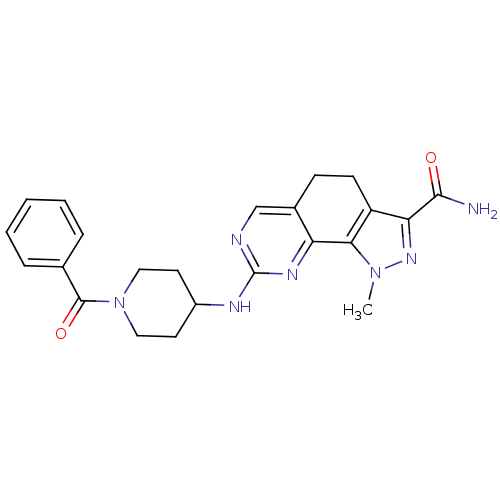 (1-Methyl-8-{[1-(phenylcarbonyl)piperidin-4-yl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307520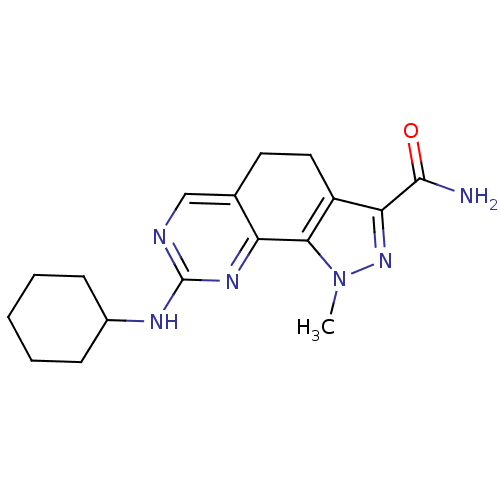 (8-(Cyclohexylamino)-1-methyl-4,5-dihydro-1H-pyrazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307545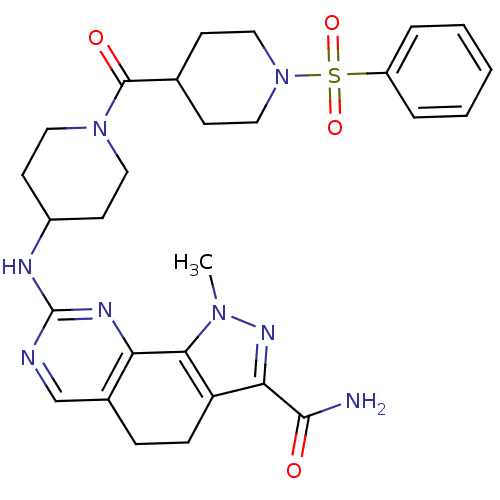 (1-Methyl-8-{[1-(phenylsulfonyl)piperidin-4-yl]amin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307509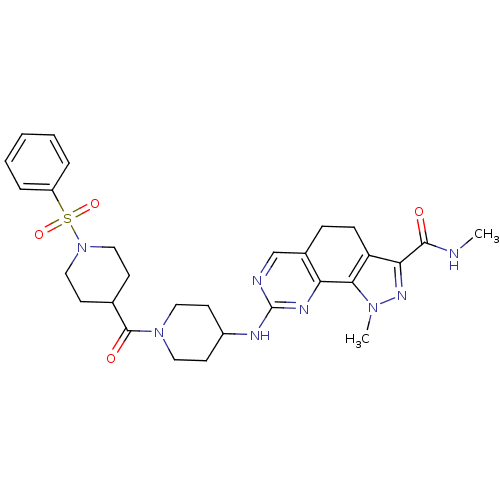 (CHEMBL592272 | N-1-Dimethyl-8-{[1-(phenylsulfonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307529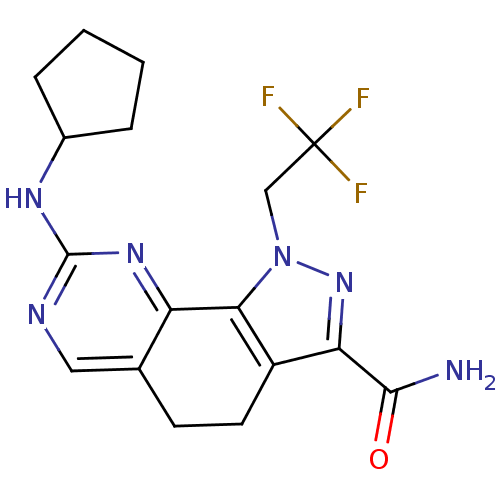 (8-(Cyclopentylamino)-1-(2,2,2-trifluoroethyl)-4,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50378657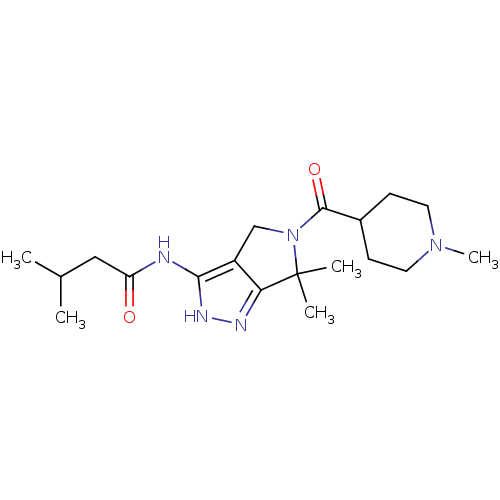 (CHEMBL1230607) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK5/p25 by scintillation proximity assay | Bioorg Med Chem 18: 1844-53 (2010) Article DOI: 10.1016/j.bmc.2010.01.042 BindingDB Entry DOI: 10.7270/Q21R6RG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307538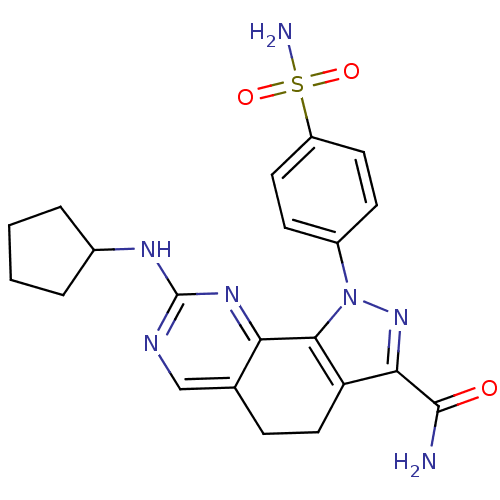 (8-(Cyclopentylamino)-1-(4-sulfamoylphenyl)-4,5-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM50307535 (8-[(1-Acetylpiperidin-4-yl)amino]-1-methyl-4,5-dih...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin B | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50027427 (CHEMBL1744453) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of human CDK2/cyclin A expressed in Escherichia coli BL21 by scintillation proximity assay | Bioorg Med Chem 18: 1844-53 (2010) Article DOI: 10.1016/j.bmc.2010.01.042 BindingDB Entry DOI: 10.7270/Q21R6RG5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM50307507 (CHEMBL597754 | N-1-Dimethyl-8-{[1-(methylsulfonyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin B | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50307530 (8-(Cyclopentylamino)-1-(1-methylpiperidin-4-yl)-4,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM50307506 (1-Methyl-8-{[1-(phenylcarbonyl)piperidin-4-yl]amin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin B | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 5 activator 1 (Homo sapiens (Human)) | BDBM50307507 (CHEMBL597754 | N-1-Dimethyl-8-{[1-(methylsulfonyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of CDK5/P25 | J Med Chem 53: 2171-87 (2010) Article DOI: 10.1021/jm901710h BindingDB Entry DOI: 10.7270/Q2N879W9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 406 total ) | Next | Last >> |