

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50304619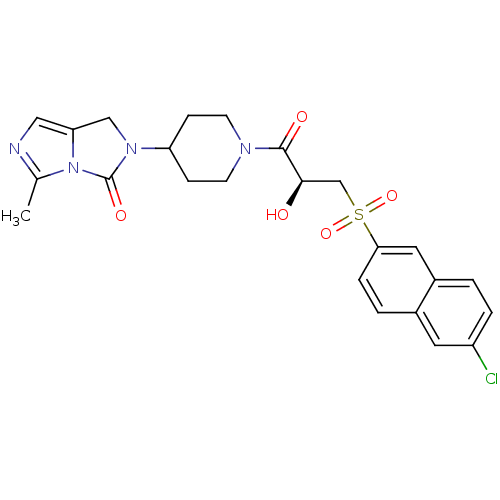 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human thrombin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50304620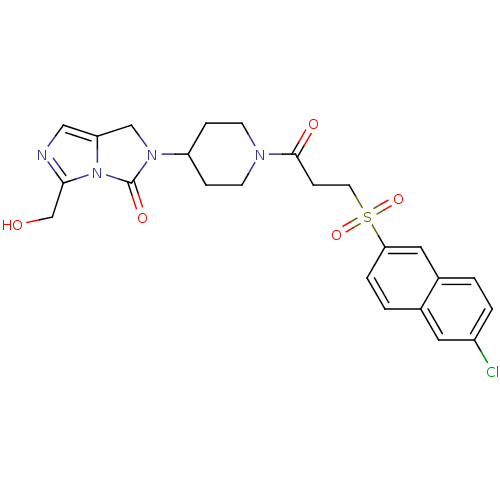 (2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human thrombin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50389606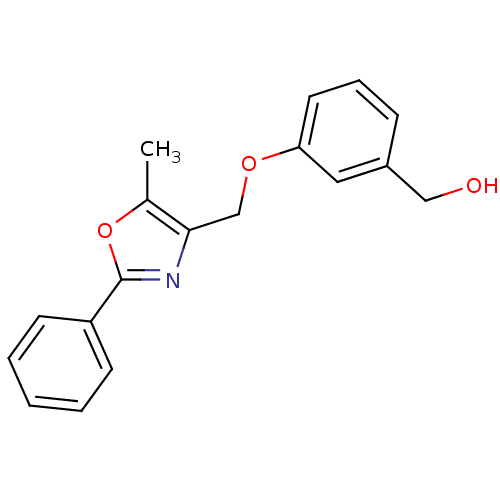 (CHEMBL2069625) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human unphosphorylated N-terminal-His6 tagged VEGFR2 catalytic domain using 5-FAM-EEPLYWSFPAKKK-CONH2 as substrate after 60 mins by mob... | ACS Med Chem Lett 3: 342-346 (2012) Article DOI: 10.1021/ml3000403 BindingDB Entry DOI: 10.7270/Q2FN1782 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50304620 (2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human plasmin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50304620 (2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human plasmin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131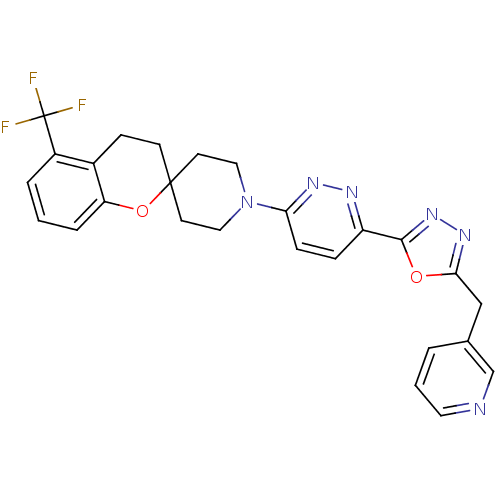 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296309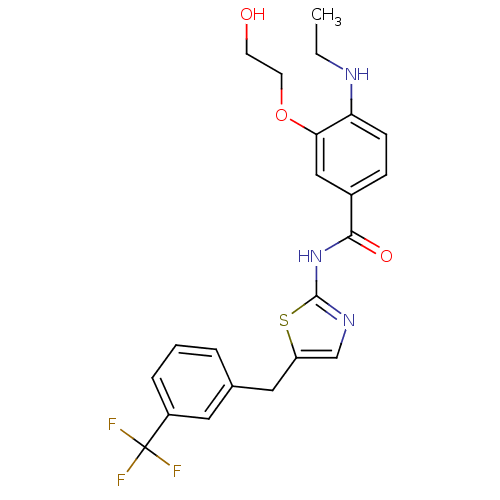 (4-(ethylamino)-3-(2-hydroxyethoxy)-N-(5-(3-(triflu...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50330378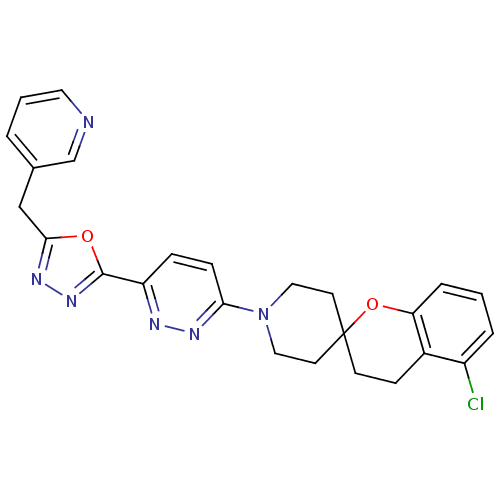 (5-Chloro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330378 (5-Chloro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306116 (CHEMBL594289 | N-(2-hydroxy-2-phenylethyl)-6-(spir...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0680 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399537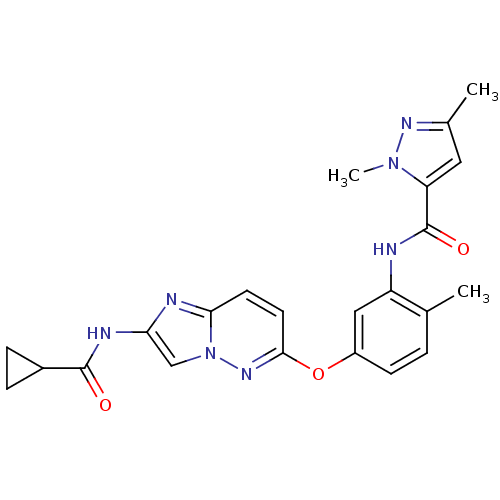 (CHEMBL2180604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 60 mins by AlphaScreen assay in presence of 1000 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330379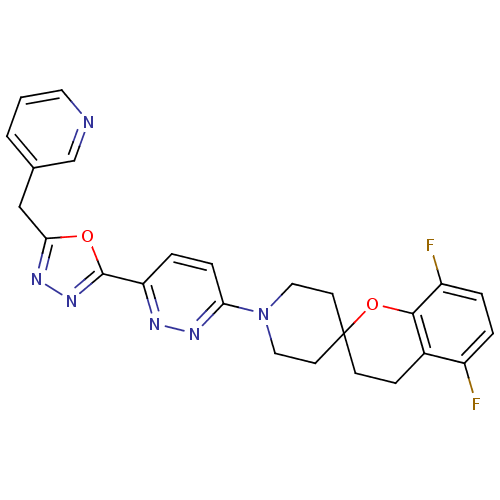 (5,8-Difluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-o...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296527 (CHEMBL552269 | N-(5-(3,5-difluorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50306129 (1'-(6-(3-(pyridin-3-ylmethyl)-1H-1,2,4-triazol-5-y...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432398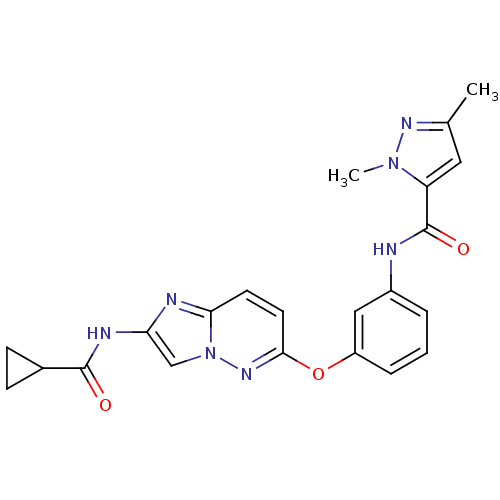 (CHEMBL2348996) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 60 mins by AlphaScreen assay in presence of 1000 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330380 (5-Methyl-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50330380 (5-Methyl-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296528 (CHEMBL557445 | N-(5-(4-fluoro-3-(trifluoromethyl)b...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50306126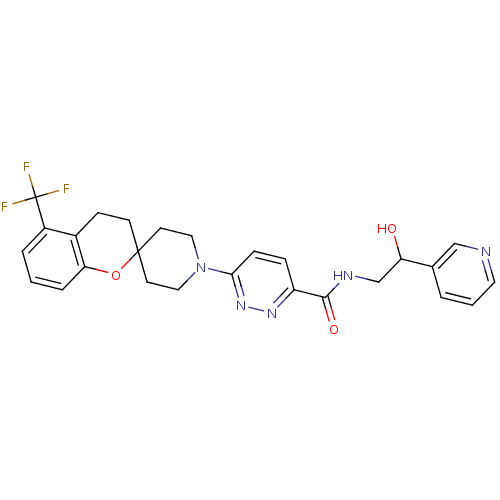 (CHEMBL605412 | N-(2-hydroxy-2-(pyridin-3-yl)ethyl)...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330381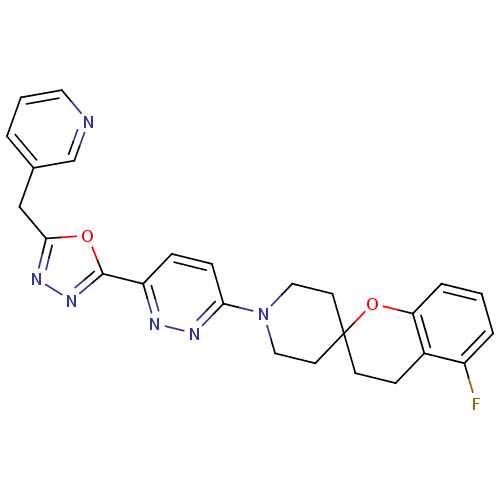 (5-Fluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in HEK293A cell microsomes assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296309 (4-(ethylamino)-3-(2-hydroxyethoxy)-N-(5-(3-(triflu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in mouse microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306126 (CHEMBL605412 | N-(2-hydroxy-2-(pyridin-3-yl)ethyl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate after 60 mins in presence of S9 microsomal fraction | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306112 (CHEMBL595247 | N-(2-hydroxy-2-phenylethyl)-6-(4-hy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50330379 (5,8-Difluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-o...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsome assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306114 (CHEMBL594084 | N-(2-hydroxy-2-phenylethyl)-6-(3-hy...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296305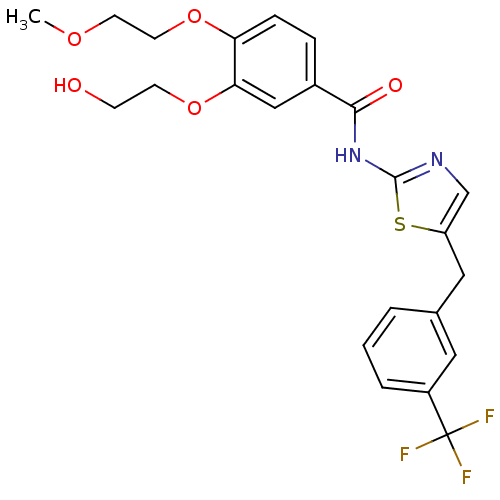 (3-(2-hydroxyethoxy)-4-(2-methoxyethoxy)-N-(5-(3-(t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in human microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50306124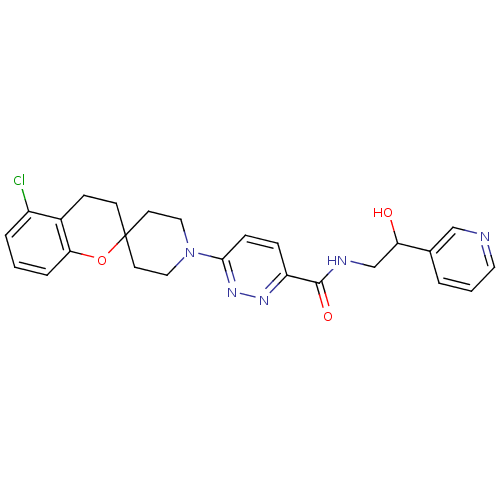 (6-(5-chlorospiro[chroman-2,4'-piperidine]-1'-yl)-N...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomal liver S9 microsomal fraction assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of stearoyl-CoA desaturase 1 in mouse microsome assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 19: 4159-66 (2009) Article DOI: 10.1016/j.bmcl.2009.05.123 BindingDB Entry DOI: 10.7270/Q2PZ58VW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296528 (CHEMBL557445 | N-(5-(4-fluoro-3-(trifluoromethyl)b...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296295 (CHEMBL552173 | N-(5-(3,5-bis(trifluoromethyl)benzy...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306110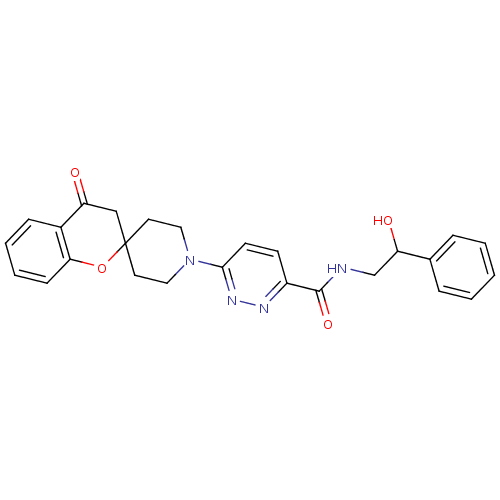 (CHEMBL607314 | N-(2-hydroxy-2-phenylethyl)-6-(4-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296526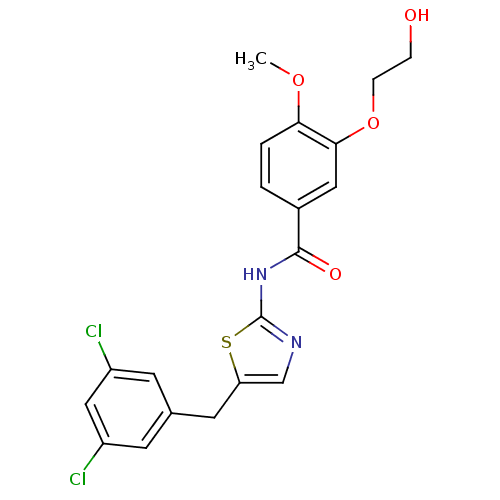 (CHEMBL552126 | N-(5-(3,5-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330380 (5-Methyl-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50330381 (5-Fluoro-1'-{6-[5-(pyridin-3-ylmethyl)-1,3,4-oxadi...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co. Ltd Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in human 293A cells assessed as conversion of [14C]stearate to [14C]oleate | Bioorg Med Chem Lett 20: 746-54 (2010) Article DOI: 10.1016/j.bmcl.2009.11.043 BindingDB Entry DOI: 10.7270/Q28C9WBJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50306131 (1'-(6-(5-(pyridin-3-ylmethyl)-1,3,4-oxadiazol-2-yl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of human SCD1 expressed in HEK293A cells assessed as reduction in conversion of [14C]stearic acid to [14C]oleic acid after 30 mins | Eur J Med Chem 45: 4788-96 (2010) Article DOI: 10.1016/j.ejmech.2010.07.044 BindingDB Entry DOI: 10.7270/Q2CZ37CN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296531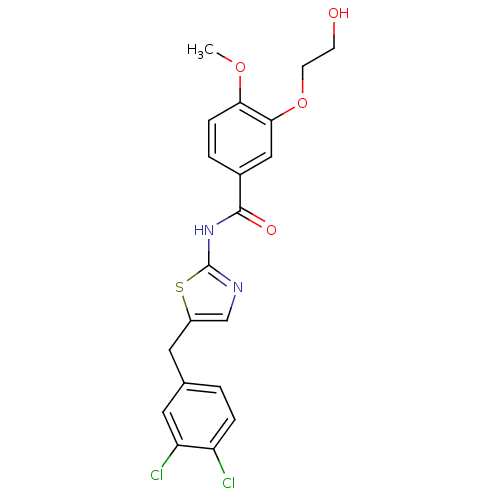 (CHEMBL550800 | N-(5-(3,4-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50399537 (CHEMBL2180604) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50432408 (CHEMBL2348998) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of VEGFR2 (unknown origin) using biotinylated poly(Glu:Tyr) as substrate after 5 mins by AlphaScreen assay in presence of 10 uM of ATP | Bioorg Med Chem 21: 2333-45 (2013) Article DOI: 10.1016/j.bmc.2013.01.074 BindingDB Entry DOI: 10.7270/Q22B90C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stearoyl-CoA desaturase (Homo sapiens (Human)) | BDBM50296526 (CHEMBL552126 | N-(5-(3,5-dichlorobenzyl)thiazol-2-...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in human microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296529 (CHEMBL552270 | N-(5-(3-chloro-4-fluorobenzyl)thiaz...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acyl-CoA desaturase 1 (Mus musculus) | BDBM50296522 (CHEMBL561588 | N-(5-(3-chlorobenzyl)thiazol-2-yl)-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of SCD1 in mouse microsomes assessed as conversion of [14C]stearate to [14C]oleate after 60 mins | Bioorg Med Chem Lett 19: 4151-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.119 BindingDB Entry DOI: 10.7270/Q21836H1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 620 total ) | Next | Last >> |