 Found 899 hits with Last Name = 'kim' and Initial = 'sc'
Found 899 hits with Last Name = 'kim' and Initial = 'sc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284986
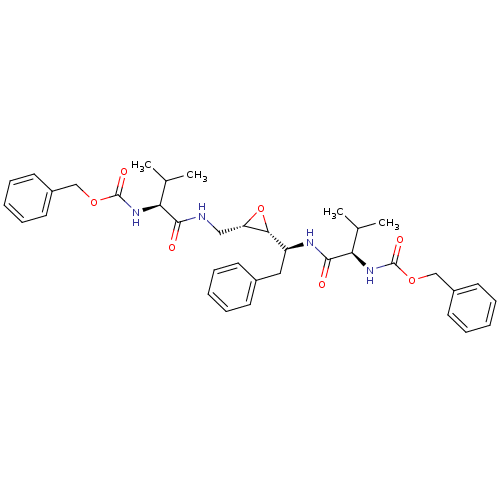
(CHEMBL55197 | [(R)-1-((S)-1-{(2R,3S)-3-[((S)-2-Ben...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C37H46N4O7/c1-24(2)31(40-36(44)46-22-27-16-10-6-11-17-27)34(42)38-21-30-33(48-30)29(20-26-14-8-5-9-15-26)39-35(43)32(25(3)4)41-37(45)47-23-28-18-12-7-13-19-28/h5-19,24-25,29-33H,20-23H2,1-4H3,(H,38,42)(H,39,43)(H,40,44)(H,41,45)/t29-,30-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288943
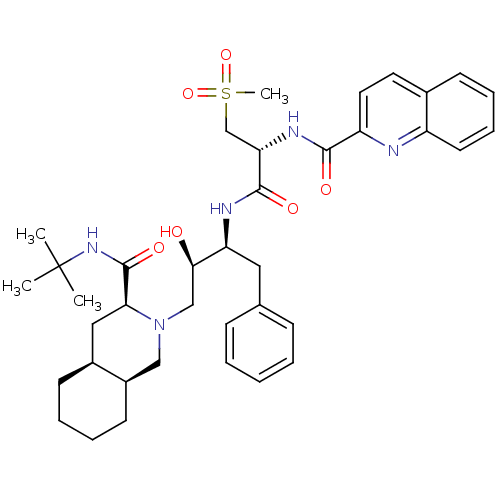
(CHEMBL154519 | Quinoline-2-carboxylic acid {(R)-1-...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CS(C)(=O)=O)NC(=O)c1ccc2ccccc2n1 Show InChI InChI=1S/C38H51N5O6S/c1-38(2,3)42-37(47)33-21-27-15-8-9-16-28(27)22-43(33)23-34(44)31(20-25-12-6-5-7-13-25)40-36(46)32(24-50(4,48)49)41-35(45)30-19-18-26-14-10-11-17-29(26)39-30/h5-7,10-14,17-19,27-28,31-34,44H,8-9,15-16,20-24H2,1-4H3,(H,40,46)(H,41,45)(H,42,47)/t27-,28+,31-,32-,33-,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288941
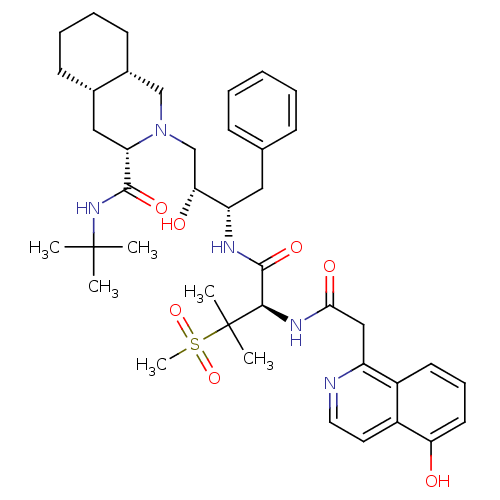
((3S,4aS,8aS)-2-((2R,3S)-2-Hydroxy-3-{(R)-2-[2-(5-h...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)Cc1nccc2c(O)cccc12)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C41H57N5O7S/c1-40(2,3)45-38(50)33-22-27-15-10-11-16-28(27)24-46(33)25-35(48)32(21-26-13-8-7-9-14-26)43-39(51)37(41(4,5)54(6,52)53)44-36(49)23-31-29-17-12-18-34(47)30(29)19-20-42-31/h7-9,12-14,17-20,27-28,32-33,35,37,47-48H,10-11,15-16,21-25H2,1-6H3,(H,43,51)(H,44,49)(H,45,50)/t27-,28+,32-,33-,35+,37+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288942
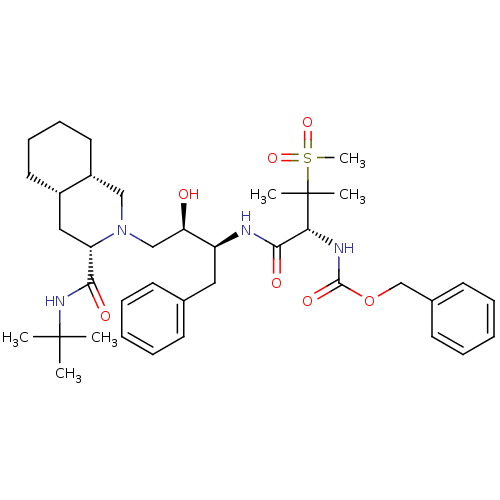
(CHEMBL154692 | {(R)-1-[(1S,2R)-1-Benzyl-3-((3S,4aS...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C38H56N4O7S/c1-37(2,3)41-34(44)31-22-28-19-13-14-20-29(28)23-42(31)24-32(43)30(21-26-15-9-7-10-16-26)39-35(45)33(38(4,5)50(6,47)48)40-36(46)49-25-27-17-11-8-12-18-27/h7-12,15-18,28-33,43H,13-14,19-25H2,1-6H3,(H,39,45)(H,40,46)(H,41,44)/t28-,29+,30-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 0.162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288939
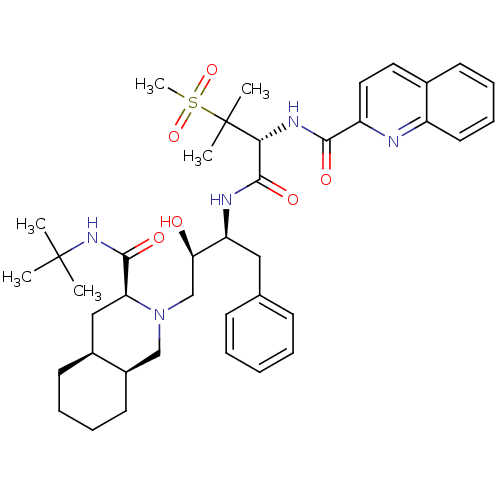
(CHEMBL345187 | Quinoline-2-carboxylic acid {(R)-1-...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C40H55N5O6S/c1-39(2,3)44-37(48)33-23-28-17-10-11-18-29(28)24-45(33)25-34(46)32(22-26-14-8-7-9-15-26)42-38(49)35(40(4,5)52(6,50)51)43-36(47)31-21-20-27-16-12-13-19-30(27)41-31/h7-9,12-16,19-21,28-29,32-35,46H,10-11,17-18,22-25H2,1-6H3,(H,42,49)(H,43,47)(H,44,48)/t28-,29+,32-,33-,34+,35+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM519

((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...)Show SMILES [H][C@@]12CCCC[C@]1([H])CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc3ccccc3n1)[C@@H](C2)C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C38H50N6O5/c1-38(2,3)43-37(49)32-20-26-14-7-8-15-27(26)22-44(32)23-33(45)30(19-24-11-5-4-6-12-24)41-36(48)31(21-34(39)46)42-35(47)29-18-17-25-13-9-10-16-28(25)40-29/h4-6,9-13,16-18,26-27,30-33,45H,7-8,14-15,19-23H2,1-3H3,(H2,39,46)(H,41,48)(H,42,47)(H,43,49)/t26-,27+,30-,31-,32-,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| 0.452 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM9294

((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...)Show SMILES CC(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C |r| Show InChI InChI=1S/C39H53N5O4/c1-25(2)35(42-36(46)31-20-19-27-15-11-12-18-30(27)40-31)38(48)41-32(21-26-13-7-6-8-14-26)34(45)24-44-23-29-17-10-9-16-28(29)22-33(44)37(47)43-39(3,4)5/h6-8,11-15,18-20,25,28-29,32-35,45H,9-10,16-17,21-24H2,1-5H3,(H,41,48)(H,42,46)(H,43,47)/t28-,29+,32-,33-,34+,35-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290520

(((R)-1-{(S)-1-[(2R,3S)-3-(2-tert-Butylsulfamoyl-et...)Show SMILES CC(C)(C)NS(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C30H43N3O8S2/c1-29(2,3)33-43(38,39)18-17-24-25(41-24)23(19-21-13-9-7-10-14-21)31-27(34)26(30(4,5)42(6,36)37)32-28(35)40-20-22-15-11-8-12-16-22/h7-16,23-26,33H,17-20H2,1-6H3,(H,31,34)(H,32,35)/t23-,24-,25+,26+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290527
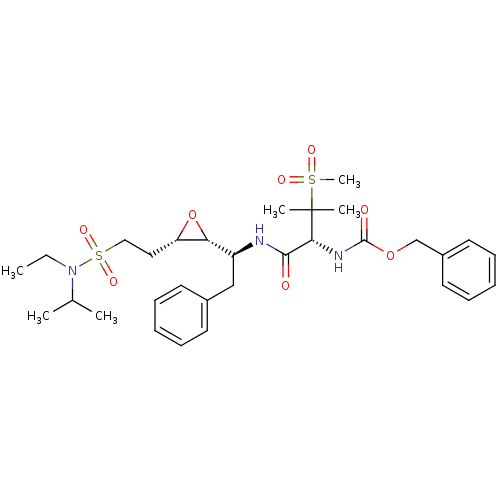
(CHEMBL86971 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-i...)Show SMILES CCN(C(C)C)S(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C31H45N3O8S2/c1-7-34(22(2)3)44(39,40)19-18-26-27(42-26)25(20-23-14-10-8-11-15-23)32-29(35)28(31(4,5)43(6,37)38)33-30(36)41-21-24-16-12-9-13-17-24/h8-17,22,25-28H,7,18-21H2,1-6H3,(H,32,35)(H,33,36)/t25-,26-,27+,28+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM197
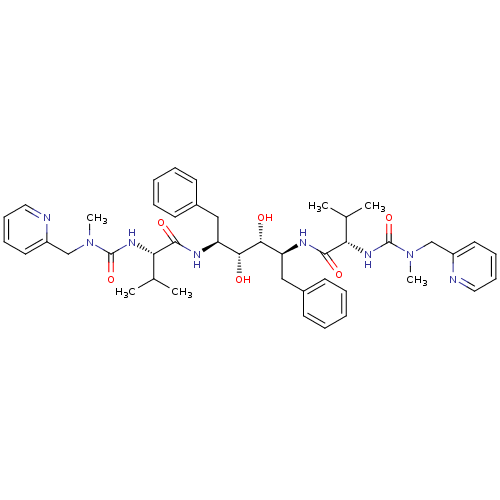
((2S)-N-[(2S,3R,4R,5S)-3,4-dihydroxy-5-[(2S)-3-meth...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)N(C)Cc1ccccn1)C(C)C |r| Show InChI InChI=1S/C44H58N8O6/c1-29(2)37(49-43(57)51(5)27-33-21-13-15-23-45-33)41(55)47-35(25-31-17-9-7-10-18-31)39(53)40(54)36(26-32-19-11-8-12-20-32)48-42(56)38(30(3)4)50-44(58)52(6)28-34-22-14-16-24-46-34/h7-24,29-30,35-40,53-54H,25-28H2,1-6H3,(H,47,55)(H,48,56)(H,49,57)(H,50,58)/t35-,36-,37-,38-,39+,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50009242
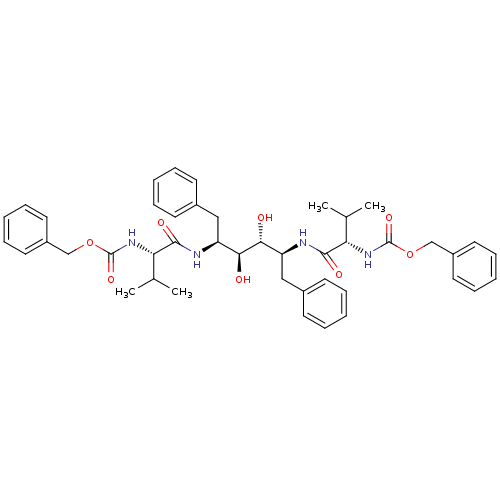
(CHEMBL48565 | {(S)-1-[(1S,2S,3R,4S)-1-Benzyl-4-((S...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C44H54N4O8/c1-29(2)37(47-43(53)55-27-33-21-13-7-14-22-33)41(51)45-35(25-31-17-9-5-10-18-31)39(49)40(50)36(26-32-19-11-6-12-20-32)46-42(52)38(30(3)4)48-44(54)56-28-34-23-15-8-16-24-34/h5-24,29-30,35-40,49-50H,25-28H2,1-4H3,(H,45,51)(H,46,52)(H,47,53)(H,48,54)/t35-,36-,37-,38-,39-,40+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288947

(CHEMBL158033 | N*1*-((S)-1-{(2R,3S)-3-[(1-Isopropy...)Show SMILES CC(C)C(NC(=O)C[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)C(CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C Show InChI InChI=1S/C33H41N5O5/c1-19(2)30(20(3)4)38-29(40)18-27-31(43-27)25(16-21-10-6-5-7-11-21)36-33(42)26(17-28(34)39)37-32(41)24-15-14-22-12-8-9-13-23(22)35-24/h5-15,19-20,25-27,30-31H,16-18H2,1-4H3,(H2,34,39)(H,36,42)(H,37,41)(H,38,40)/t25-,26?,27-,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease was determined |
Bioorg Med Chem Lett 6: 589-594 (1996)
Article DOI: 10.1016/0960-894X(96)00087-X
BindingDB Entry DOI: 10.7270/Q2FQ9WM4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288940

(CHEMBL154416 | Quinoline-2-carboxylic acid {(R)-1-...)Show SMILES CSC(C)(C)[C@H](NC(=O)c1ccc2ccccc2n1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@H]2CCCC[C@H]2C[C@H]1C(=O)NC(C)(C)C Show InChI InChI=1S/C40H55N5O4S/c1-39(2,3)44-37(48)33-23-28-17-10-11-18-29(28)24-45(33)25-34(46)32(22-26-14-8-7-9-15-26)42-38(49)35(40(4,5)50-6)43-36(47)31-21-20-27-16-12-13-19-30(27)41-31/h7-9,12-16,19-21,28-29,32-35,46H,10-11,17-18,22-25H2,1-6H3,(H,42,49)(H,43,47)(H,44,48)/t28-,29+,32-,33-,34+,35+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against P2 site in HIV protease. |
Bioorg Med Chem Lett 6: 585-588 (1996)
Article DOI: 10.1016/0960-894X(96)00086-8
BindingDB Entry DOI: 10.7270/Q2KH0N9Z |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290525

(CHEMBL92097 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-i...)Show SMILES CCN(CC(C)C)S(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C32H47N3O8S2/c1-7-35(21-23(2)3)45(40,41)19-18-27-28(43-27)26(20-24-14-10-8-11-15-24)33-30(36)29(32(4,5)44(6,38)39)34-31(37)42-22-25-16-12-9-13-17-25/h8-17,23,26-29H,7,18-22H2,1-6H3,(H,33,36)(H,34,37)/t26-,27-,28+,29+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290523

(CHEMBL327222 | Thiophene-2-carboxylic acid [(R)-1-...)Show SMILES CCN(C(C)C)S(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1cccs1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C28H41N3O7S3/c1-7-31(19(2)3)41(36,37)17-15-22-24(38-22)21(18-20-12-9-8-10-13-20)29-27(33)25(28(4,5)40(6,34)35)30-26(32)23-14-11-16-39-23/h8-14,16,19,21-22,24-25H,7,15,17-18H2,1-6H3,(H,29,33)(H,30,32)/t21-,22-,24+,25+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290524
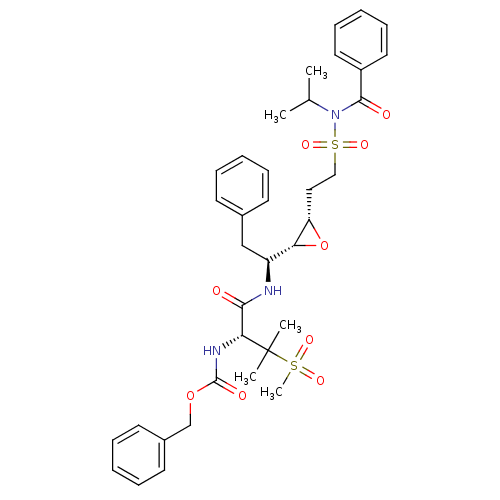
(CHEMBL404933 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Benzoy...)Show SMILES CC(C)N(C(=O)c1ccccc1)S(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C36H45N3O9S2/c1-25(2)39(34(41)28-19-13-8-14-20-28)50(45,46)22-21-30-31(48-30)29(23-26-15-9-6-10-16-26)37-33(40)32(36(3,4)49(5,43)44)38-35(42)47-24-27-17-11-7-12-18-27/h6-20,25,29-32H,21-24H2,1-5H3,(H,37,40)(H,38,42)/t29-,30-,31+,32+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284988
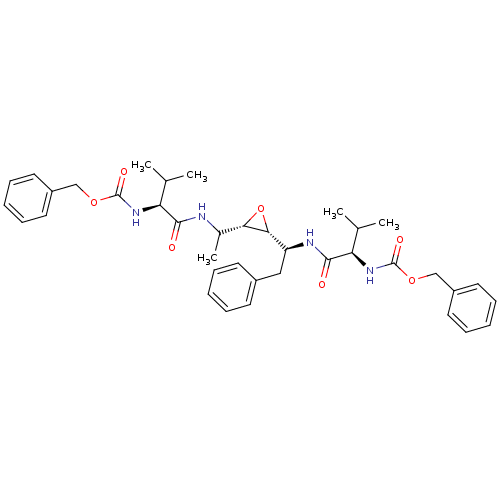
(CHEMBL52809 | [(S)-1-(1-{(2S,3R)-3-[(S)-1-((R)-2-B...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)NC(C)[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C38H48N4O7/c1-24(2)31(41-37(45)47-22-28-17-11-7-12-18-28)35(43)39-26(5)33-34(49-33)30(21-27-15-9-6-10-16-27)40-36(44)32(25(3)4)42-38(46)48-23-29-19-13-8-14-20-29/h6-20,24-26,30-34H,21-23H2,1-5H3,(H,39,43)(H,40,44)(H,41,45)(H,42,46)/t26?,30-,31-,32+,33-,34+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290526

(CHEMBL328036 | [(R)-1-((S)-1-{(2R,3S)-3-[2-(Ethyl-...)Show SMILES CCN(c1ccccc1)S(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C34H43N3O8S2/c1-5-37(27-19-13-8-14-20-27)47(42,43)22-21-29-30(45-29)28(23-25-15-9-6-10-16-25)35-32(38)31(34(2,3)46(4,40)41)36-33(39)44-24-26-17-11-7-12-18-26/h6-20,28-31H,5,21-24H2,1-4H3,(H,35,38)(H,36,39)/t28-,29-,30+,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290522

(CHEMBL313237 | Quinoline-2-carboxylic acid [(R)-1-...)Show SMILES CCN(c1ccccc1)S(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C36H42N4O7S2/c1-5-40(27-17-10-7-11-18-27)49(45,46)23-22-31-32(47-31)30(24-25-14-8-6-9-15-25)38-35(42)33(36(2,3)48(4,43)44)39-34(41)29-21-20-26-16-12-13-19-28(26)37-29/h6-21,30-33H,5,22-24H2,1-4H3,(H,38,42)(H,39,41)/t30-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290521
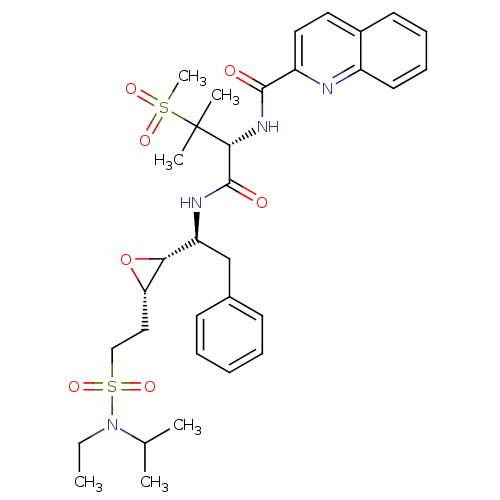
(CHEMBL89076 | Quinoline-2-carboxylic acid [(R)-1-(...)Show SMILES CCN(C(C)C)S(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)C(C)(C)S(C)(=O)=O Show InChI InChI=1S/C33H44N4O7S2/c1-7-37(22(2)3)46(42,43)20-19-28-29(44-28)27(21-23-13-9-8-10-14-23)35-32(39)30(33(4,5)45(6,40)41)36-31(38)26-18-17-24-15-11-12-16-25(24)34-26/h8-18,22,27-30H,7,19-21H2,1-6H3,(H,35,39)(H,36,38)/t27-,28-,29+,30+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284989
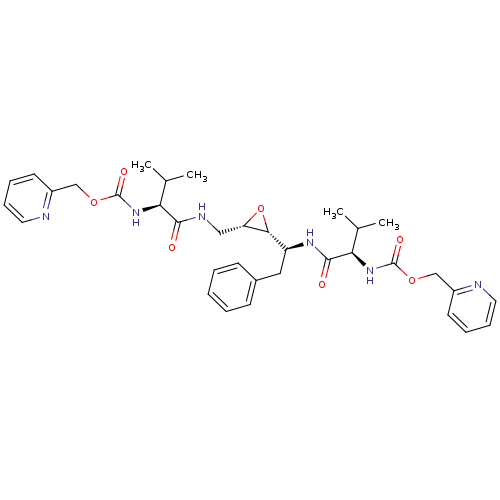
(CHEMBL300705 | {(R)-2-Methyl-1-[(S)-1-((2R,3S)-3-{...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccn1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1ccccn1)C(C)C Show InChI InChI=1S/C35H44N6O7/c1-22(2)29(40-34(44)46-20-25-14-8-10-16-36-25)32(42)38-19-28-31(48-28)27(18-24-12-6-5-7-13-24)39-33(43)30(23(3)4)41-35(45)47-21-26-15-9-11-17-37-26/h5-17,22-23,27-31H,18-21H2,1-4H3,(H,38,42)(H,39,43)(H,40,44)(H,41,45)/t27-,28-,29-,30+,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50290528

(CHEMBL261900 | N*1*-{(S)-1-[(2R,3S)-3-(2-tert-Buty...)Show SMILES CC(C)(C)NS(=O)(=O)CC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)C(CC(N)=O)NC(=O)c1ccc2ccccc2n1 Show InChI InChI=1S/C30H37N5O6S/c1-30(2,3)35-42(39,40)16-15-25-27(41-25)23(17-19-9-5-4-6-10-19)33-29(38)24(18-26(31)36)34-28(37)22-14-13-20-11-7-8-12-21(20)32-22/h4-14,23-25,27,35H,15-18H2,1-3H3,(H2,31,36)(H,33,38)(H,34,37)/t23-,24?,25-,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 7: 2635-2638 (1997)
Article DOI: 10.1016/S0960-894X(97)10054-3
BindingDB Entry DOI: 10.7270/Q2G44Q84 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284984
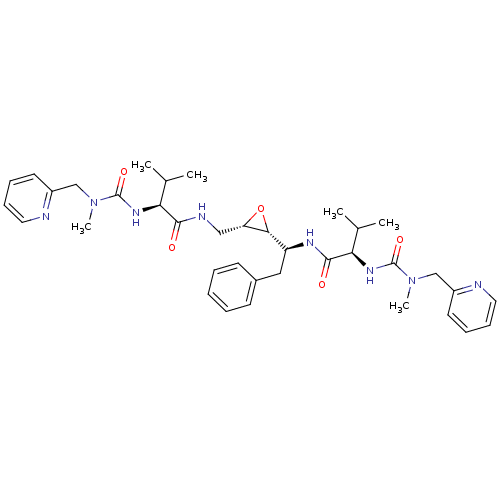
((R)-3-Methyl-N-[(S)-1-((2R,3S)-3-{[(S)-3-methyl-2-...)Show SMILES CC(C)[C@H](NC(=O)N(C)Cc1ccccn1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)N(C)Cc1ccccn1)C(C)C Show InChI InChI=1S/C37H50N8O5/c1-24(2)31(42-36(48)44(5)22-27-16-10-12-18-38-27)34(46)40-21-30-33(50-30)29(20-26-14-8-7-9-15-26)41-35(47)32(25(3)4)43-37(49)45(6)23-28-17-11-13-19-39-28/h7-19,24-25,29-33H,20-23H2,1-6H3,(H,40,46)(H,41,47)(H,42,48)(H,43,49)/t29-,30-,31-,32+,33+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288946
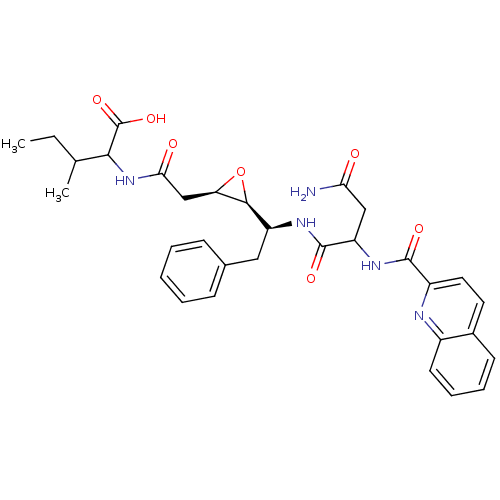
(2-{2-[(2R,3S)-3-((S)-1-{3-Carbamoyl-2-[(quinoline-...)Show SMILES CCC(C)C(NC(=O)C[C@H]1O[C@H]1[C@H](Cc1ccccc1)NC(=O)C(CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(O)=O Show InChI InChI=1S/C32H37N5O7/c1-3-18(2)28(32(42)43)37-27(39)17-25-29(44-25)23(15-19-9-5-4-6-10-19)35-31(41)24(16-26(33)38)36-30(40)22-14-13-20-11-7-8-12-21(20)34-22/h4-14,18,23-25,28-29H,3,15-17H2,1-2H3,(H2,33,38)(H,35,41)(H,36,40)(H,37,39)(H,42,43)/t18?,23-,24?,25+,28?,29-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease was determined |
Bioorg Med Chem Lett 6: 589-594 (1996)
Article DOI: 10.1016/0960-894X(96)00087-X
BindingDB Entry DOI: 10.7270/Q2FQ9WM4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284987

(CHEMBL293061 | {(R)-2-Methyl-1-[(S)-1-((2R,3S)-3-{...)Show SMILES CC(C)[C@H](NC(=O)OCc1cccnc1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1cccnc1)C(C)C Show InChI InChI=1S/C35H44N6O7/c1-22(2)29(40-34(44)46-20-25-12-8-14-36-17-25)32(42)38-19-28-31(48-28)27(16-24-10-6-5-7-11-24)39-33(43)30(23(3)4)41-35(45)47-21-26-13-9-15-37-18-26/h5-15,17-18,22-23,27-31H,16,19-21H2,1-4H3,(H,38,42)(H,39,43)(H,40,44)(H,41,45)/t27-,28-,29-,30+,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284991
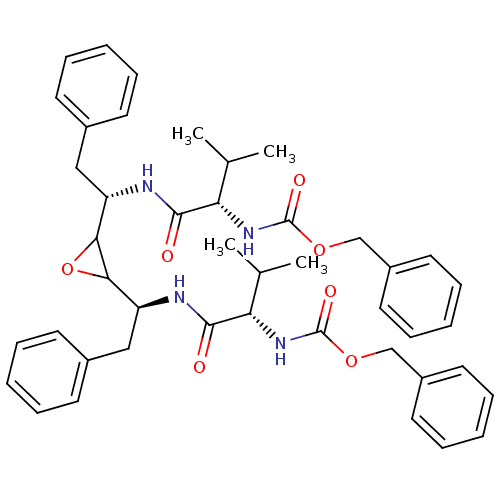
(CHEMBL295499 | [(S)-1-((S)-1-{3-[(S)-1-((S)-2-Benz...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C1OC1[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)C(C)C Show InChI InChI=1S/C44H52N4O7/c1-29(2)37(47-43(51)53-27-33-21-13-7-14-22-33)41(49)45-35(25-31-17-9-5-10-18-31)39-40(55-39)36(26-32-19-11-6-12-20-32)46-42(50)38(30(3)4)48-44(52)54-28-34-23-15-8-16-24-34/h5-24,29-30,35-40H,25-28H2,1-4H3,(H,45,49)(H,46,50)(H,47,51)(H,48,52)/t35-,36-,37-,38-,39?,40?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284990
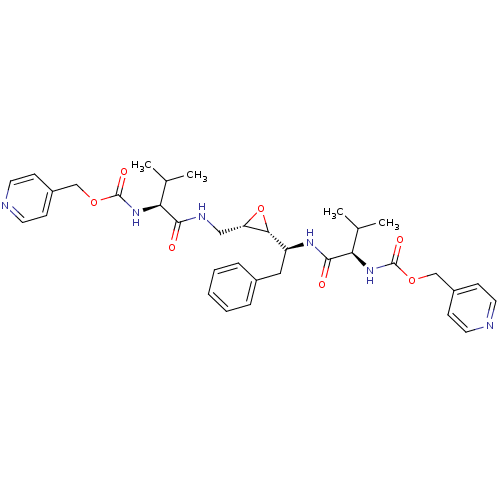
(CHEMBL54394 | {(R)-2-Methyl-1-[(S)-1-((2R,3S)-3-{[...)Show SMILES CC(C)[C@H](NC(=O)OCc1ccncc1)C(=O)NC[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)[C@H](NC(=O)OCc1ccncc1)C(C)C Show InChI InChI=1S/C35H44N6O7/c1-22(2)29(40-34(44)46-20-25-10-14-36-15-11-25)32(42)38-19-28-31(48-28)27(18-24-8-6-5-7-9-24)39-33(43)30(23(3)4)41-35(45)47-21-26-12-16-37-17-13-26/h5-17,22-23,27-31H,18-21H2,1-4H3,(H,38,42)(H,39,43)(H,40,44)(H,41,45)/t27-,28-,29-,30+,31+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288945
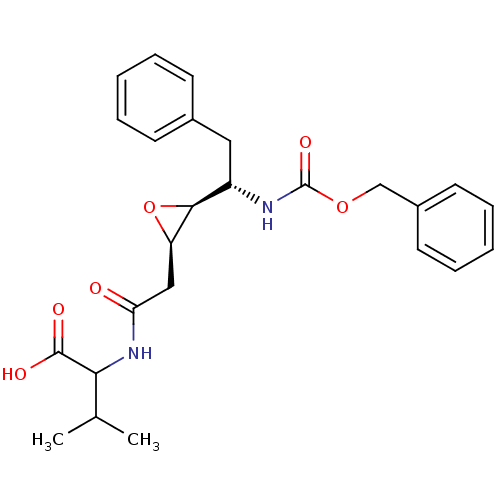
(2-{2-[(2R,3S)-3-((S)-1-Benzyloxycarbonylamino-2-ph...)Show SMILES CC(C)C(NC(=O)C[C@H]1O[C@H]1[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C25H30N2O6/c1-16(2)22(24(29)30)27-21(28)14-20-23(33-20)19(13-17-9-5-3-6-10-17)26-25(31)32-15-18-11-7-4-8-12-18/h3-12,16,19-20,22-23H,13-15H2,1-2H3,(H,26,31)(H,27,28)(H,29,30)/t19-,20+,22?,23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 1.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease was determined |
Bioorg Med Chem Lett 6: 589-594 (1996)
Article DOI: 10.1016/0960-894X(96)00087-X
BindingDB Entry DOI: 10.7270/Q2FQ9WM4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288948
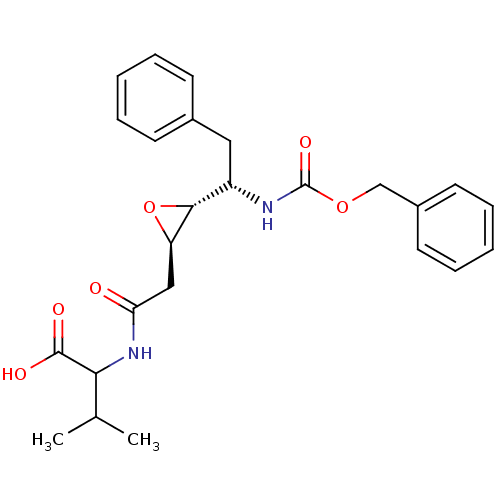
(2-{2-[(2R,3R)-3-((S)-1-Benzyloxycarbonylamino-2-ph...)Show SMILES CC(C)C(NC(=O)C[C@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C25H30N2O6/c1-16(2)22(24(29)30)27-21(28)14-20-23(33-20)19(13-17-9-5-3-6-10-17)26-25(31)32-15-18-11-7-4-8-12-18/h3-12,16,19-20,22-23H,13-15H2,1-2H3,(H,26,31)(H,27,28)(H,29,30)/t19-,20+,22?,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease was determined |
Bioorg Med Chem Lett 6: 589-594 (1996)
Article DOI: 10.1016/0960-894X(96)00087-X
BindingDB Entry DOI: 10.7270/Q2FQ9WM4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288944
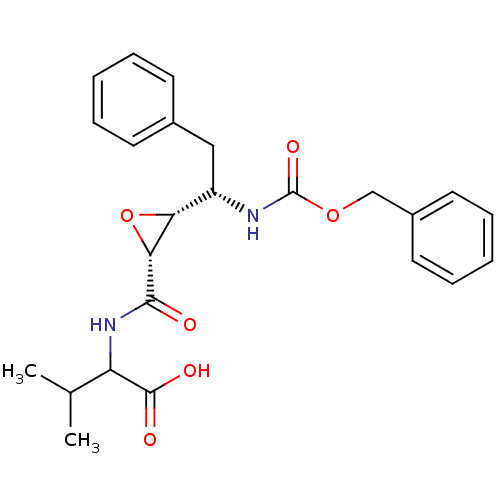
(2-{[(2R,3R)-3-((S)-1-Benzyloxycarbonylamino-2-phen...)Show SMILES CC(C)C(NC(=O)[C@@H]1O[C@@H]1[C@H](Cc1ccccc1)NC(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C24H28N2O6/c1-15(2)19(23(28)29)26-22(27)21-20(32-21)18(13-16-9-5-3-6-10-16)25-24(30)31-14-17-11-7-4-8-12-17/h3-12,15,18-21H,13-14H2,1-2H3,(H,25,30)(H,26,27)(H,28,29)/t18-,19?,20+,21+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| >1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV protease was determined |
Bioorg Med Chem Lett 6: 589-594 (1996)
Article DOI: 10.1016/0960-894X(96)00087-X
BindingDB Entry DOI: 10.7270/Q2FQ9WM4 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50284985
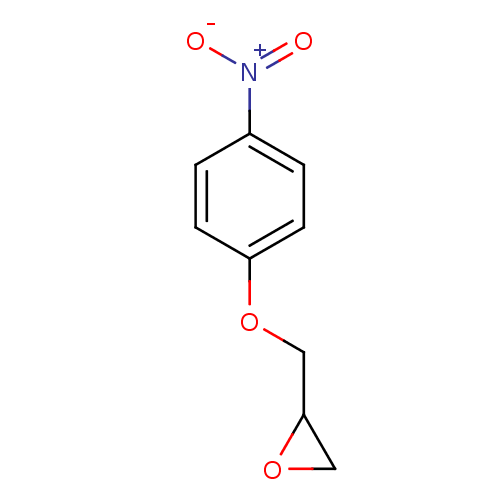
(1,2-Epoxy-3-(4'-nitrophenoxy)propane | 1,2-Epoxy-3...)Show InChI InChI=1S/C9H9NO4/c11-10(12)7-1-3-8(4-2-7)13-5-9-6-14-9/h1-4,9H,5-6H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| 1.10E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition activity against HIV-1 protease |
Bioorg Med Chem Lett 5: 1843-1848 (1995)
Article DOI: 10.1016/0960-894X(95)00306-E
BindingDB Entry DOI: 10.7270/Q2KW5G0J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1/3
(Homo sapiens (Human)) | BDBM28123

(3-cyanoquinoline, 8 | 4-({3-chloro-4-[(1-methyl-1H...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Cl)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C28H29ClN6O3S/c1-34-8-6-31-28(34)39-26-5-4-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(36-2)15-21(23)27)38-11-3-7-35-9-12-37-13-10-35/h4-6,8,14-16,18H,3,7,9-13H2,1-2H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 phosphorylation in LoVo cells |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50132262
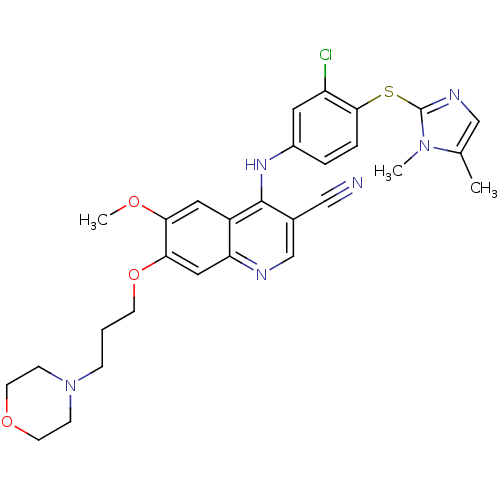
(4-[3-Chloro-4-(1,5-dimethyl-1H-imidazol-2-ylsulfan...)Show SMILES COc1cc2c(Nc3ccc(Sc4ncc(C)n4C)c(Cl)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C29H31ClN6O3S/c1-19-17-33-29(35(19)2)40-27-6-5-21(13-23(27)30)34-28-20(16-31)18-32-24-15-26(25(37-3)14-22(24)28)39-10-4-7-36-8-11-38-12-9-36/h5-6,13-15,17-18H,4,7-12H2,1-3H3,(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM28124
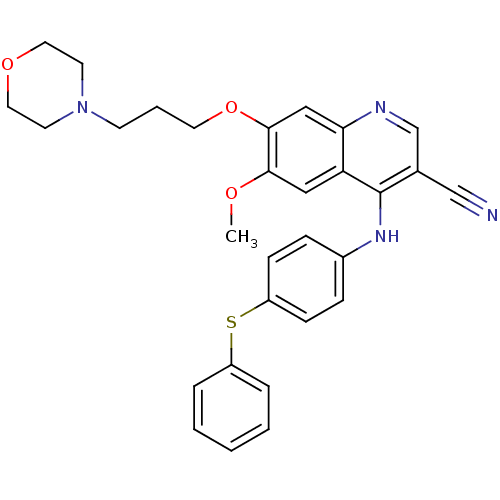
(3-cyanoquinoline, 9 | 6-methoxy-7-[3-(morpholin-4-...)Show SMILES COc1cc2c(Nc3ccc(Sc4ccccc4)cc3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C30H30N4O3S/c1-35-28-18-26-27(19-29(28)37-15-5-12-34-13-16-36-17-14-34)32-21-22(20-31)30(26)33-23-8-10-25(11-9-23)38-24-6-3-2-4-7-24/h2-4,6-11,18-19,21H,5,12-17H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dual specificity mitogen-activated protein kinase kinase using coupled MEK assay (which uses activated Raf to activate an... |
Bioorg Med Chem Lett 11: 1407-10 (2001)
BindingDB Entry DOI: 10.7270/Q2RB73WF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50132264

(4-[3-Chloro-4-(1,4,5-trimethyl-1H-imidazol-2-ylsul...)Show SMILES COc1cc2c(Nc3ccc(Sc4nc(C)c(C)n4C)c(Cl)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C30H33ClN6O3S/c1-19-20(2)36(3)30(34-19)41-28-7-6-22(14-24(28)31)35-29-21(17-32)18-33-25-16-27(26(38-4)15-23(25)29)40-11-5-8-37-9-12-39-13-10-37/h6-7,14-16,18H,5,8-13H2,1-4H3,(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50132266
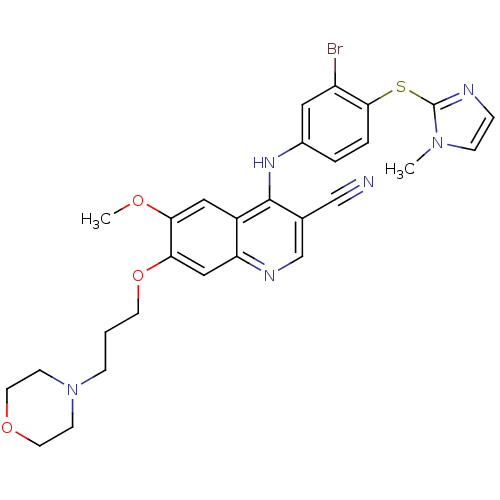
(4-[3-Bromo-4-(1-methyl-1H-imidazol-2-ylsulfanyl)-p...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Br)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C28H29BrN6O3S/c1-34-8-6-31-28(34)39-26-5-4-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(36-2)15-21(23)27)38-11-3-7-35-9-12-37-13-10-35/h4-6,8,14-16,18H,3,7,9-13H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM28123

(3-cyanoquinoline, 8 | 4-({3-chloro-4-[(1-methyl-1H...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Cl)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C28H29ClN6O3S/c1-34-8-6-31-28(34)39-26-5-4-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(36-2)15-21(23)27)38-11-3-7-35-9-12-37-13-10-35/h4-6,8,14-16,18H,3,7,9-13H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50132260
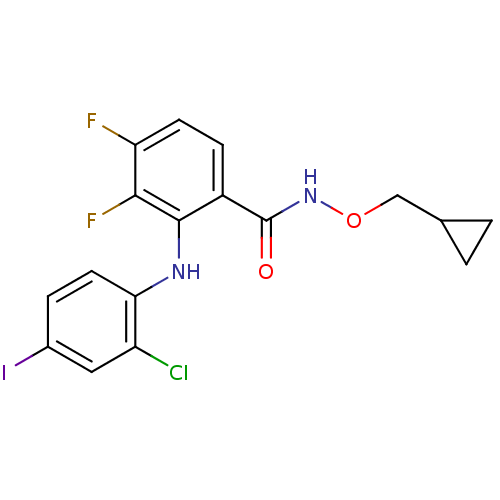
(2-(2-Chloro-4-iodo-phenylamino)-N-cyclopropylmetho...)Show InChI InChI=1S/C17H14ClF2IN2O2/c18-12-7-10(21)3-6-14(12)22-16-11(4-5-13(19)15(16)20)17(24)23-25-8-9-1-2-9/h3-7,9,22H,1-2,8H2,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50132261
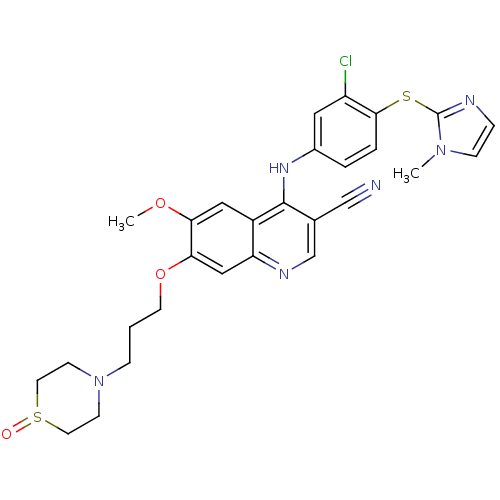
(4-[3-Chloro-4-(1-methyl-1H-imidazol-2-ylsulfanyl)-...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Cl)c3)c(cnc2cc1OCCCN1CCS(=O)CC1)C#N Show InChI InChI=1S/C28H29ClN6O3S2/c1-34-8-6-31-28(34)39-26-5-4-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(37-2)15-21(23)27)38-11-3-7-35-9-12-40(36)13-10-35/h4-6,8,14-16,18H,3,7,9-13H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM28123

(3-cyanoquinoline, 8 | 4-({3-chloro-4-[(1-methyl-1H...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Cl)c3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C28H29ClN6O3S/c1-34-8-6-31-28(34)39-26-5-4-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(36-2)15-21(23)27)38-11-3-7-35-9-12-37-13-10-35/h4-6,8,14-16,18H,3,7,9-13H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of MEK1 phosphorylation by activated human recombinant Raf |
J Med Chem 45: 529-32 (2002)
BindingDB Entry DOI: 10.7270/Q2FQ9XCD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50059889

((staurosporine)3-methoxy-2-methyl-4-methylamino-(2...)Show SMILES CN[C@@H]1CC2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah
Curated by ChEMBL
| Assay Description
Inhibition of Mitogen-activated protein kinase (MAPK)phosphorylation by activated MEK-1 |
J Med Chem 45: 529-32 (2002)
BindingDB Entry DOI: 10.7270/Q2FQ9XCD |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50099987
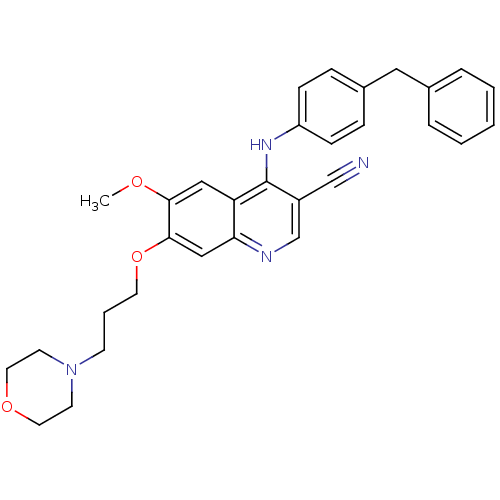
(4-(4-Benzyl-phenylamino)-6-methoxy-7-(3-morpholin-...)Show SMILES COc1cc2c(Nc3ccc(Cc4ccccc4)cc3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C31H32N4O3/c1-36-29-19-27-28(20-30(29)38-15-5-12-35-13-16-37-17-14-35)33-22-25(21-32)31(27)34-26-10-8-24(9-11-26)18-23-6-3-2-4-7-23/h2-4,6-11,19-20,22H,5,12-18H2,1H3,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dual specificity mitogen-activated protein kinase kinase using coupled MEK assay (which uses activated Raf to activate an... |
Bioorg Med Chem Lett 11: 1407-10 (2001)
BindingDB Entry DOI: 10.7270/Q2RB73WF |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM28118
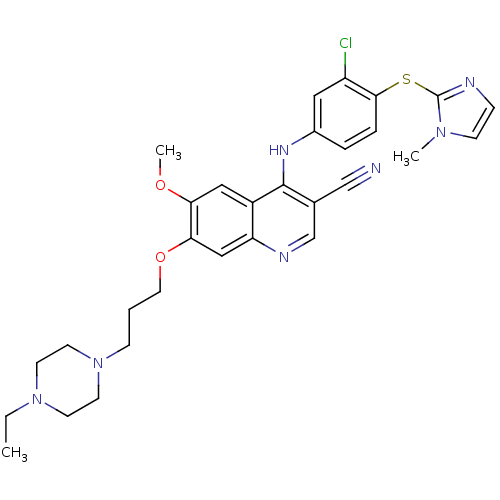
(3-cyanoquinoline, 3 | 4-({3-chloro-4-[(1-methyl-1H...)Show SMILES CCN1CCN(CCCOc2cc3ncc(C#N)c(Nc4ccc(Sc5nccn5C)c(Cl)c4)c3cc2OC)CC1 Show InChI InChI=1S/C30H34ClN7O2S/c1-4-37-11-13-38(14-12-37)9-5-15-40-27-18-25-23(17-26(27)39-3)29(21(19-32)20-34-25)35-22-6-7-28(24(31)16-22)41-30-33-8-10-36(30)2/h6-8,10,16-18,20H,4-5,9,11-15H2,1-3H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543787
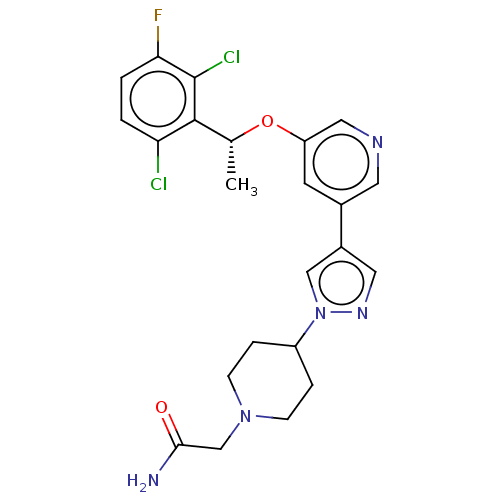
(CHEMBL4645582)Show SMILES C[C@@H](Oc1cncc(c1)-c1cnn(c1)C1CCN(CC(N)=O)CC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C23H24Cl2FN5O2/c1-14(22-19(24)2-3-20(26)23(22)25)33-18-8-15(9-28-11-18)16-10-29-31(12-16)17-4-6-30(7-5-17)13-21(27)32/h2-3,8-12,14,17H,4-7,13H2,1H3,(H2,27,32)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50099990
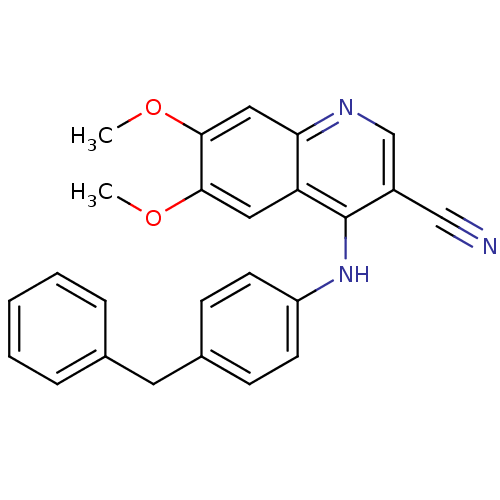
(4-(4-Benzyl-phenylamino)-6,7-dimethoxy-quinoline-3...)Show SMILES COc1cc2ncc(C#N)c(Nc3ccc(Cc4ccccc4)cc3)c2cc1OC Show InChI InChI=1S/C25H21N3O2/c1-29-23-13-21-22(14-24(23)30-2)27-16-19(15-26)25(21)28-20-10-8-18(9-11-20)12-17-6-4-3-5-7-17/h3-11,13-14,16H,12H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against Dual specificity mitogen-activated protein kinase kinase using coupled MEK assay (which uses activated Raf to activate an... |
Bioorg Med Chem Lett 11: 1407-10 (2001)
BindingDB Entry DOI: 10.7270/Q2RB73WF |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50543784
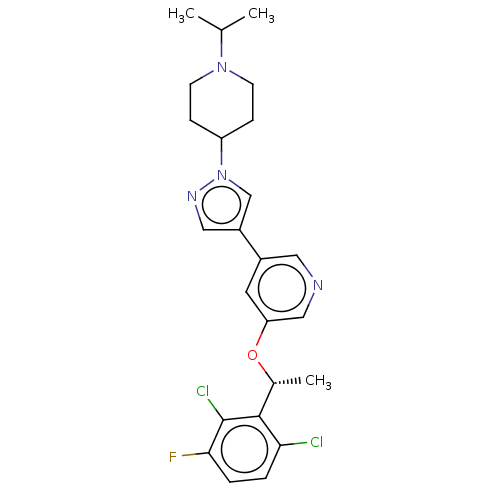
(CHEMBL4649193)Show SMILES CC(C)N1CCC(CC1)n1cc(cn1)-c1cncc(O[C@H](C)c2c(Cl)ccc(F)c2Cl)c1 |r| Show InChI InChI=1S/C24H27Cl2FN4O/c1-15(2)30-8-6-19(7-9-30)31-14-18(12-29-31)17-10-20(13-28-11-17)32-16(3)23-21(25)4-5-22(27)24(23)26/h4-5,10-16,19H,6-9H2,1-3H3/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sungkyunkwan University
Curated by ChEMBL
| Assay Description
Inhibition of c-Met (unknown origin) by ADP-Glo kinase assay |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.127189
BindingDB Entry DOI: 10.7270/Q23F4T7M |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM28119

(3-cyanoquinoline, 4 | 4-({3-chloro-4-[(1-methyl-1H...)Show SMILES COc1cc2c(Nc3ccc(Sc4nccn4C)c(Cl)c3)c(cnc2cc1OCCCN1CCCC1)C#N Show InChI InChI=1S/C28H29ClN6O2S/c1-34-12-8-31-28(34)38-26-7-6-20(14-22(26)29)33-27-19(17-30)18-32-23-16-25(24(36-2)15-21(23)27)37-13-5-11-35-9-3-4-10-35/h6-8,12,14-16,18H,3-5,9-11,13H2,1-2H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 1
(Homo sapiens (Human)) | BDBM50095232
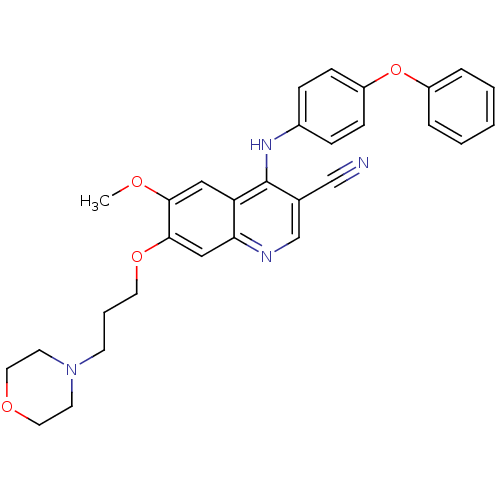
(6-Methoxy-7-(3-morpholin-4-yl-propoxy)-4-(4-phenox...)Show SMILES COc1cc2c(Nc3ccc(Oc4ccccc4)cc3)c(cnc2cc1OCCCN1CCOCC1)C#N Show InChI InChI=1S/C30H30N4O4/c1-35-28-18-26-27(19-29(28)37-15-5-12-34-13-16-36-17-14-34)32-21-22(20-31)30(26)33-23-8-10-25(11-9-23)38-24-6-3-2-4-7-24/h2-4,6-11,18-19,21H,5,12-17H2,1H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against Mitogen activated protein kinase kinase kinase 1 was determined using Raf/MEK1 coupled assay |
Bioorg Med Chem Lett 13: 3031-4 (2003)
BindingDB Entry DOI: 10.7270/Q2P84B8T |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data