

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222932 (US9315500, 2.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145270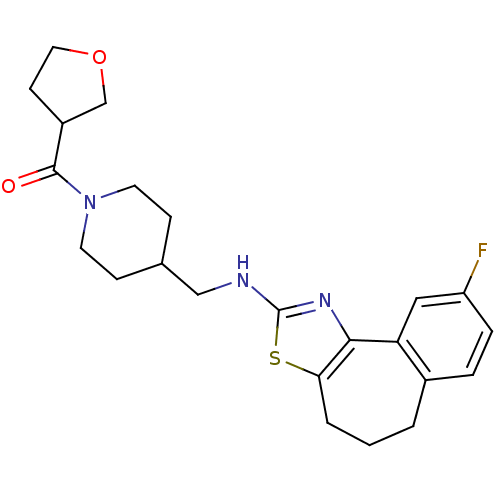 (CHEMBL81994 | {4-[(9-Fluoro-5,6-dihydro-4H-3-thia-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222933 (US9315500, 2.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222934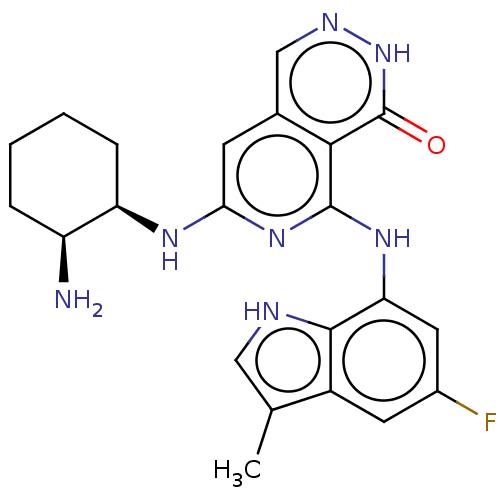 (US9315500, 2.4) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222931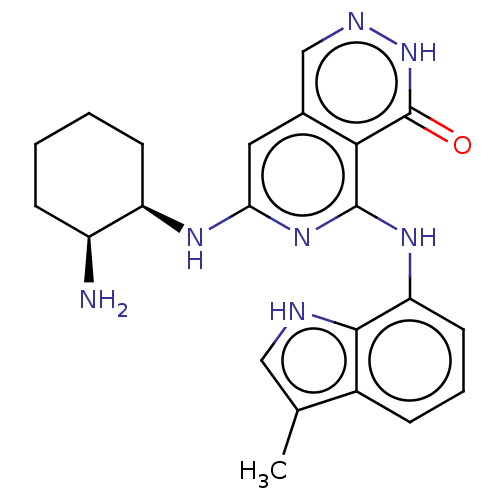 (US9315500, 2.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222935 (US9315500, 2.5) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222952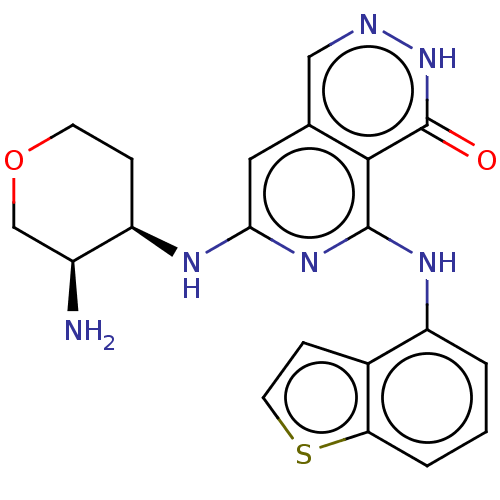 (US9315500, 3.2) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145282 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145277 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145279 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145280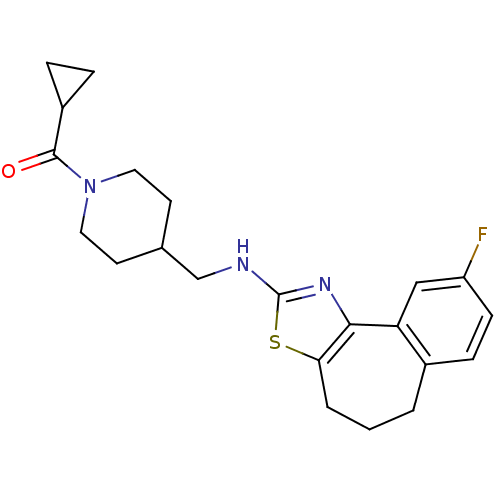 (CHEMBL84453 | Cyclopropyl-{4-[(9-fluoro-5,6-dihydr...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145289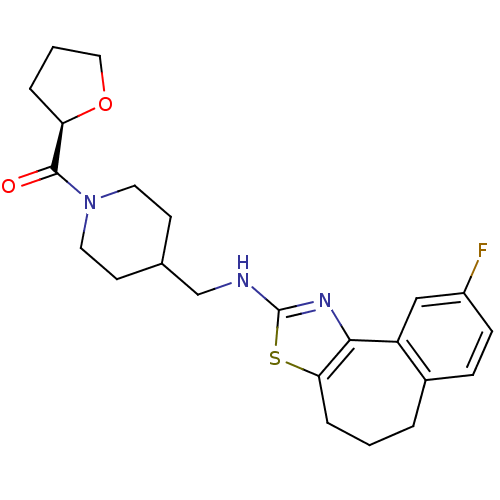 (CHEMBL312362 | {4-[(9-Fluoro-5,6-dihydro-4H-3-thia...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proto-oncogene tyrosine-protein kinase Src (Homo sapiens (Human)) | BDBM50320214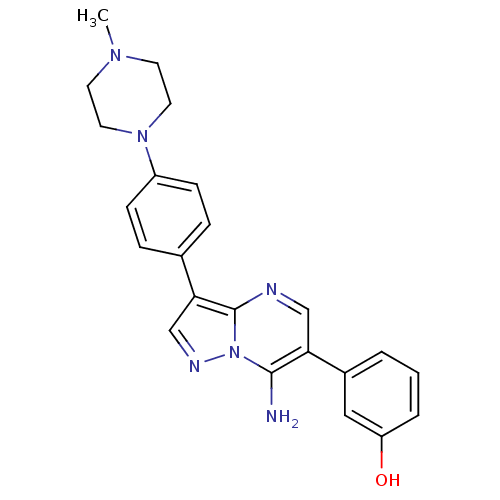 (3-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of SRC | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222936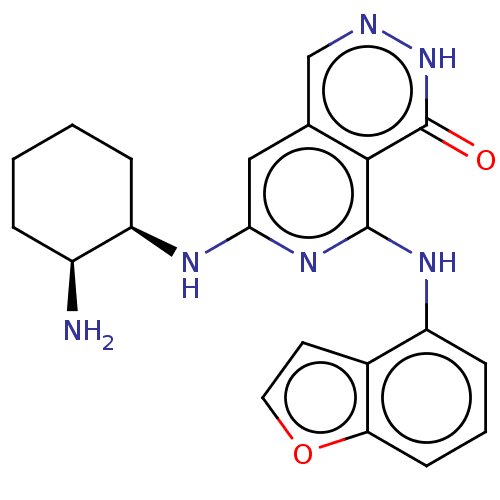 (US9315500, 2.6) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222953 (US9315500, 3.3) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145266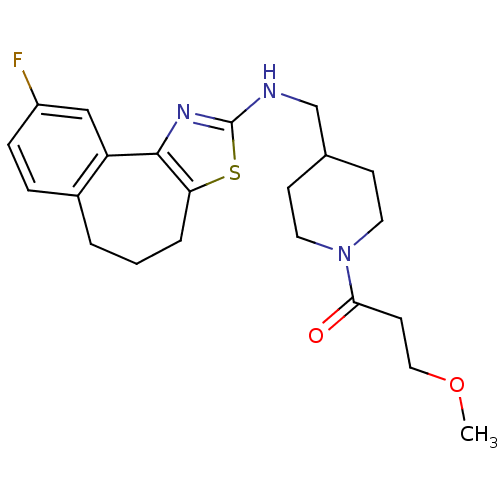 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145235 (CHEMBL432169 | {4-[(9-Fluoro-5,6-dihydro-4H-3-thia...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222955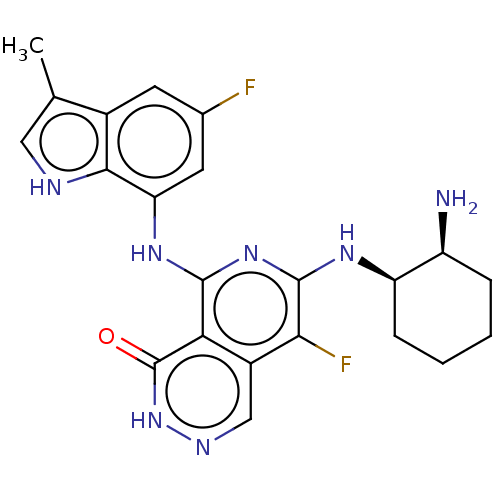 (US9315500, 1.1) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145252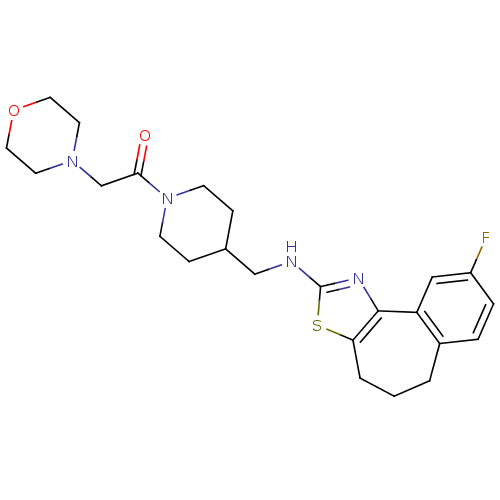 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145244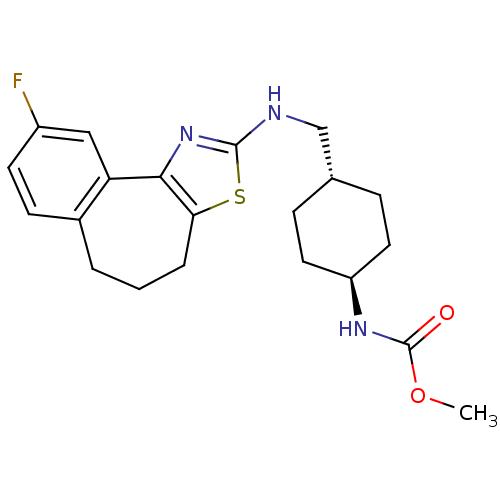 (CHEMBL82158 | {4-[(9-Fluoro-5,6-dihydro-4H-3-thia-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50399042 (CHEMBL2178812) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay | J Med Chem 55: 9722-34 (2012) Article DOI: 10.1021/jm3009508 BindingDB Entry DOI: 10.7270/Q2K938PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222937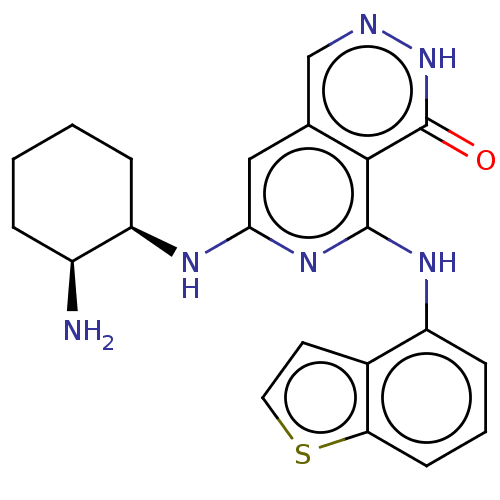 (US9315500, 2.7) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222938 (US9315500, 2.8) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50399045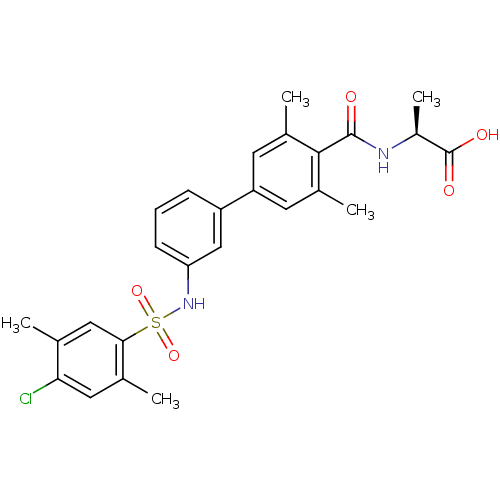 (CHEMBL2178809) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay | J Med Chem 55: 9722-34 (2012) Article DOI: 10.1021/jm3009508 BindingDB Entry DOI: 10.7270/Q2K938PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222939 (US9315500, 2.9) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50145232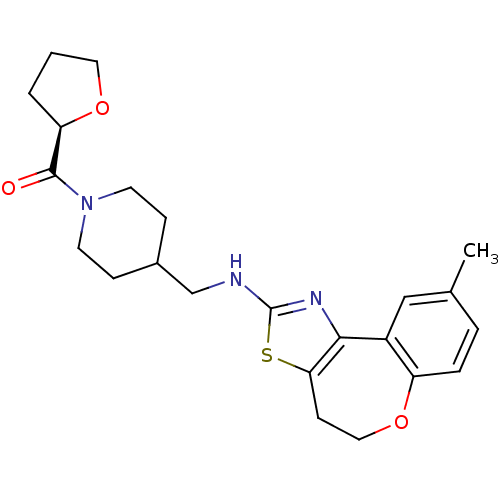 (CHEMBL311229 | {4-[(9-Methyl-4,5-dihydro-6-oxa-3-t...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against rat neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50089060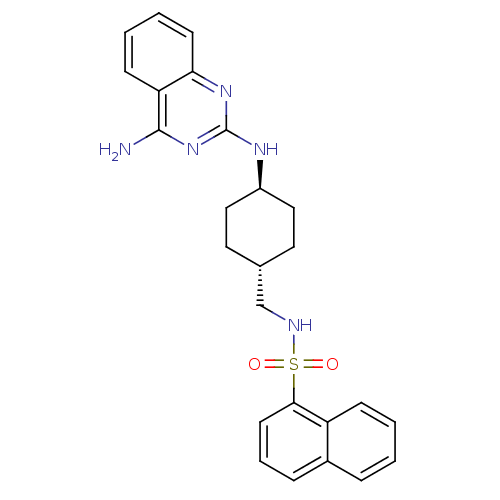 (CHEMBL273597 | Naphthalene-1-sulfonic acid [4-(4-a...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145243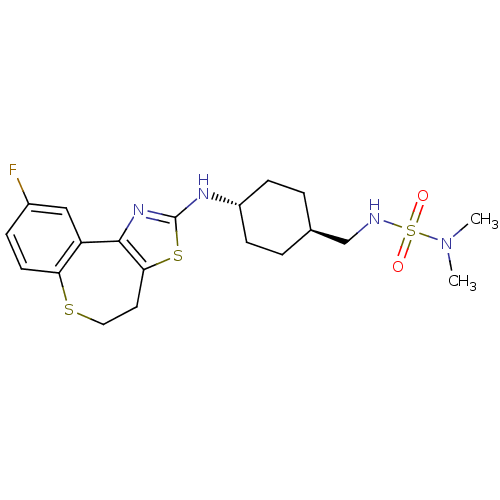 (CHEMBL83830 | N,N-dimethyl-N'-({4-[(19-Fluoro-2-me...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145272 (2-(Cyclopropylmethyl-methyl-amino)-1-{4-[(9-fluoro...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145256 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50399039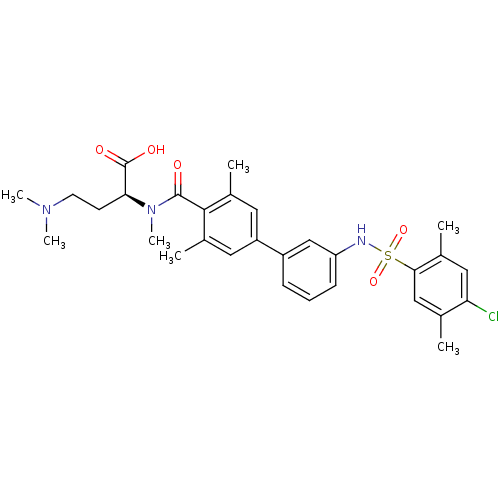 (CHEMBL2178814) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description Antagonist activity at human S1P1R receptor expressed in CHO cell membranes by [35S]GTPgamma binding assay | J Med Chem 55: 9722-34 (2012) Article DOI: 10.1021/jm3009508 BindingDB Entry DOI: 10.7270/Q2K938PD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145267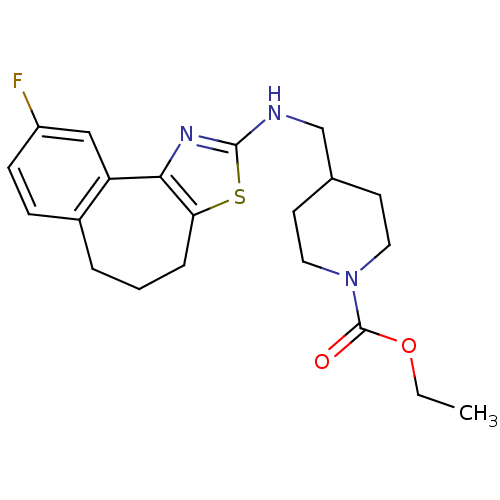 (4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[e]a...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145241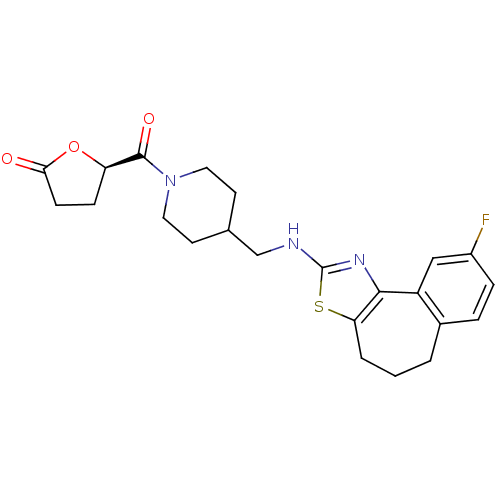 ((R)-5-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-be...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50320225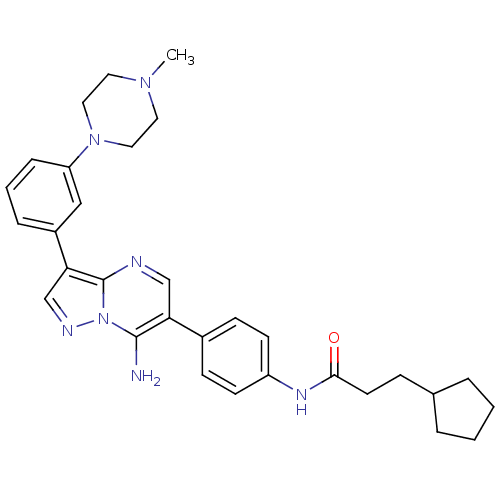 (CHEMBL1085316 | N-(4-(7-amino-3-(3-(4-methylpipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LCK | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145259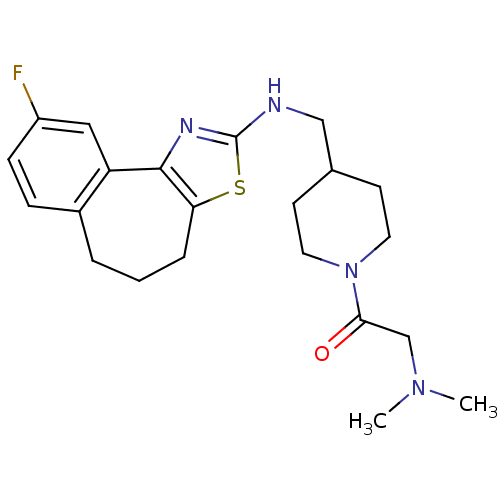 (2-Dimethylamino-1-{4-[(9-fluoro-5,6-dihydro-4H-3-t...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50320205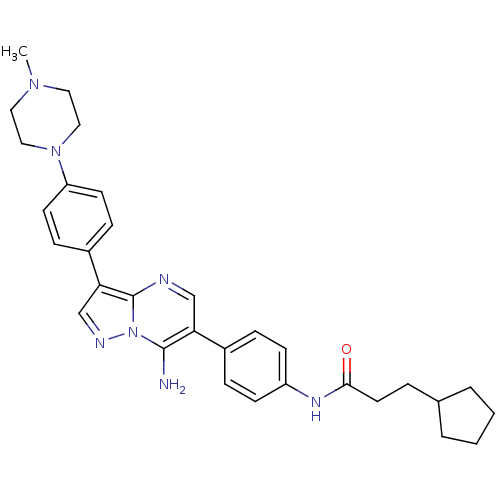 (CHEMBL1083001 | N-(4-(7-amino-3-(4-(4-methylpipera...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LCK | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145295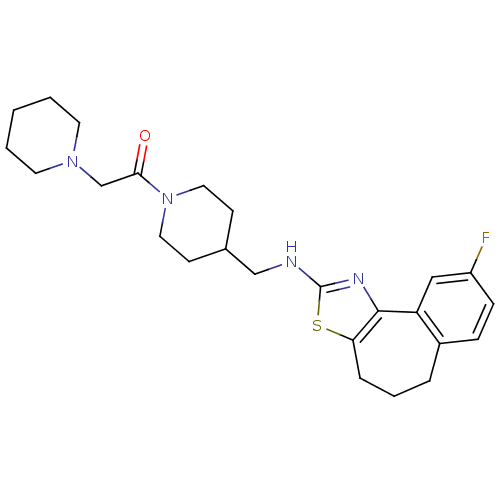 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145232 (CHEMBL311229 | {4-[(9-Methyl-4,5-dihydro-6-oxa-3-t...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50320209 (1-(4-(7-amino-3-(4-(4-methylpiperazin-1-yl)phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LCK | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145242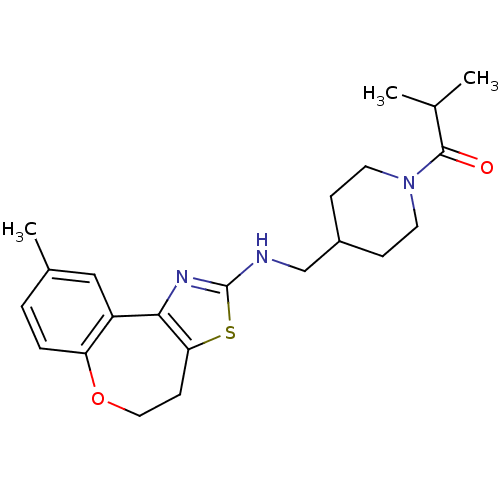 (2-Methyl-1-{4-[(9-methyl-4,5-dihydro-6-oxa-3-thia-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor measured as Ca+ response | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145293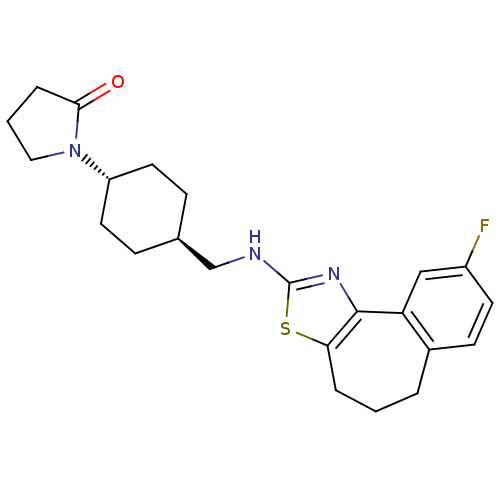 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145287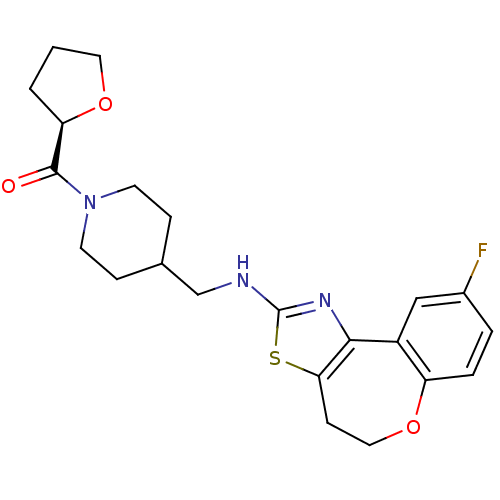 (CHEMBL309685 | {4-[(9-Fluoro-4,5-dihydro-6-oxa-3-t...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222940 (US9315500, 2.10) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase SYK [2-635] (Homo sapiens (Human)) | BDBM222941 (US9315500, 2.11) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
NOVARTIS AG US Patent | Assay Description All assays were performed in 384 well microtiter plates. Each assay plate contained 8-point serial dilutions for 40 test compounds, as well as four 8... | US Patent US9315500 (2016) BindingDB Entry DOI: 10.7270/Q2V986WN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Rat 6B) | BDBM50145236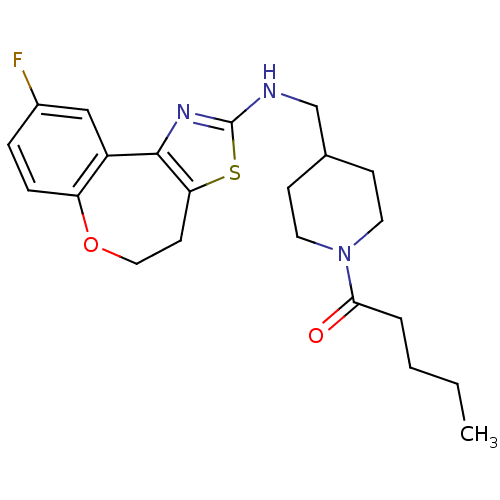 (1-{4-[(9-Fluoro-4,5-dihydro-6-oxa-3-thia-1-aza-ben...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against rat neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145275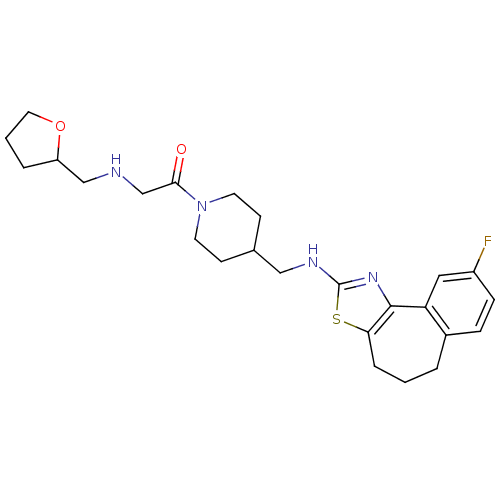 (1-{4-[(9-Fluoro-5,6-dihydro-4H-3-thia-1-aza-benzo[...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145283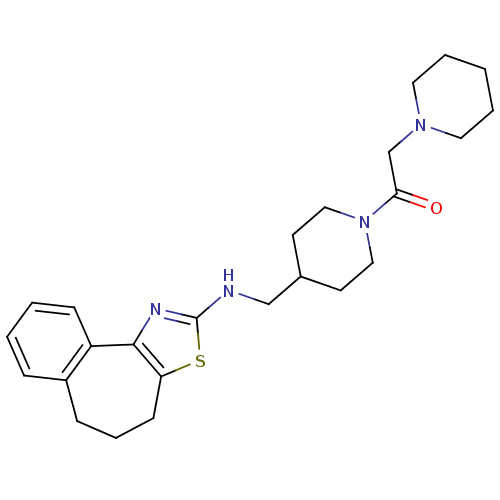 (1-{4-[(5,6-Dihydro-4H-3-thia-1-aza-benzo[e]azulen-...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145297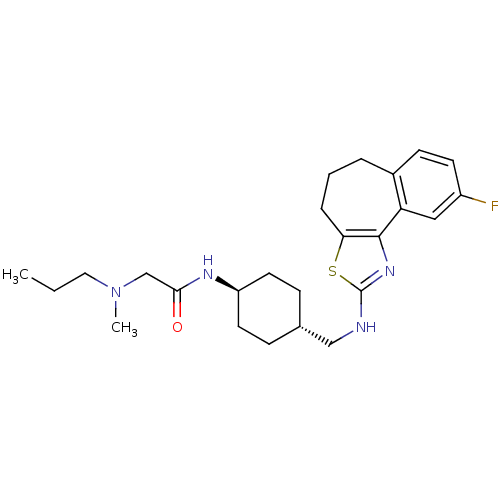 (CHEMBL79308 | N-{4-[(9-Fluoro-5,6-dihydro-4H-3-thi...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Lck (Homo sapiens (Human)) | BDBM50320224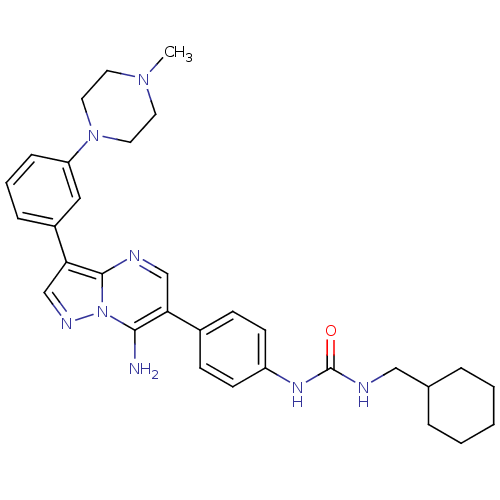 (1-(4-(7-amino-3-(3-(4-methylpiperazin-1-yl)phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute of Biomedical Research Curated by ChEMBL | Assay Description Inhibition of LCK | Bioorg Med Chem Lett 20: 3628-31 (2010) Article DOI: 10.1016/j.bmcl.2010.04.112 BindingDB Entry DOI: 10.7270/Q2XP754D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 5 (Homo sapiens (Human)) | BDBM50145238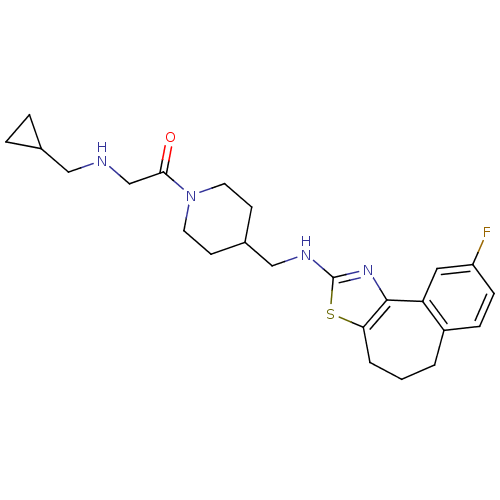 (2-(Cyclopropylmethyl-amino)-1-{4-[(9-fluoro-5,6-di...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG Curated by ChEMBL | Assay Description In vitro binding affinity of the compound against human neuropeptide Y5 receptor | Bioorg Med Chem Lett 14: 2451-7 (2004) Article DOI: 10.1016/j.bmcl.2004.03.014 BindingDB Entry DOI: 10.7270/Q2WM1CV1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 268 total ) | Next | Last >> |