

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185799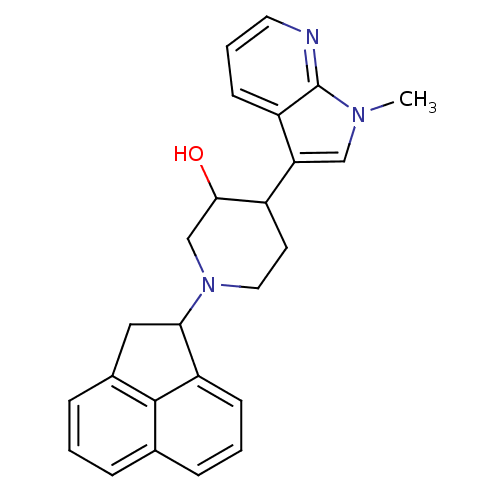 (1-(1,2-dihydroacenaphthylen-1-yl)-4-(1-methyl-1H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185800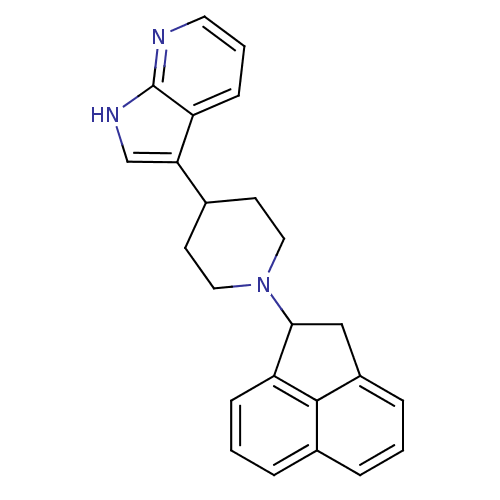 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185796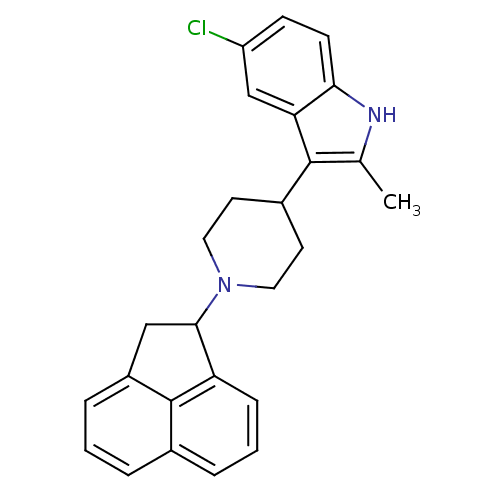 (5-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185805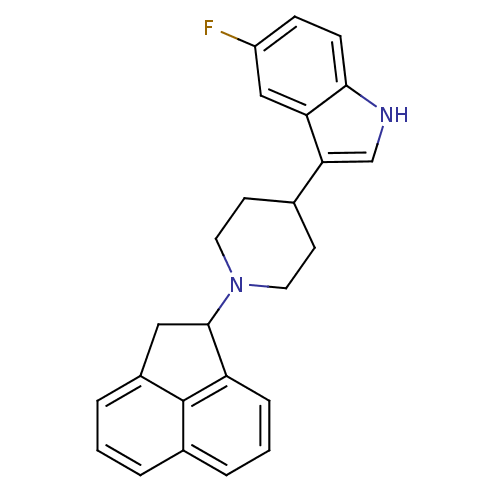 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from ORL1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185813 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185811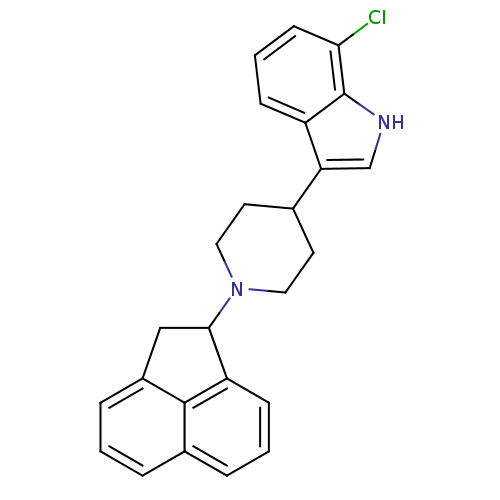 (7-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [125I]Tyr14-nociceptin from ORL1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185788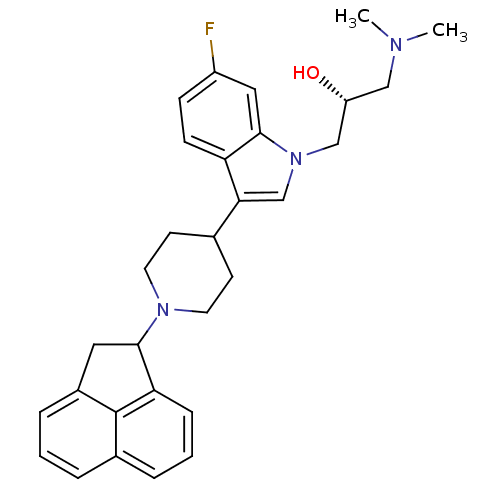 ((2R)-1-(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185803 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185812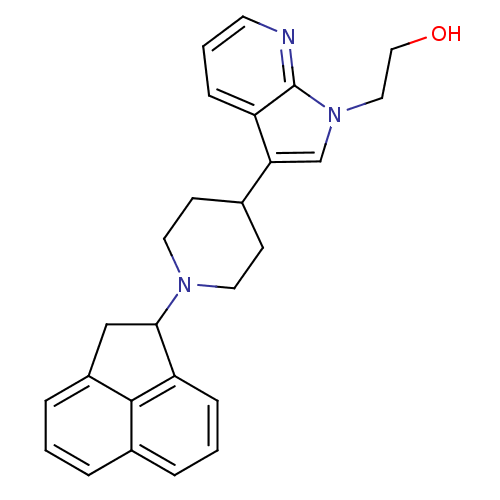 (2-(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185786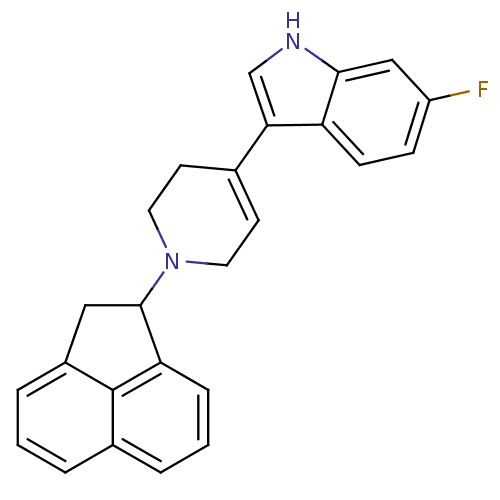 (3-(1-(1,2-dihydroacenaphthylen-1-yl)-1,2,3,6-tetra...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185799 (1-(1,2-dihydroacenaphthylen-1-yl)-4-(1-methyl-1H-p...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185810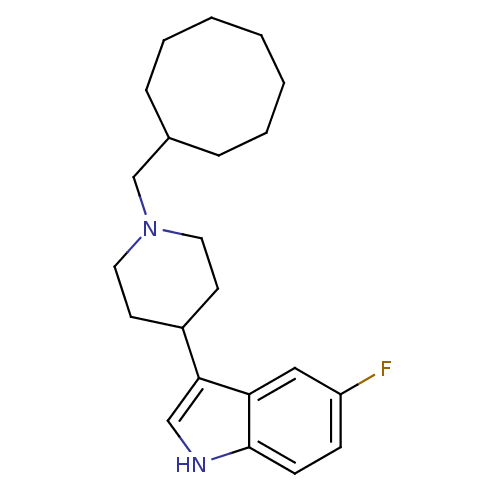 (3-(1-(cyclooctylmethyl)piperidin-4-yl)-5-fluoro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185792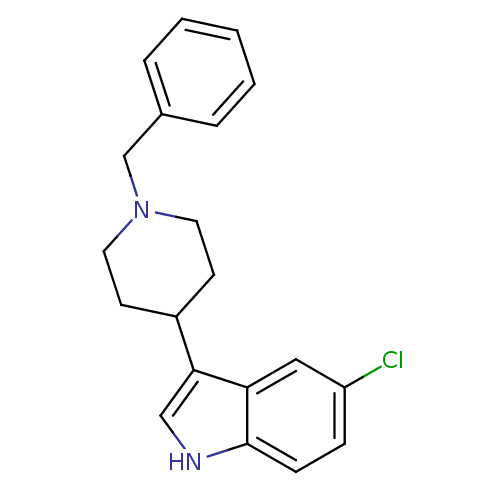 (3-(1-benzylpiperidin-4-yl)-5-chloro-1H-indole | CH...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185796 (5-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185811 (7-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185810 (3-(1-(cyclooctylmethyl)piperidin-4-yl)-5-fluoro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185808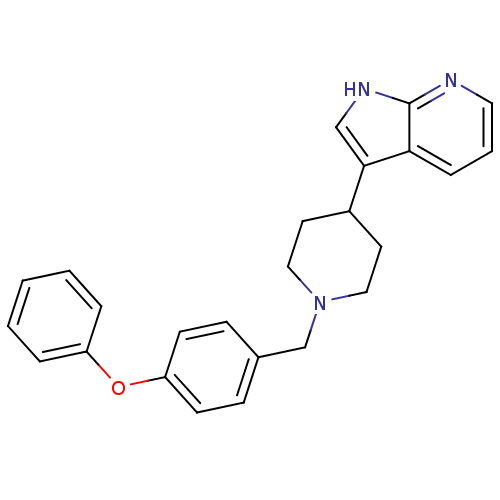 (3-(1-(4-phenoxybenzyl)piperidin-4-yl)-1H-pyrrolo[2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185792 (3-(1-benzylpiperidin-4-yl)-5-chloro-1H-indole | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185807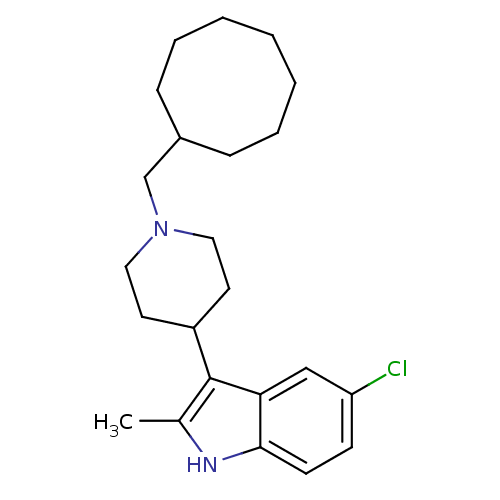 (5-chloro-3-(1-(cyclooctylmethyl)piperidin-4-yl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185802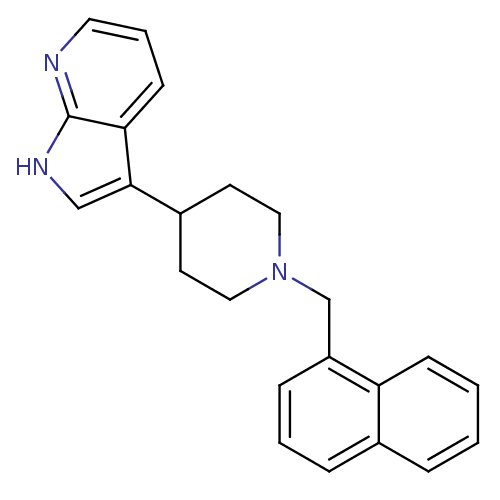 (3-(1-(naphthalen-1-ylmethyl)piperidin-4-yl)-1H-pyr...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185800 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185798 (7-chloro-3-(1-(cyclooctylmethyl)piperidin-4-yl)-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185794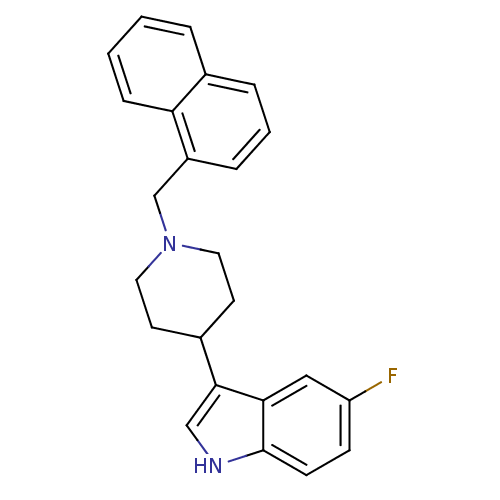 (5-fluoro-3-(1-(naphthalen-1-ylmethyl)piperidin-4-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185805 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185809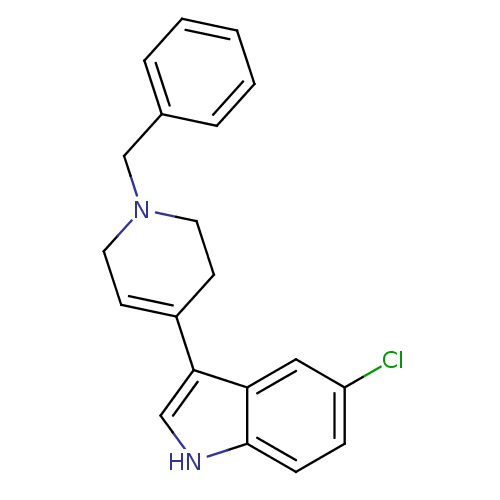 (3-(1-benzyl-1,2,3,6-tetrahydropyridin-4-yl)-5-chlo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185812 (2-(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185806 (3-(1-(cyclooctylmethyl)piperidin-4-yl)-1H-pyrrolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185798 (7-chloro-3-(1-(cyclooctylmethyl)piperidin-4-yl)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185796 (5-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185807 (5-chloro-3-(1-(cyclooctylmethyl)piperidin-4-yl)-2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185802 (3-(1-(naphthalen-1-ylmethyl)piperidin-4-yl)-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185795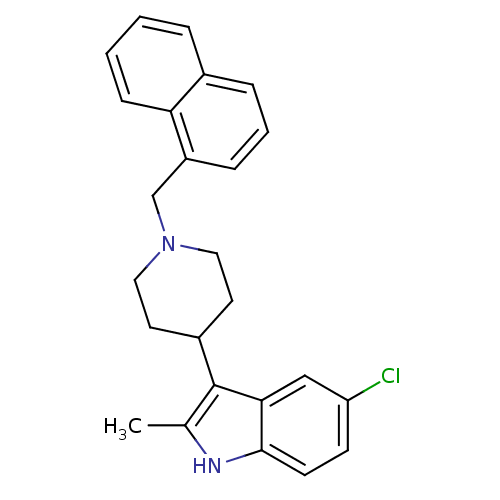 (5-chloro-2-methyl-3-(1-(naphthalen-1-ylmethyl)pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185788 ((2R)-1-(3-(1-(1,2-dihydroacenaphthylen-1-yl)piperi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185794 (5-fluoro-3-(1-(naphthalen-1-ylmethyl)piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185802 (3-(1-(naphthalen-1-ylmethyl)piperidin-4-yl)-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185794 (5-fluoro-3-(1-(naphthalen-1-ylmethyl)piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185795 (5-chloro-2-methyl-3-(1-(naphthalen-1-ylmethyl)pipe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185813 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185803 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185789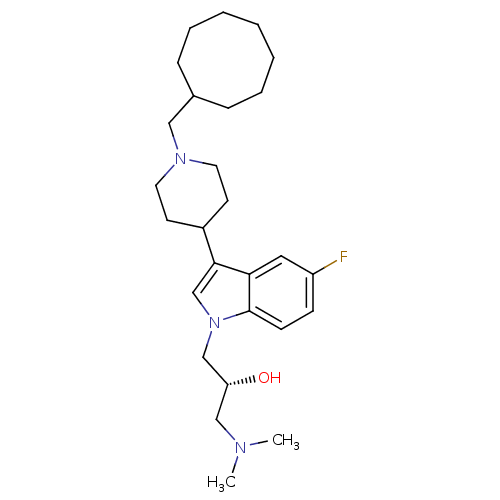 ((R)-1-(3-(1-(cyclooctylmethyl)piperidin-4-yl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185809 (3-(1-benzyl-1,2,3,6-tetrahydropyridin-4-yl)-5-chlo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185810 (3-(1-(cyclooctylmethyl)piperidin-4-yl)-5-fluoro-1H...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185792 (3-(1-benzylpiperidin-4-yl)-5-chloro-1H-indole | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185798 (7-chloro-3-(1-(cyclooctylmethyl)piperidin-4-yl)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185807 (5-chloro-3-(1-(cyclooctylmethyl)piperidin-4-yl)-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nociceptin receptor (Homo sapiens (Human)) | BDBM50185797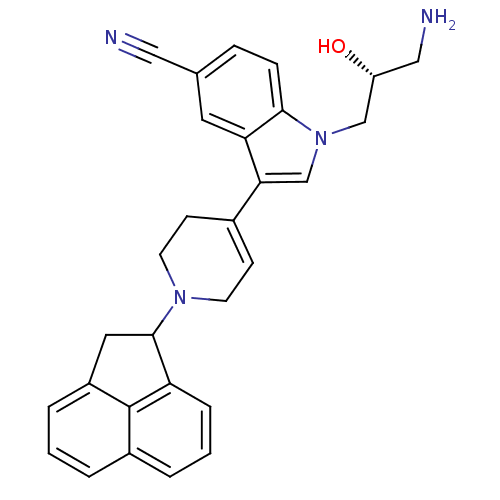 (1-((R)-3-amino-2-hydroxypropyl)-3-(1-(1,2-dihydroa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Agonist activity at ORL1 receptor expressed in HEK293 cells by calciun flux assay | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185805 (3-(1-(1,2-dihydroacenaphthylen-1-yl)piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185789 ((R)-1-(3-(1-(cyclooctylmethyl)piperidin-4-yl)-5-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50185806 (3-(1-(cyclooctylmethyl)piperidin-4-yl)-1H-pyrrolo[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50185811 (7-chloro-3-(1-(1,2-dihydroacenaphthylen-1-yl)piper...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]U-69593 from kappa opioid receptor expressed in HEK293 cells | Bioorg Med Chem Lett 16: 3524-8 (2006) Article DOI: 10.1016/j.bmcl.2006.03.094 BindingDB Entry DOI: 10.7270/Q2VT1RQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 250 total ) | Next | Last >> |