

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orotidine 5'-phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM50199178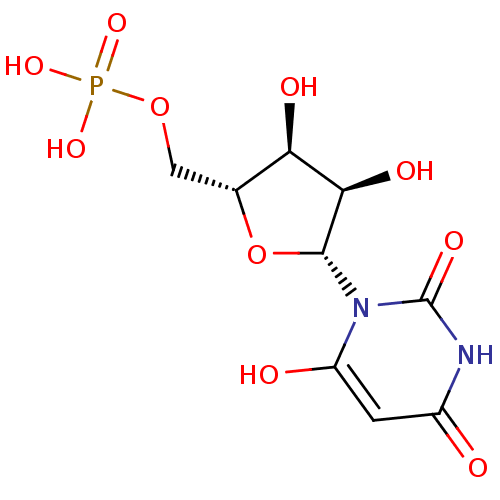 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisia uridine 5'-monophosphate synthase | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM50199178 (1-beta-D-ribofuranosyl(3H)pyrimidine-2,4,6-trione ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.00900 | -63.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisia uridine 5'-monophosphate synthase after overnight incubation at room temperature by VP-ITC microcalorimetry | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM50378784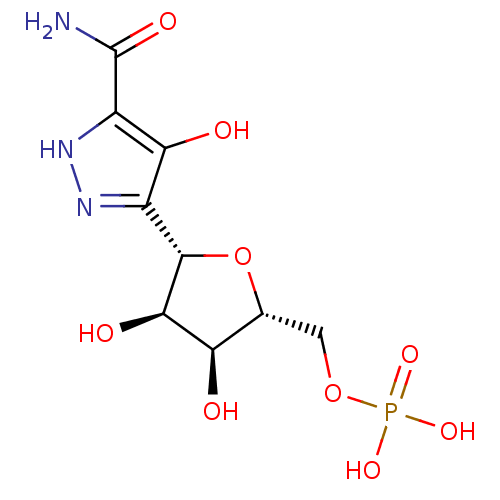 (CHEMBL1164953) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisia uridine 5'-monophosphate synthase after overnight incubation at room temperature by VP-ITC microcalorimetry | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM50378784 (CHEMBL1164953) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | 17 | -44.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University Health Network Curated by ChEMBL | Assay Description Inhibition of human uridine 5'-monophosphate synthase after overnight incubation at room temperature by UV spectroscopy | Bioorg Med Chem 18: 4032-41 (2010) Article DOI: 10.1016/j.bmc.2010.04.017 BindingDB Entry DOI: 10.7270/Q24T6KBX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Saccharomyces cerevisiae) | BDBM21340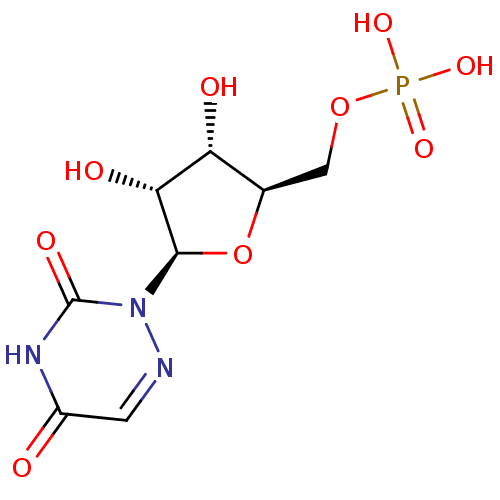 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae ODCase at 25 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21337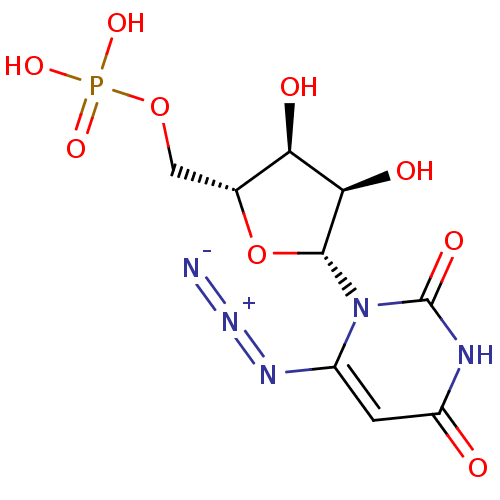 (6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21337 (6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 200 | -42.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27946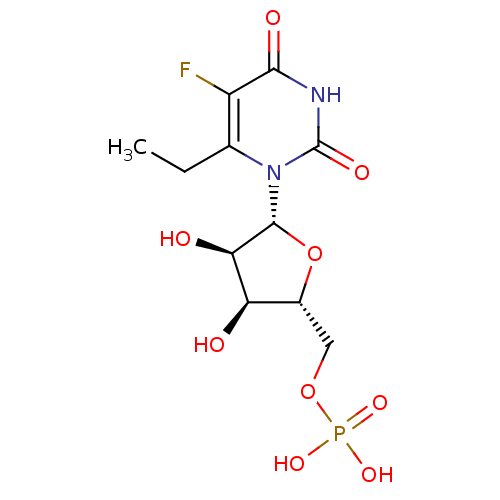 (uridine derivative, 43 | {[(2R,3S,4R,5R)-5-(6-ethy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | -38.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27944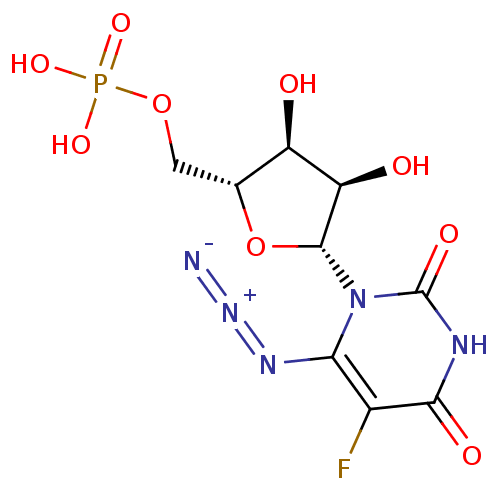 (uridine derivative, 41 | {[(2R,3S,4R,5R)-5-(6-azid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM50341908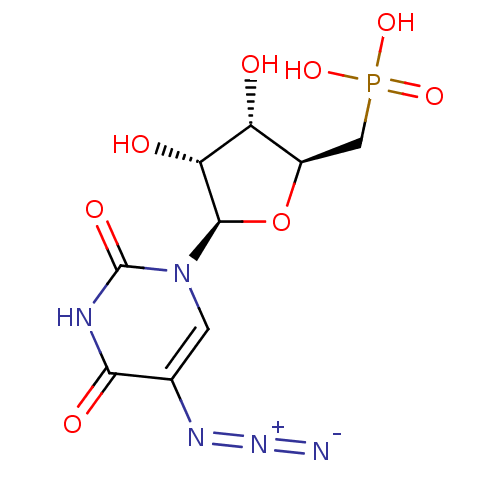 (((2S,3S,4R,5R)-5-(5-azido-2,4-dioxo-3,4-dihydropyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Irreversible inhibition of Methanobacterium thermoautotrophicum 5'-monophosphate decarboxylase | J Med Chem 54: 2891-901 (2011) Article DOI: 10.1021/jm101642g BindingDB Entry DOI: 10.7270/Q2V98924 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21338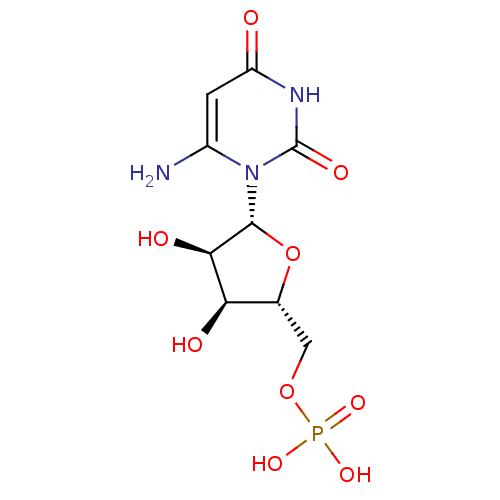 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 840 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21338 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 840 | -38.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21338 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase at 55 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21340 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Plasmodium falciparum ODCase at 25 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21340 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.10E+3 | -35.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21337 (6-Azido-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 2.00E+3 | -33.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21338 (6-Amino-uridine 5-O-Monophosphate | C6-Uridine Der...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.10E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27945 (uridine derivative, 42 | {[(2R,3S,4R,5R)-5-(6-amin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.14E+4 | -31.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21340 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase at 55 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21340 (6-aza-UMP | C6-Uridine Derivative, 18 | {[(2R,3S,4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.24E+4 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27945 (uridine derivative, 42 | {[(2R,3S,4R,5R)-5-(6-amin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.66E+4 | -28.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM21339 (6-Methyl-uridine 5-O-Monophosphate | C6-Uridine De...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21335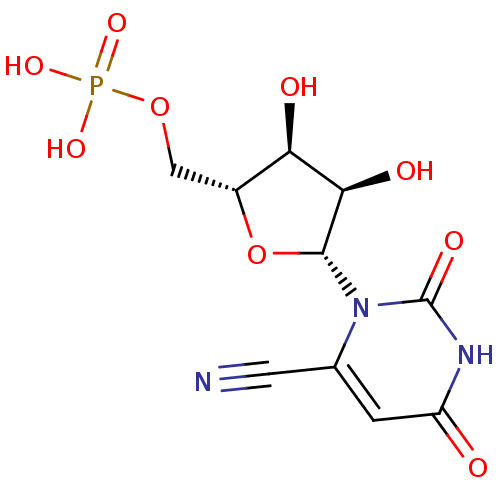 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.60E+4 | -27.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21335 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM27946 (uridine derivative, 43 | {[(2R,3S,4R,5R)-5-(6-ethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21335 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.90E+4 | -28.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21335 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum ODCase at 55 degreeC by competitive binding assay | J Med Chem 49: 4937-45 (2006) Article DOI: 10.1021/jm060202r BindingDB Entry DOI: 10.7270/Q2J9676J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-arginine deiminase type-2 (Mus musculus) | BDBM50248829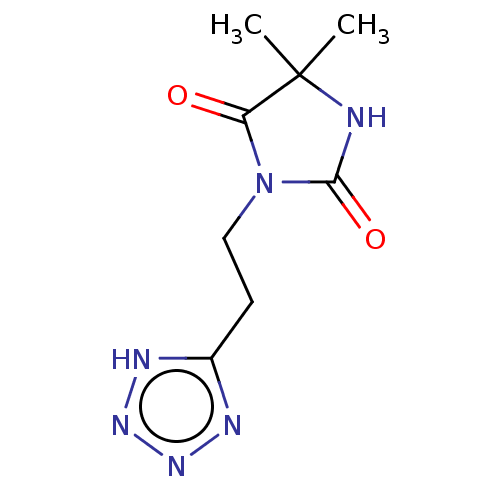 (CHEMBL4084229) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of full length recombinant mouse N-terminal GST-tagged PAD2 expressed in baculovirus infected Sf9 cells using benzoyl arginine ethyl ester... | Bioorg Med Chem 25: 2643-2656 (2017) Article DOI: 10.1016/j.bmc.2017.03.006 BindingDB Entry DOI: 10.7270/Q2HM5BW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium falciparum (malaria parasite P. falcipa...) | BDBM21339 (6-Methyl-uridine 5-O-Monophosphate | C6-Uridine De...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 3.41E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM50341905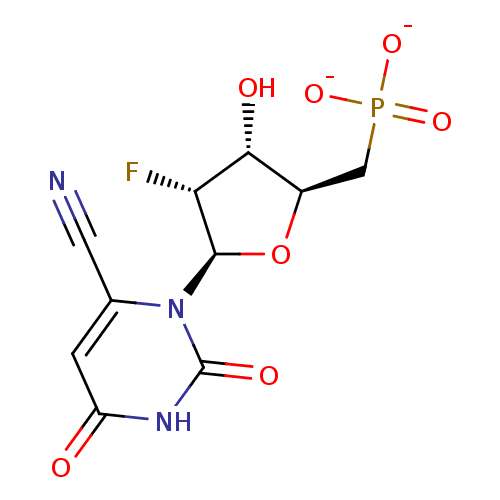 (CHEMBL1765124 | ammonium 6-Cyano-2'-deoxy-2'-fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of Methanobacterium thermoautotrophicum 5'-monophosphate decarboxylase by VP-ITC microcalorimeter | J Med Chem 54: 2891-901 (2011) Article DOI: 10.1021/jm101642g BindingDB Entry DOI: 10.7270/Q2V98924 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50248829 (CHEMBL4084229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal GST-tagged PAD4 expressed in baculovirus infected Sf9 cells using benzoyl arginine ethylester ... | Bioorg Med Chem 25: 2643-2656 (2017) Article DOI: 10.1016/j.bmc.2017.03.006 BindingDB Entry DOI: 10.7270/Q2HM5BW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-2 (Mus musculus) | BDBM454169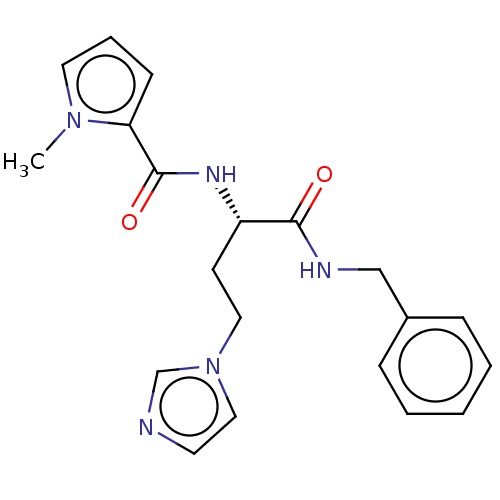 (BDBM50246180 | US10716791, Code KP-302) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of full length mouse GST-tagged PAD2 expressed in baculovirus infected Sf9 insect cells preincubated for 30 mins followed by substrate add... | J Med Chem 60: 8876-8887 (2017) Article DOI: 10.1021/acs.jmedchem.7b01102 BindingDB Entry DOI: 10.7270/Q2QF8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine-5'-phosphate decarboxylase (Plasmodium vivax (malaria parasite P. vivax)) | BDBM21335 (6-cyanouridine 5-monophosphate | C6-Uridine Deriva...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 6.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50246191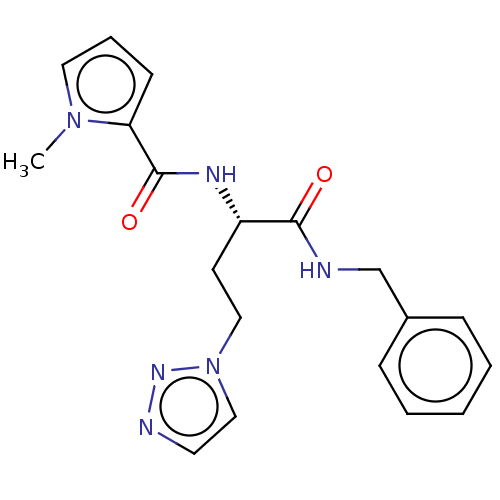 (CHEMBL4089260) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of full length human GST-tagged PAD4 expressed in baculovirus infected Sf9 insect cells preincubated for 30 mins followed by substrate add... | J Med Chem 60: 8876-8887 (2017) Article DOI: 10.1021/acs.jmedchem.7b01102 BindingDB Entry DOI: 10.7270/Q2QF8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50426301 (CHEMBL1402123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of PAD4 (unknown origin) at 52 degC | J Med Chem 56: 1715-22 (2013) Article DOI: 10.1021/jm301755q BindingDB Entry DOI: 10.7270/Q2ZS2XTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50426300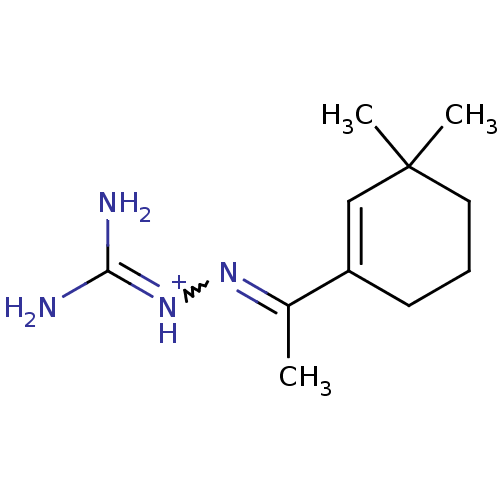 (CHEMBL2312699) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of PAD4 (unknown origin) at 52 degC | J Med Chem 56: 1715-22 (2013) Article DOI: 10.1021/jm301755q BindingDB Entry DOI: 10.7270/Q2ZS2XTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-2 (Mus musculus) | BDBM50246191 (CHEMBL4089260) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of full length mouse GST-tagged PAD2 expressed in baculovirus infected Sf9 insect cells preincubated for 30 mins followed by substrate add... | J Med Chem 60: 8876-8887 (2017) Article DOI: 10.1021/acs.jmedchem.7b01102 BindingDB Entry DOI: 10.7270/Q2QF8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM454162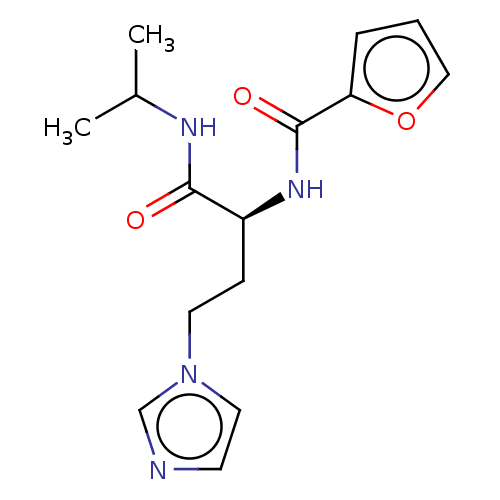 (BDBM50246174 | US10716791, Code KP-286) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of full length human GST-tagged PAD4 expressed in baculovirus infected Sf9 insect cells preincubated for 30 mins followed by substrate add... | J Med Chem 60: 8876-8887 (2017) Article DOI: 10.1021/acs.jmedchem.7b01102 BindingDB Entry DOI: 10.7270/Q2QF8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uridine 5'-monophosphate synthase (Homo sapiens (Human)) | BDBM27943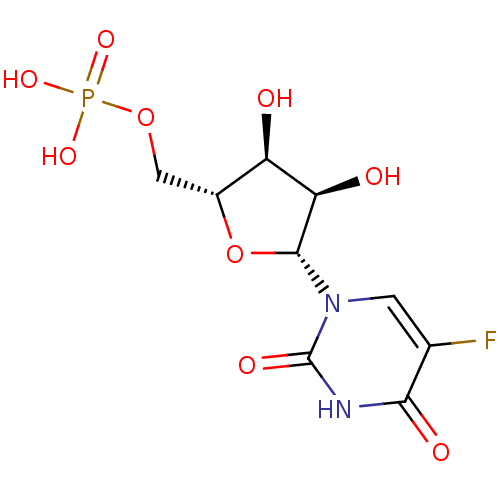 (uridine derivative, 39 | {[(2R,3S,4R,5R)-5-(5-fluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 9.80E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein-arginine deiminase type-1 (Homo sapiens (Human)) | BDBM50246191 (CHEMBL4089260) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of full length human GST-tagged PAD1 expressed in baculovirus infected Sf9 insect cells preincubated for 30 mins followed by substrate add... | J Med Chem 60: 8876-8887 (2017) Article DOI: 10.1021/acs.jmedchem.7b01102 BindingDB Entry DOI: 10.7270/Q2QF8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50426303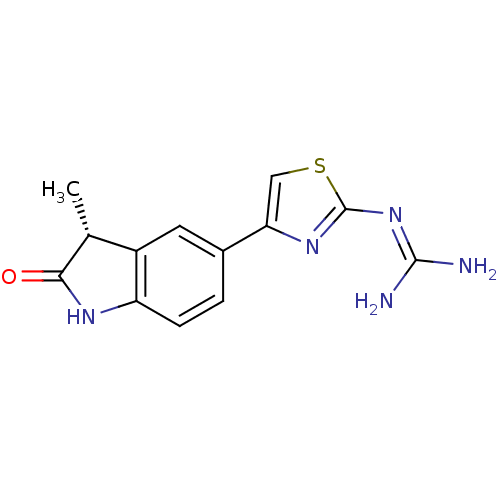 (CHEMBL2312697) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of PAD4 (unknown origin) at 37 degC | J Med Chem 56: 1715-22 (2013) Article DOI: 10.1021/jm301755q BindingDB Entry DOI: 10.7270/Q2ZS2XTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50426300 (CHEMBL2312699) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.27E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of PAD4 (unknown origin) at 37 degC | J Med Chem 56: 1715-22 (2013) Article DOI: 10.1021/jm301755q BindingDB Entry DOI: 10.7270/Q2ZS2XTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50426302 (CHEMBL2312698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of PAD4 (unknown origin) at 37 degC | J Med Chem 56: 1715-22 (2013) Article DOI: 10.1021/jm301755q BindingDB Entry DOI: 10.7270/Q2ZS2XTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21339 (6-Methyl-uridine 5-O-Monophosphate | C6-Uridine De...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.34E+5 | -24.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 52: 1648-58 (2009) Article DOI: 10.1021/jm801224t BindingDB Entry DOI: 10.7270/Q2RR1WJB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orotidine 5'-phosphate decarboxylase (Methanobacterium thermoautotrophicum) | BDBM21339 (6-Methyl-uridine 5-O-Monophosphate | C6-Uridine De...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.34E+5 | -24.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 55 |
Toronto General Research Institute | Assay Description An enzyme assay method using isothermal titration calorimetry (ITC) was developed to investigate the inhibition kinetics of ODCase. Titrations were p... | J Med Chem 51: 439-48 (2008) Article DOI: 10.1021/jm7010673 BindingDB Entry DOI: 10.7270/Q27H1GWS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cAMP-specific 3',5'-cyclic phosphodiesterase 4A (Homo sapiens (Human)) | BDBM50426301 (CHEMBL1402123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.53E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of PAD4 (unknown origin) at 37 degC | J Med Chem 56: 1715-22 (2013) Article DOI: 10.1021/jm301755q BindingDB Entry DOI: 10.7270/Q2ZS2XTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-1 (Homo sapiens (Human)) | BDBM454169 (BDBM50246180 | US10716791, Code KP-302) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.55E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of full length human GST-tagged PAD1 expressed in baculovirus infected Sf9 insect cells preincubated for 30 mins followed by substrate add... | J Med Chem 60: 8876-8887 (2017) Article DOI: 10.1021/acs.jmedchem.7b01102 BindingDB Entry DOI: 10.7270/Q2QF8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-2 (Mus musculus) | BDBM50426302 (CHEMBL2312698) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of full length recombinant mouse N-terminal GST-tagged PAD2 expressed in baculovirus infected Sf9 cells using benzoyl arginine ethyl ester... | Bioorg Med Chem 25: 2643-2656 (2017) Article DOI: 10.1016/j.bmc.2017.03.006 BindingDB Entry DOI: 10.7270/Q2HM5BW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM454169 (BDBM50246180 | US10716791, Code KP-302) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of full length human GST-tagged PAD4 expressed in baculovirus infected Sf9 insect cells preincubated for 30 mins followed by substrate add... | J Med Chem 60: 8876-8887 (2017) Article DOI: 10.1021/acs.jmedchem.7b01102 BindingDB Entry DOI: 10.7270/Q2QF8W9F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein-arginine deiminase type-4 (Homo sapiens (Human)) | BDBM50426302 (CHEMBL2312698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.83E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Health Network Curated by ChEMBL | Assay Description Inhibition of full length recombinant human N-terminal GST-tagged PAD4 expressed in baculovirus infected Sf9 cells using benzoyl arginine ethylester ... | Bioorg Med Chem 25: 2643-2656 (2017) Article DOI: 10.1016/j.bmc.2017.03.006 BindingDB Entry DOI: 10.7270/Q2HM5BW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 100 total ) | Next | Last >> |