

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase | J Med Chem 31: 500-3 (1988) BindingDB Entry DOI: 10.7270/Q28P61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy) rate of inactivation by NpcA | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368890 (CHEMBL1790792) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against biotinylated human HIV-1 protease | J Med Chem 37: 293-304 (1994) BindingDB Entry DOI: 10.7270/Q27S7PD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50367250 (3-DEAZAARISTEROMYCIN A | CHEMBL268272) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against S-adenosyl-homocysteine hydrolase | J Med Chem 28: 471-7 (1985) BindingDB Entry DOI: 10.7270/Q21C1XF3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368891 (CHEMBL1790796) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against biotinylated human HIV-1 protease | J Med Chem 37: 293-304 (1994) BindingDB Entry DOI: 10.7270/Q27S7PD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006218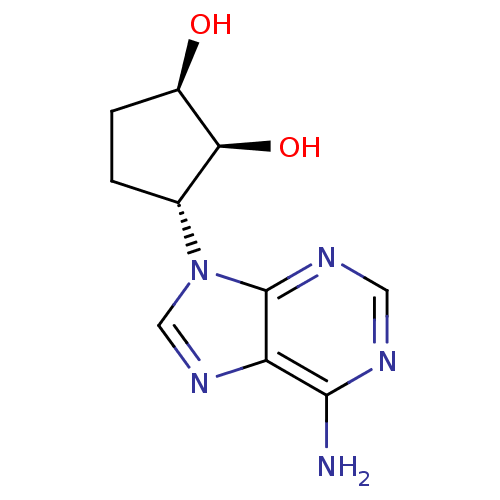 ((1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368893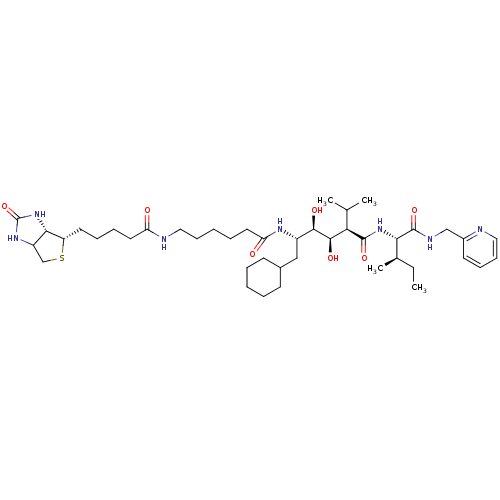 (CHEMBL1790794) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against biotinylated human HIV-1 protease | J Med Chem 37: 293-304 (1994) BindingDB Entry DOI: 10.7270/Q27S7PD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006220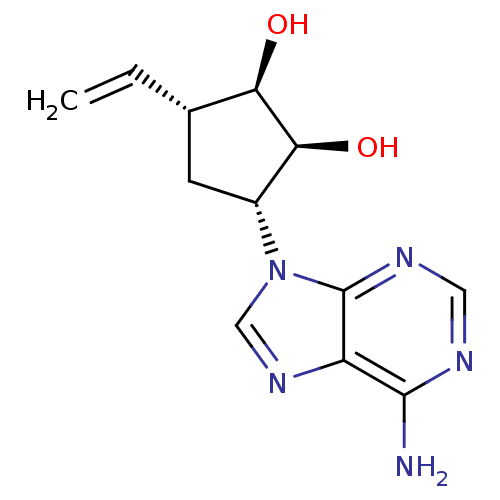 (3-(6-Amino-purin-9-yl)-5-vinyl-cyclopentane-1,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006221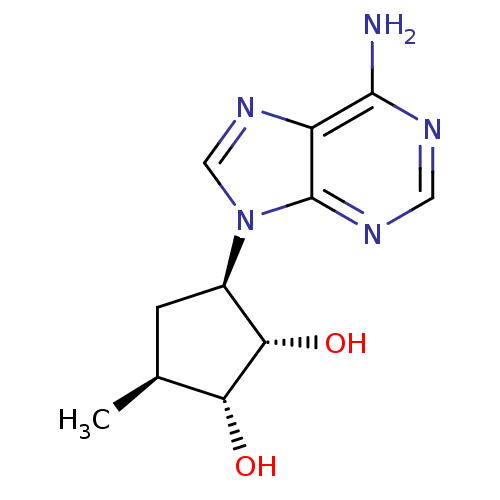 (3-(6-Amino-purin-9-yl)-5-methyl-cyclopentane-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50405655 (CHEMBL147260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase | J Med Chem 31: 500-3 (1988) BindingDB Entry DOI: 10.7270/Q28P61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50051435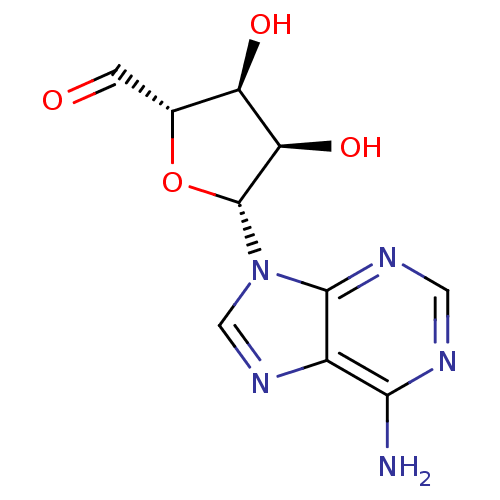 (5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method | J Med Chem 40: 1608-18 (1997) Article DOI: 10.1021/jm960828p BindingDB Entry DOI: 10.7270/Q2PR7WPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280299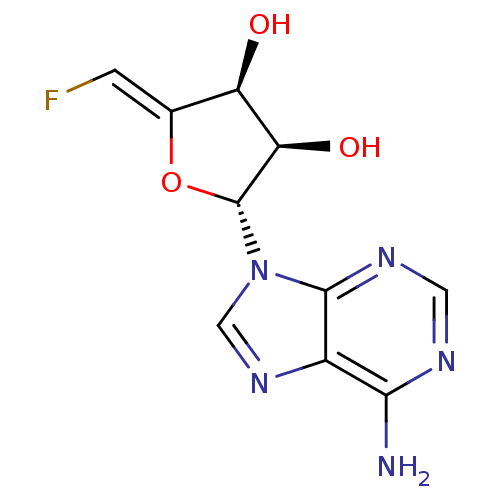 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50051436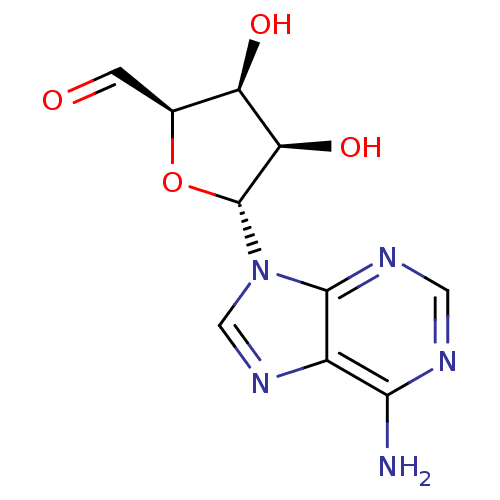 ((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50046747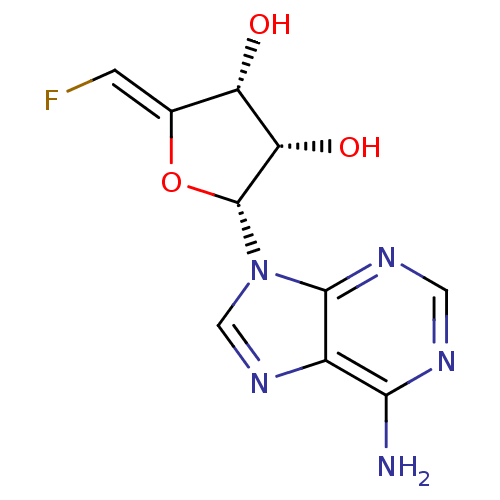 (2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50051435 (5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Inhibition of S-adenosyl-L-homocysteine hydrolase, inactivation rate constant. | J Med Chem 39: 4162-6 (1996) Article DOI: 10.1021/jm960313y BindingDB Entry DOI: 10.7270/Q2DN45QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50051436 ((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase | J Med Chem 39: 2347-53 (1996) Article DOI: 10.1021/jm950916u BindingDB Entry DOI: 10.7270/Q20Z72CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATP-dependent translocase ABCB1 (Homo sapiens (Human)) | BDBM50206310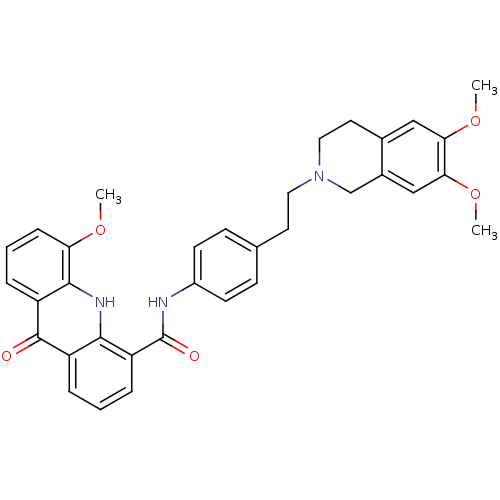 (5-Methoxy-9-oxo-9,10-dihydro-acridine-4-carboxylic...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Kansas Curated by ChEMBL | Assay Description TP_TRANSPORTER: transepithelial transport of digoxin (basal to apical) in Caco-2 cells | Pharm Res 20: 161-8 (2003) Article DOI: 10.1023/a:1022359300826 BindingDB Entry DOI: 10.7270/Q2BP05N6 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280301 ((2R,3R,4S)-2-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50046748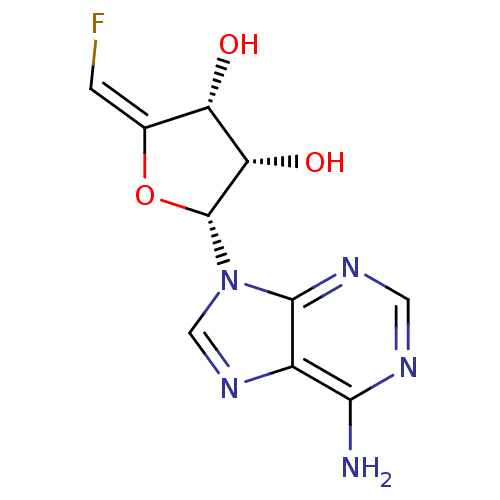 (2-(6-Amino-purin-9-yl)-5-fluoromethylene-tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006215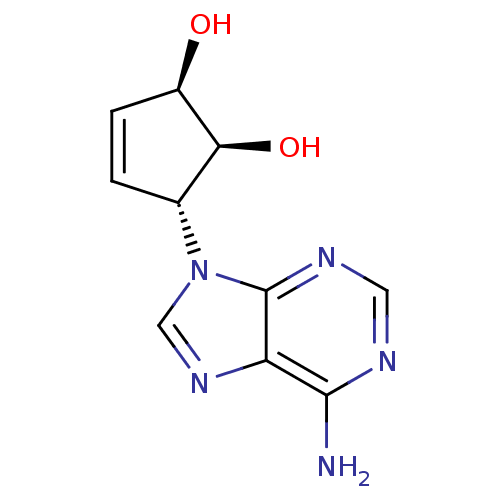 ((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase | J Med Chem 31: 500-3 (1988) BindingDB Entry DOI: 10.7270/Q28P61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50051436 ((2R,3S,4R,5R)-5-(6-Amino-purin-9-yl)-3,4-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method | J Med Chem 40: 1608-18 (1997) Article DOI: 10.1021/jm960828p BindingDB Entry DOI: 10.7270/Q2PR7WPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50051435 (5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity against recombinant rat liver S-adenosyl-homocysteine hydrolase | J Med Chem 36: 883-7 (1993) BindingDB Entry DOI: 10.7270/Q2HM593F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50051435 (5'-Dehydroadenosine | 5'-deoxy-5'-oxoadenosine | 9...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG MMDB PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Binding affinity of the compound towards purified recombinant human placental S-adenosyl-L-homocysteine hydrolase | J Med Chem 39: 2347-53 (1996) Article DOI: 10.1021/jm950916u BindingDB Entry DOI: 10.7270/Q20Z72CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368889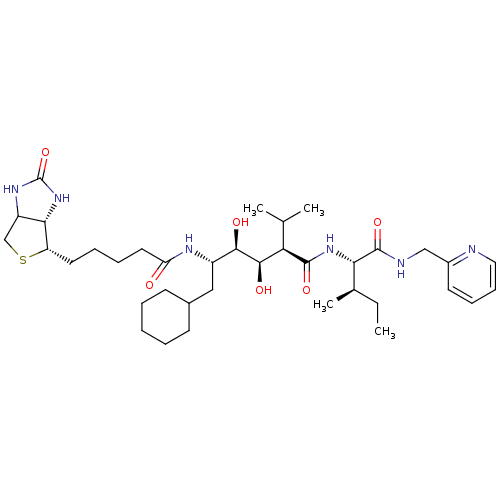 (CHEMBL1790790) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against biotinylated human HIV-1 protease | J Med Chem 37: 293-304 (1994) BindingDB Entry DOI: 10.7270/Q27S7PD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006215 ((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368892 (CHEMBL1790797) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against biotinylated human HIV-1 protease | J Med Chem 37: 293-304 (1994) BindingDB Entry DOI: 10.7270/Q27S7PD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280300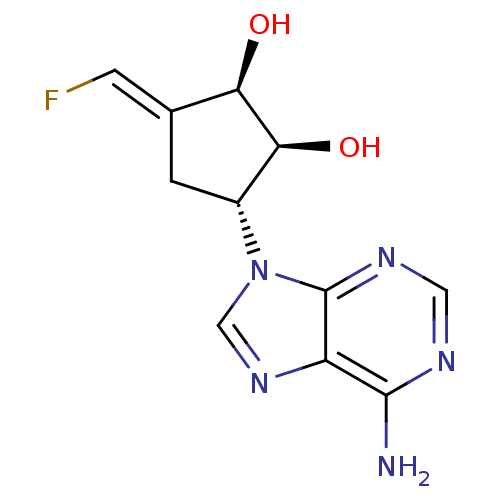 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023889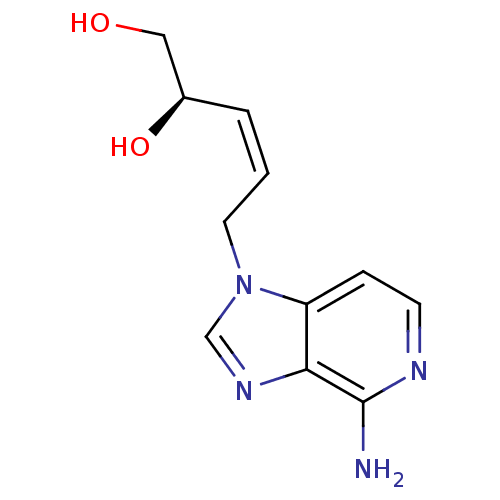 (5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-pent-3-ene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50369258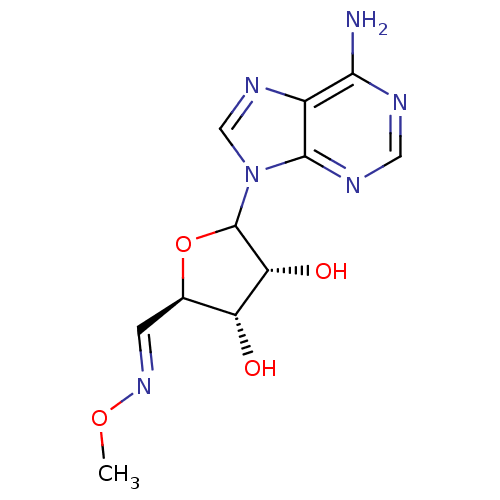 (CHEMBL606276) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method | J Med Chem 40: 1608-18 (1997) Article DOI: 10.1021/jm960828p BindingDB Entry DOI: 10.7270/Q2PR7WPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50368896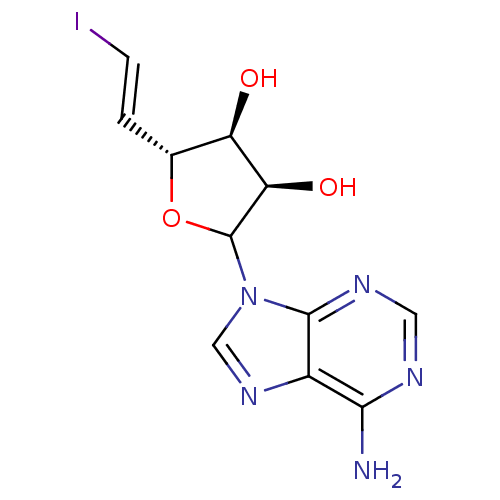 (CHEMBL608056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase | J Med Chem 37: 3579-87 (1994) BindingDB Entry DOI: 10.7270/Q2BK1D0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368887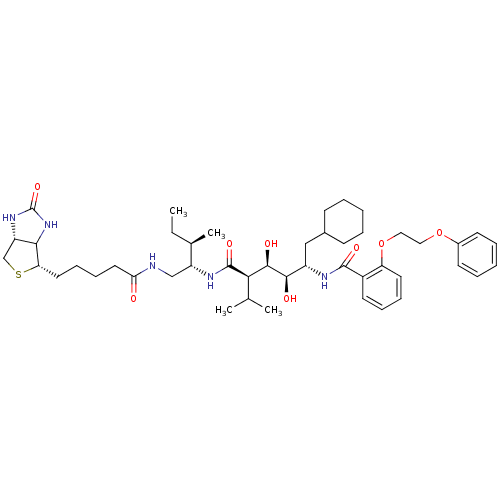 (CHEMBL1790791) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against biotinylated human HIV-1 protease | J Med Chem 37: 293-304 (1994) BindingDB Entry DOI: 10.7270/Q27S7PD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50369257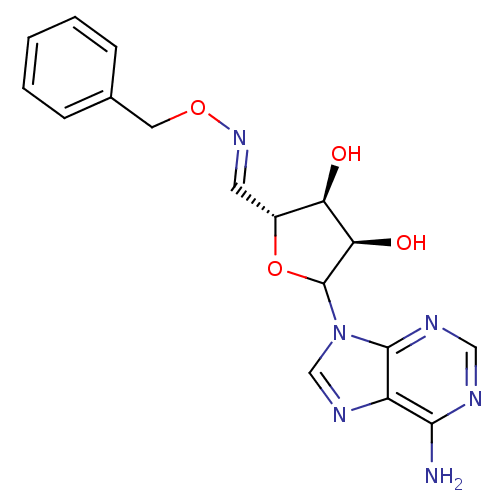 (CHEMBL605902) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method | J Med Chem 40: 1608-18 (1997) Article DOI: 10.1021/jm960828p BindingDB Entry DOI: 10.7270/Q2PR7WPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50088426 ((1R,2S,3R,5R)-3-(6-amino-9H-purin-9-yl)-5-(hydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity against S-adenosyl-homocysteine hydrolase | J Med Chem 28: 471-7 (1985) BindingDB Entry DOI: 10.7270/Q21C1XF3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023879 (2-[2-(6-Amino-purin-9-yl)-ethylidene]-propane-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50407233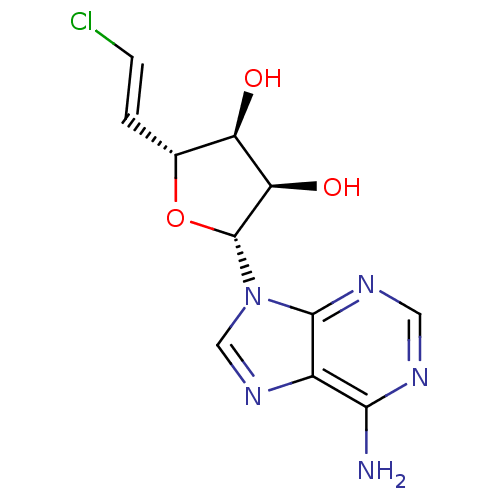 (CHEMBL2092790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase | J Med Chem 37: 3579-87 (1994) BindingDB Entry DOI: 10.7270/Q2BK1D0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50369255 (CHEMBL605900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 111 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description concentration dependent inhibition of S-Adenosyl-homocysteine hydrolase was determined by Kitz and Wilson method | J Med Chem 40: 1608-18 (1997) Article DOI: 10.1021/jm960828p BindingDB Entry DOI: 10.7270/Q2PR7WPS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Rattus norvegicus) | BDBM50280298 ((1R,2S,3R)-3-(6-Amino-purin-9-yl)-5-[1-fluoro-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its inhibitory activity against recombinant rat liver S-adenosylhomocysteine hydrolase | Bioorg Med Chem Lett 2: 1741-1744 (1992) Article DOI: 10.1016/S0960-894X(00)80467-9 BindingDB Entry DOI: 10.7270/Q21Z44B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50011148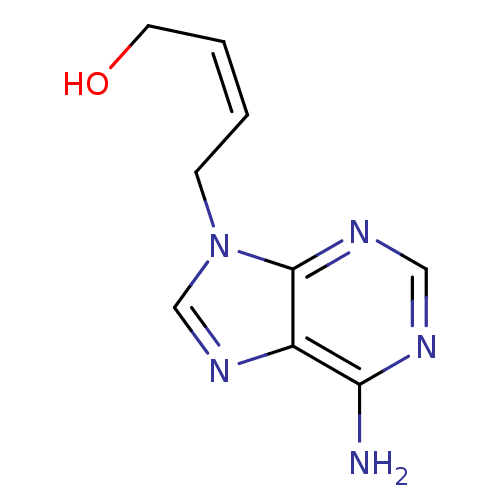 ((Z)-4-(6-Amino-purin-9-yl)-but-2-en-1-ol | 4-(6-Am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50368888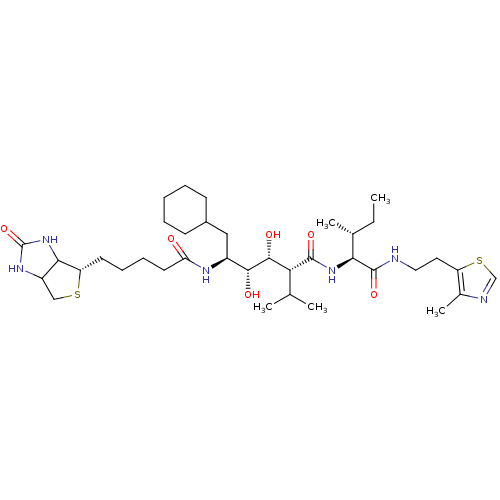 (CHEMBL1790793) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against biotinylated human HIV-1 protease | J Med Chem 37: 293-304 (1994) BindingDB Entry DOI: 10.7270/Q27S7PD1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50407232 (CHEMBL2092789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 134 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Brigham Young University Curated by ChEMBL | Assay Description Tested for kinetic constant for the inhibition of recombinant human placental S-Adenosyl-L-homocysteine Hydrolase | J Med Chem 37: 3579-87 (1994) BindingDB Entry DOI: 10.7270/Q2BK1D0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023885 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-yn-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023886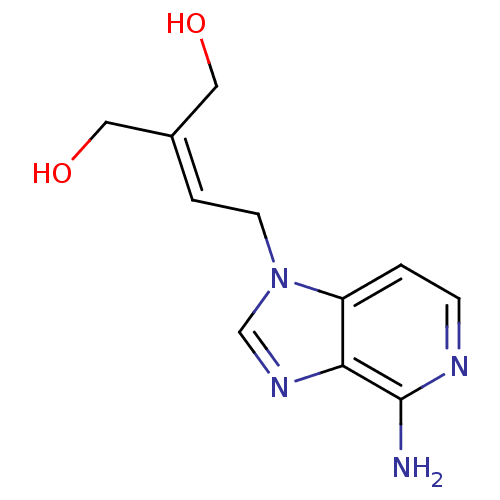 (2-[2-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-ethylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023887 (4-(6-Amino-purin-9-yl)-2-methyl-but-2-en-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50139892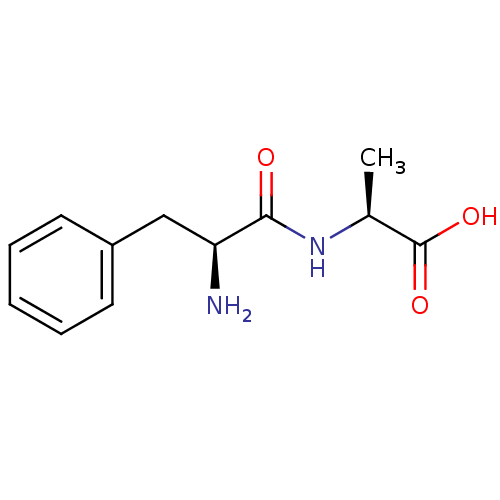 ((S)-2-((S)-2-Amino-3-phenyl-propionylamino)-propio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tromsø Curated by ChEMBL | Assay Description Affinity fo the compound against human Intestinal peptide transporter PepT1 in Caco-2 cells was measured as inhibition of [14C]-Gly-Sar uptake | J Med Chem 47: 1060-9 (2004) Article DOI: 10.1021/jm031022+ BindingDB Entry DOI: 10.7270/Q2DF6QMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023877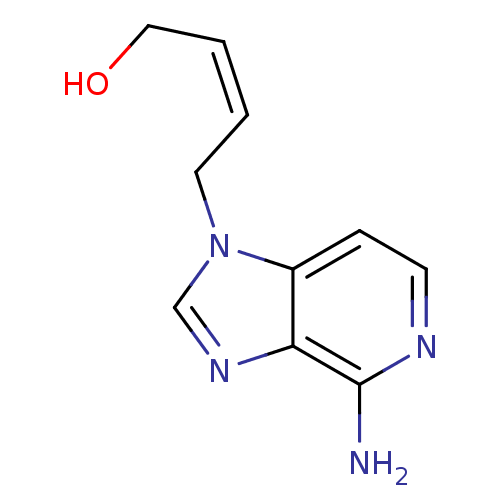 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-en-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023882 (5-(6-Amino-purin-9-yl)-pent-3-ene-1,2-diol | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50139894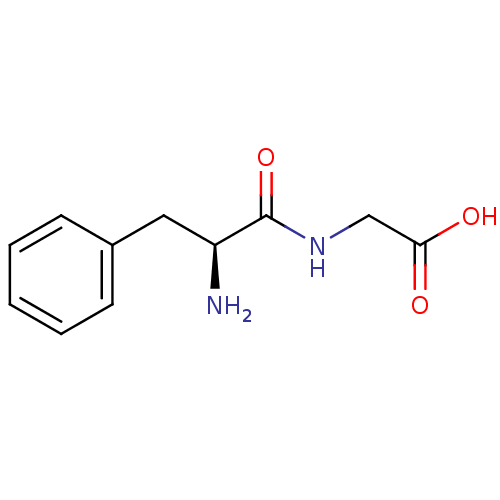 (((S)-2-Amino-3-phenyl-propionylamino)-acetic acid ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tromsø Curated by ChEMBL | Assay Description Affinity fo the compound against human Intestinal peptide transporter PepT1 in Caco-2 cells was measured as inhibition of [14C]-Gly-Sar uptake | J Med Chem 47: 1060-9 (2004) Article DOI: 10.1021/jm031022+ BindingDB Entry DOI: 10.7270/Q2DF6QMV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Bos taurus (bovine)) | BDBM50080520 (3-Fluoromethyl-7-iodo-1,2,3,4-tetrahydro-isoquinol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Affinity for bovine Phenylethanolamine N-Methyltransferase | J Med Chem 42: 3588-601 (1999) Article DOI: 10.1021/jm990045e BindingDB Entry DOI: 10.7270/Q2NV9HFK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylethanolamine N-methyltransferase (Bos taurus (bovine)) | BDBM13014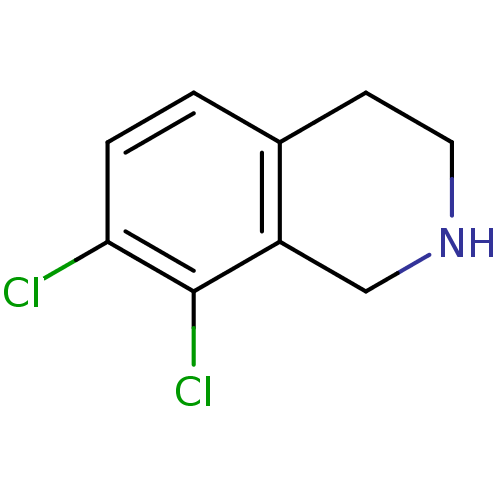 (7,8-Dichloro-1,2,3,4-tetrahydro-isoquinoline; hydr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Affinity for bovine Phenylethanolamine N-Methyltransferase (PNMT) | J Med Chem 42: 3588-601 (1999) Article DOI: 10.1021/jm990045e BindingDB Entry DOI: 10.7270/Q2NV9HFK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 307 total ) | Next | Last >> |