 Found 1535 hits with Last Name = 'chamoin' and Initial = 's'
Found 1535 hits with Last Name = 'chamoin' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191945
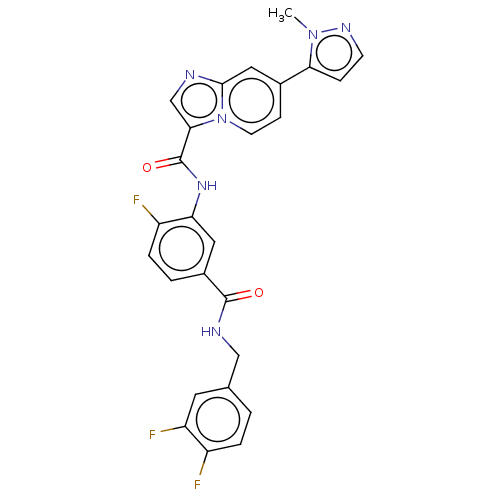
(CHEMBL3904768)Show SMILES Cn1nccc1-c1ccn2c(cnc2c1)C(=O)Nc1cc(ccc1F)C(=O)NCc1ccc(F)c(F)c1 Show InChI InChI=1S/C26H19F3N6O2/c1-34-22(6-8-32-34)16-7-9-35-23(14-30-24(35)12-16)26(37)33-21-11-17(3-5-19(21)28)25(36)31-13-15-2-4-18(27)20(29)10-15/h2-12,14H,13H2,1H3,(H,31,36)(H,33,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191977
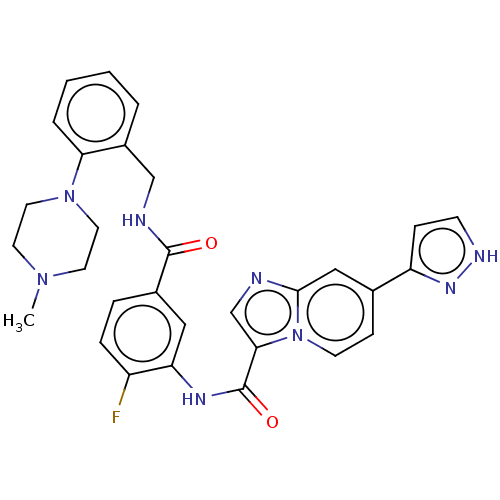
(CHEMBL3983564)Show SMILES CN1CCN(CC1)c1ccccc1CNC(=O)c1ccc(F)c(NC(=O)c2cnc3cc(ccn23)-c2cc[nH]n2)c1 Show InChI InChI=1S/C30H29FN8O2/c1-37-12-14-38(15-13-37)26-5-3-2-4-22(26)18-33-29(40)21-6-7-23(31)25(16-21)35-30(41)27-19-32-28-17-20(9-11-39(27)28)24-8-10-34-36-24/h2-11,16-17,19H,12-15,18H2,1H3,(H,33,40)(H,34,36)(H,35,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191981
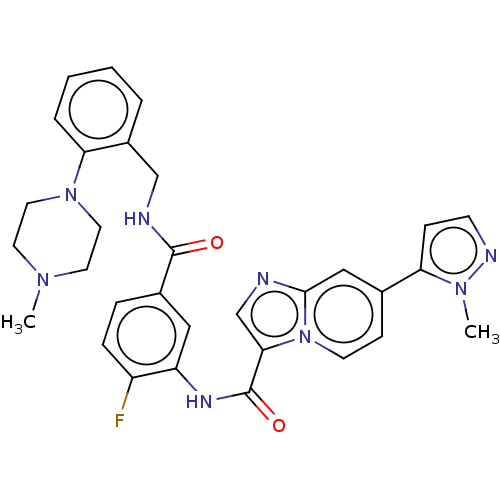
(CHEMBL3979322)Show SMILES CN1CCN(CC1)c1ccccc1CNC(=O)c1ccc(F)c(NC(=O)c2cnc3cc(ccn23)-c2ccnn2C)c1 Show InChI InChI=1S/C31H31FN8O2/c1-37-13-15-39(16-14-37)27-6-4-3-5-23(27)19-34-30(41)22-7-8-24(32)25(17-22)36-31(42)28-20-33-29-18-21(10-12-40(28)29)26-9-11-35-38(26)2/h3-12,17-18,20H,13-16,19H2,1-2H3,(H,34,41)(H,36,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191973
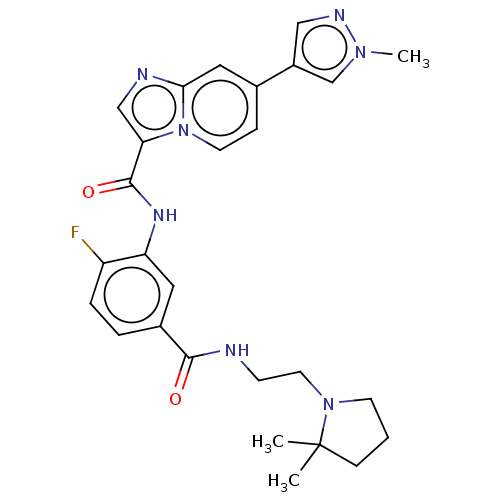
(CHEMBL3940697)Show SMILES Cn1cc(cn1)-c1ccn2c(cnc2c1)C(=O)Nc1cc(ccc1F)C(=O)NCCN1CCCC1(C)C Show InChI InChI=1S/C27H30FN7O2/c1-27(2)8-4-10-34(27)12-9-29-25(36)19-5-6-21(28)22(13-19)32-26(37)23-16-30-24-14-18(7-11-35(23)24)20-15-31-33(3)17-20/h5-7,11,13-17H,4,8-10,12H2,1-3H3,(H,29,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191978
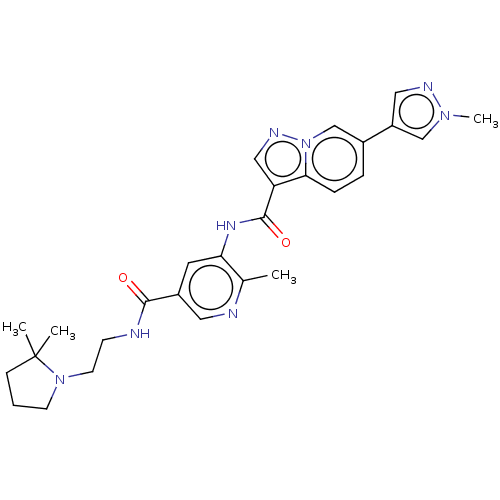
(CHEMBL3915941)Show SMILES Cc1ncc(cc1NC(=O)c1cnn2cc(ccc12)-c1cnn(C)c1)C(=O)NCCN1CCCC1(C)C Show InChI InChI=1S/C27H32N8O2/c1-18-23(12-20(13-29-18)25(36)28-9-11-34-10-5-8-27(34,2)3)32-26(37)22-15-31-35-17-19(6-7-24(22)35)21-14-30-33(4)16-21/h6-7,12-17H,5,8-11H2,1-4H3,(H,28,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191974
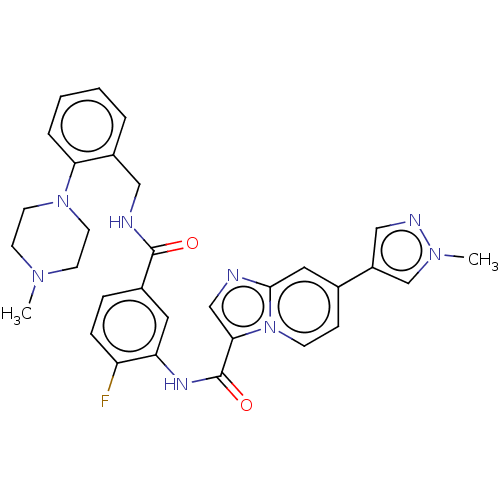
(CHEMBL3913766)Show SMILES CN1CCN(CC1)c1ccccc1CNC(=O)c1ccc(F)c(NC(=O)c2cnc3cc(ccn23)-c2cnn(C)c2)c1 Show InChI InChI=1S/C31H31FN8O2/c1-37-11-13-39(14-12-37)27-6-4-3-5-23(27)17-34-30(41)22-7-8-25(32)26(15-22)36-31(42)28-19-33-29-16-21(9-10-40(28)29)24-18-35-38(2)20-24/h3-10,15-16,18-20H,11-14,17H2,1-2H3,(H,34,41)(H,36,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191975
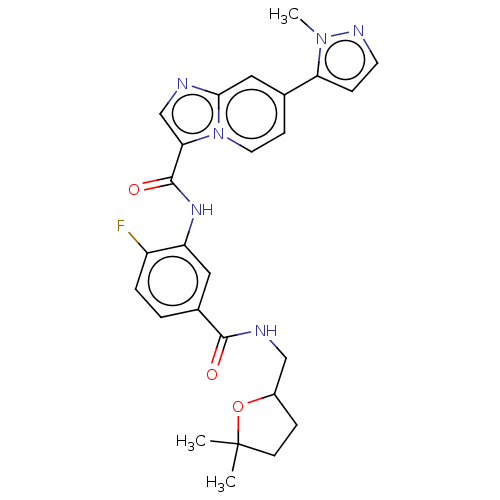
(CHEMBL3975580)Show SMILES Cn1nccc1-c1ccn2c(cnc2c1)C(=O)Nc1cc(ccc1F)C(=O)NCC1CCC(C)(C)O1 Show InChI InChI=1S/C26H27FN6O3/c1-26(2)9-6-18(36-26)14-29-24(34)17-4-5-19(27)20(12-17)31-25(35)22-15-28-23-13-16(8-11-33(22)23)21-7-10-30-32(21)3/h4-5,7-8,10-13,15,18H,6,9,14H2,1-3H3,(H,29,34)(H,31,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191946
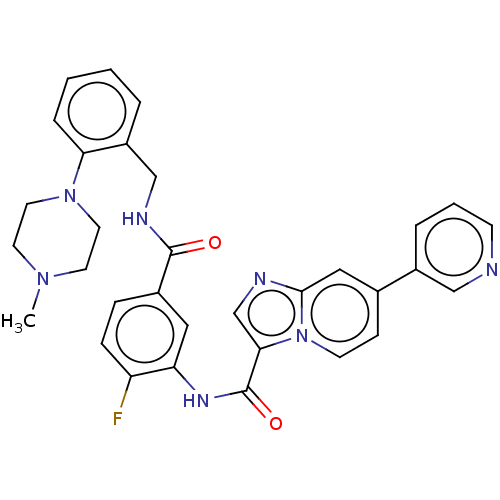
(CHEMBL3950278)Show SMILES CN1CCN(CC1)c1ccccc1CNC(=O)c1ccc(F)c(NC(=O)c2cnc3cc(ccn23)-c2cccnc2)c1 Show InChI InChI=1S/C32H30FN7O2/c1-38-13-15-39(16-14-38)28-7-3-2-5-25(28)20-36-31(41)23-8-9-26(33)27(17-23)37-32(42)29-21-35-30-18-22(10-12-40(29)30)24-6-4-11-34-19-24/h2-12,17-19,21H,13-16,20H2,1H3,(H,36,41)(H,37,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191980
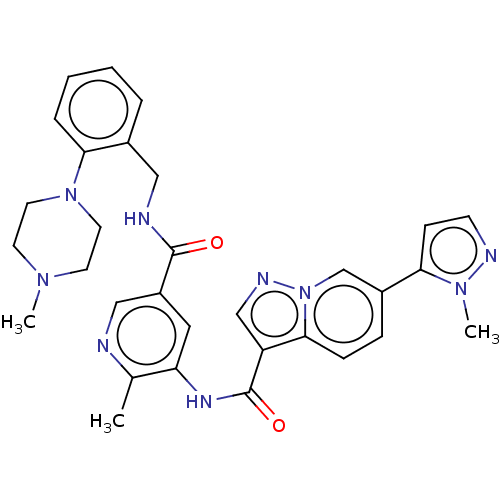
(CHEMBL3985689)Show SMILES CN1CCN(CC1)c1ccccc1CNC(=O)c1cnc(C)c(NC(=O)c2cnn3cc(ccc23)-c2ccnn2C)c1 Show InChI InChI=1S/C31H33N9O2/c1-21-26(36-31(42)25-19-35-40-20-23(8-9-29(25)40)27-10-11-34-38(27)3)16-24(18-32-21)30(41)33-17-22-6-4-5-7-28(22)39-14-12-37(2)13-15-39/h4-11,16,18-20H,12-15,17H2,1-3H3,(H,33,41)(H,36,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50192005

(CHEMBL3947262)Show SMILES CN1CCN(CC1)c1ccccc1CNC(=O)c1ccc(F)c(NC(=O)c2cnn3cc(ccc23)-c2ccnn2C)c1 Show InChI InChI=1S/C31H31FN8O2/c1-37-13-15-39(16-14-37)28-6-4-3-5-22(28)18-33-30(41)21-7-9-25(32)26(17-21)36-31(42)24-19-35-40-20-23(8-10-29(24)40)27-11-12-34-38(27)2/h3-12,17,19-20H,13-16,18H2,1-2H3,(H,33,41)(H,36,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50192006
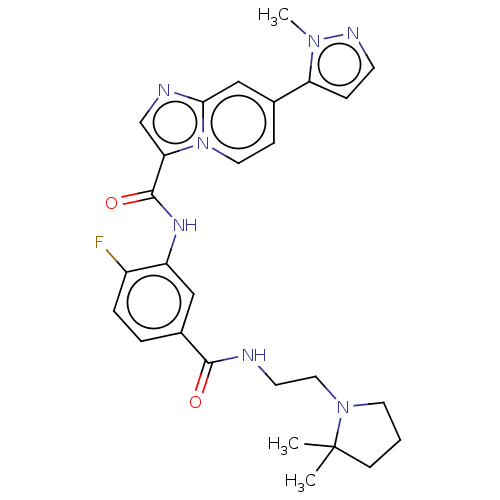
(CHEMBL3955987)Show SMILES Cn1nccc1-c1ccn2c(cnc2c1)C(=O)Nc1cc(ccc1F)C(=O)NCCN1CCCC1(C)C Show InChI InChI=1S/C27H30FN7O2/c1-27(2)9-4-12-34(27)14-11-29-25(36)19-5-6-20(28)21(15-19)32-26(37)23-17-30-24-16-18(8-13-35(23)24)22-7-10-31-33(22)3/h5-8,10,13,15-17H,4,9,11-12,14H2,1-3H3,(H,29,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50191972
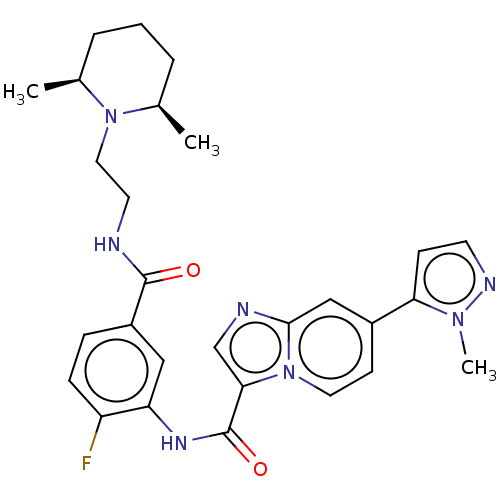
(CHEMBL3895824)Show SMILES C[C@H]1CCC[C@@H](C)N1CCNC(=O)c1ccc(F)c(NC(=O)c2cnc3cc(ccn23)-c2ccnn2C)c1 |r| Show InChI InChI=1S/C28H32FN7O2/c1-18-5-4-6-19(2)35(18)14-12-30-27(37)21-7-8-22(29)23(15-21)33-28(38)25-17-31-26-16-20(10-13-36(25)26)24-9-11-32-34(24)3/h7-11,13,15-19H,4-6,12,14H2,1-3H3,(H,30,37)(H,33,38)/t18-,19+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor alpha
(Homo sapiens (Human)) | BDBM50192007
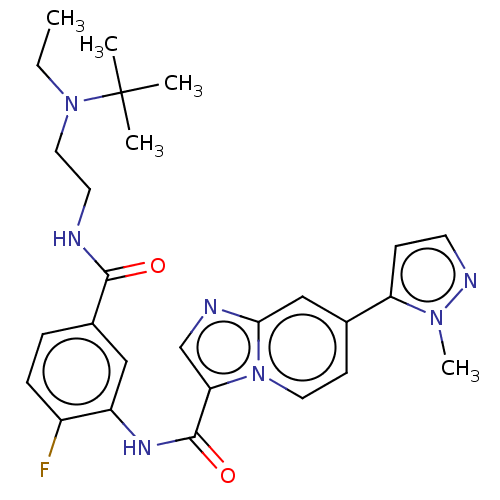
(CHEMBL3902237)Show SMILES CCN(CCNC(=O)c1ccc(F)c(NC(=O)c2cnc3cc(ccn23)-c2ccnn2C)c1)C(C)(C)C Show InChI InChI=1S/C27H32FN7O2/c1-6-34(27(2,3)4)14-12-29-25(36)19-7-8-20(28)21(15-19)32-26(37)23-17-30-24-16-18(10-13-35(23)24)22-9-11-31-33(22)5/h7-11,13,15-17H,6,12,14H2,1-5H3,(H,29,36)(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFRalpha kinase domain (unknown origin) assessed as reduction in probe peptide substrate phosphorylation by capillary electrophoresis |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Rattus norvegicus) | BDBM50191973

(CHEMBL3940697)Show SMILES Cn1cc(cn1)-c1ccn2c(cnc2c1)C(=O)Nc1cc(ccc1F)C(=O)NCCN1CCCC1(C)C Show InChI InChI=1S/C27H30FN7O2/c1-27(2)8-4-10-34(27)12-9-29-25(36)19-5-6-21(28)22(13-19)32-26(37)23-16-30-24-14-18(7-11-35(23)24)20-15-31-33(3)17-20/h5-7,11,13-17H,4,8-10,12H2,1-3H3,(H,29,36)(H,32,37) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Platelet-derived growth factor receptor beta
(Rattus norvegicus) | BDBM50191975

(CHEMBL3975580)Show SMILES Cn1nccc1-c1ccn2c(cnc2c1)C(=O)Nc1cc(ccc1F)C(=O)NCC1CCC(C)(C)O1 Show InChI InChI=1S/C26H27FN6O3/c1-26(2)9-6-18(36-26)14-29-24(34)17-4-5-19(27)20(12-17)31-25(35)22-15-28-23-13-16(8-11-33(22)23)21-7-10-30-32(21)3/h4-5,7-8,10-13,15,18H,6,9,14H2,1-3H3,(H,29,34)(H,31,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes of Biomedical Research (NIBR)
Curated by ChEMBL
| Assay Description
Inhibition of PDGFR-beta driven proliferation of rat A10 cells after 68 hrs in presence of rat recombinant PDGF-BB by cell titer-glo luminescence ass... |
J Med Chem 59: 7901-14 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00703
BindingDB Entry DOI: 10.7270/Q25X2BW1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204077

(US9539260, E3 | US9763952, Example E3)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H30N4O5/c1-16-11-18(13-26-23(16)30-2)28-7-10-32-21-14-25-22(12-20(21)28)33-19-3-6-27(15-19)24(29)17-4-8-31-9-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM202513
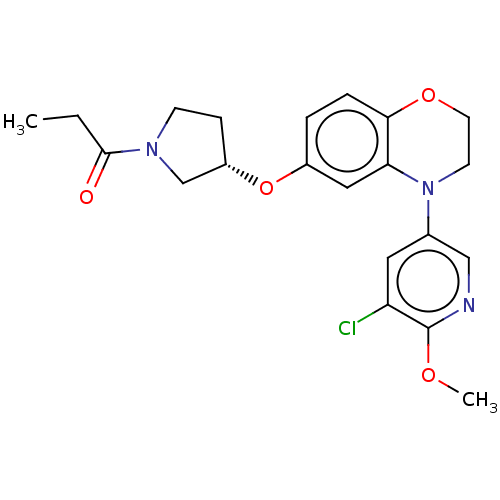
(US9539260, A12 | US9763952, Example A12)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(Cl)c3)c2c1 |r| Show InChI InChI=1S/C21H24ClN3O4/c1-3-20(26)24-7-6-16(13-24)29-15-4-5-19-18(11-15)25(8-9-28-19)14-10-17(22)21(27-2)23-12-14/h4-5,10-12,16H,3,6-9,13H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM341005
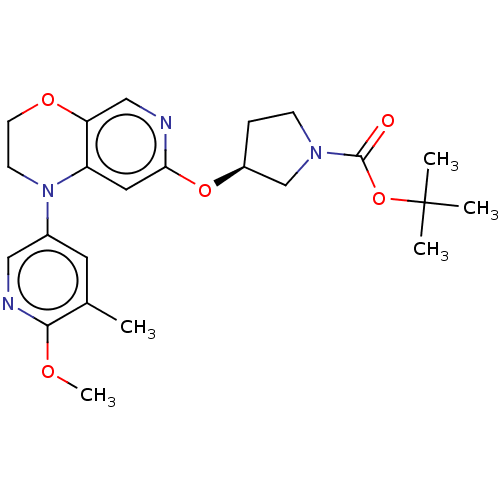
(US9763952, Example E1 | US9763952, Example F1 | {(...)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)OC(C)(C)C)cc12 |r| Show InChI InChI=1S/C23H30N4O5/c1-15-10-16(12-25-21(15)29-5)27-8-9-30-19-13-24-20(11-18(19)27)31-17-6-7-26(14-17)22(28)32-23(2,3)4/h10-13,17H,6-9,14H2,1-5H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204087

(US9539260, F4 | US9763952, Example F4)Show SMILES COc1ncc(cc1C#N)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H27N5O5/c1-31-23-17(12-25)10-18(13-27-23)29-6-9-33-21-14-26-22(11-20(21)29)34-19-2-5-28(15-19)24(30)16-3-7-32-8-4-16/h10-11,13-14,16,19H,2-9,15H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204089

(US9539260, F6 | US9763952, Example F6)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H30N4O6S/c1-16-11-18(13-26-23(16)35(2,30)31)28-7-10-33-21-14-25-22(12-20(21)28)34-19-3-6-27(15-19)24(29)17-4-8-32-9-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204090
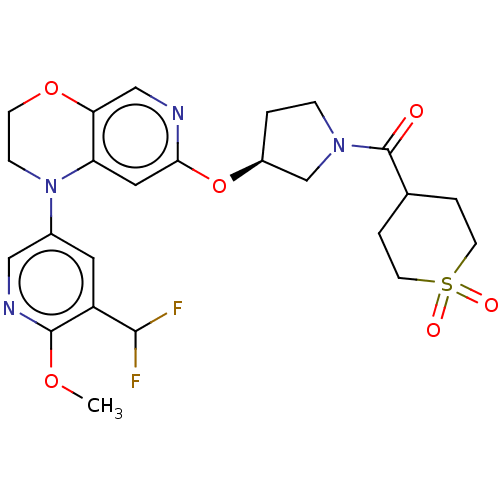
(US9539260, F7 | US9763952, Example F7)Show SMILES COc1ncc(cc1C(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCS(=O)(=O)CC3)cc12 |r| Show InChI InChI=1S/C24H28F2N4O6S/c1-34-23-18(22(25)26)10-16(12-28-23)30-6-7-35-20-13-27-21(11-19(20)30)36-17-2-5-29(14-17)24(31)15-3-8-37(32,33)9-4-15/h10-13,15,17,22H,2-9,14H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204092
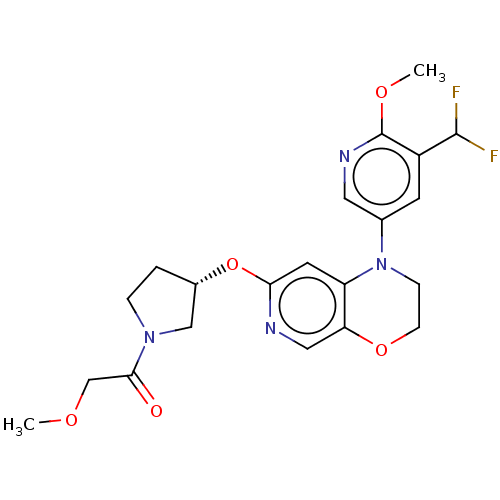
(US9539260, F9 | US9763952, Example F9)Show SMILES COCC(=O)N1CC[C@@H](C1)Oc1cc2N(CCOc2cn1)c1cnc(OC)c(c1)C(F)F |r| Show InChI InChI=1S/C21H24F2N4O5/c1-29-12-19(28)26-4-3-14(11-26)32-18-8-16-17(10-24-18)31-6-5-27(16)13-7-15(20(22)23)21(30-2)25-9-13/h7-10,14,20H,3-6,11-12H2,1-2H3/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204093
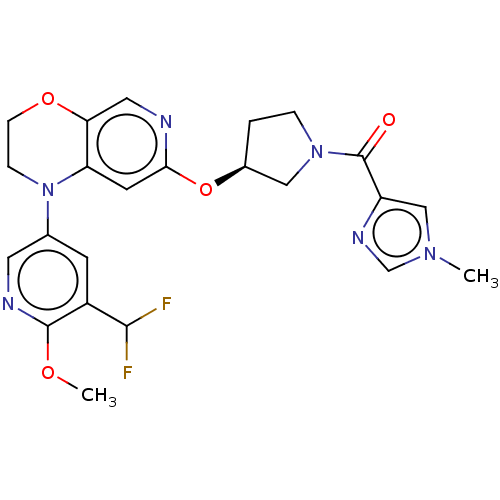
(US9539260, F10 | US9763952, Example F10)Show SMILES COc1ncc(cc1C(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)c3cn(C)cn3)cc12 |r| Show InChI InChI=1S/C23H24F2N6O4/c1-29-12-17(28-13-29)23(32)30-4-3-15(11-30)35-20-8-18-19(10-26-20)34-6-5-31(18)14-7-16(21(24)25)22(33-2)27-9-14/h7-10,12-13,15,21H,3-6,11H2,1-2H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204095

(US9539260, F12 | US9763952, Example F12)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(OC3CCN(C3)C(=O)C3CCS(=O)(=O)CC3)cc12 Show InChI InChI=1S/C24H30N4O6S/c1-16-11-18(13-26-23(16)32-2)28-7-8-33-21-14-25-22(12-20(21)28)34-19-3-6-27(15-19)24(29)17-4-9-35(30,31)10-5-17/h11-14,17,19H,3-10,15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM202490
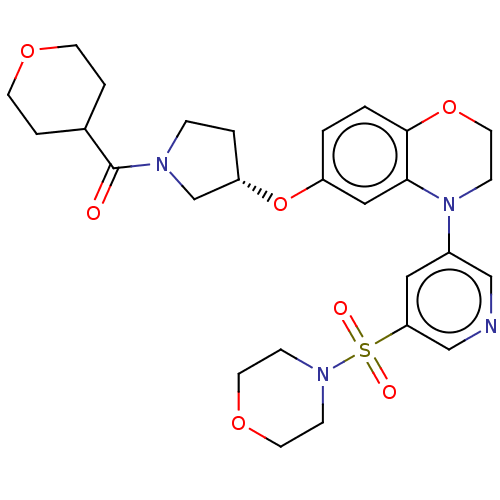
(US9539260, A3 | US9763952, Example A3)Show SMILES O=C(C1CCOCC1)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cncc(c3)S(=O)(=O)N3CCOCC3)c2c1 |r| Show InChI InChI=1S/C27H34N4O7S/c32-27(20-4-10-35-11-5-20)29-6-3-23(19-29)38-22-1-2-26-25(16-22)31(9-14-37-26)21-15-24(18-28-17-21)39(33,34)30-7-12-36-13-8-30/h1-2,15-18,20,23H,3-14,19H2/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM202513

(US9539260, A12 | US9763952, Example A12)Show SMILES CCC(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(Cl)c3)c2c1 |r| Show InChI InChI=1S/C21H24ClN3O4/c1-3-20(26)24-7-6-16(13-24)29-15-4-5-19-18(11-15)25(8-9-28-19)14-10-17(22)21(27-2)23-12-14/h4-5,10-12,16H,3,6-9,13H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204120

(US9539260, K | US9763952, Example K)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)[C@@H]3CCN(C3)C(C)=O)cc12 |r| Show InChI InChI=1S/C26H29N5O5/c1-17(32)29-7-5-18(15-29)26(33)30-8-6-22(16-30)36-21-3-4-24-23(12-21)31(9-10-35-24)20-11-19(13-27)25(34-2)28-14-20/h3-4,11-12,14,18,22H,5-10,15-16H2,1-2H3/t18-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203663
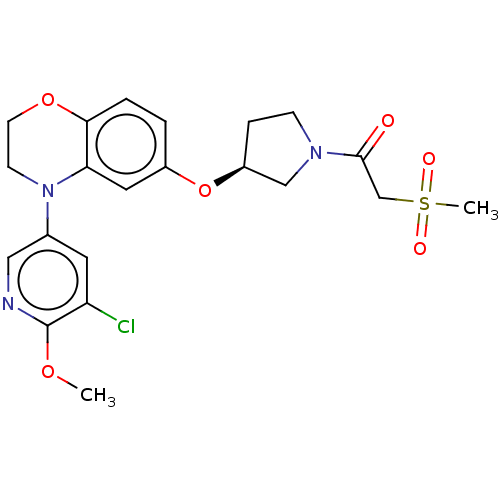
(US9539260, B110 | US9763952, Example B110)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)CS(C)(=O)=O)cc12 |r| Show InChI InChI=1S/C21H24ClN3O6S/c1-29-21-17(22)9-14(11-23-21)25-7-8-30-19-4-3-15(10-18(19)25)31-16-5-6-24(12-16)20(26)13-32(2,27)28/h3-4,9-11,16H,5-8,12-13H2,1-2H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203908

(US9539260, C24 | US9539260, C25 | US9763952, Examp...)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3COCCO3)cc12 |r,w:24.27| Show InChI InChI=1S/C24H26N4O6/c1-30-23-16(12-25)10-17(13-26-23)28-6-7-32-21-3-2-18(11-20(21)28)34-19-4-5-27(14-19)24(29)22-15-31-8-9-33-22/h2-3,10-11,13,19,22H,4-9,14-15H2,1H3/t19-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204008
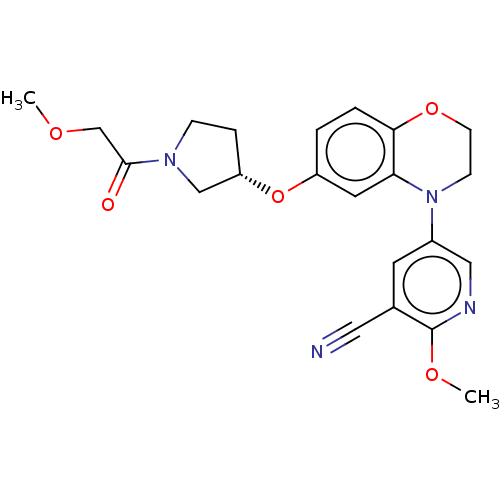
(US9539260, D12 | US9763952, Example D12)Show SMILES COCC(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r| Show InChI InChI=1S/C22H24N4O5/c1-28-14-21(27)25-6-5-18(13-25)31-17-3-4-20-19(10-17)26(7-8-30-20)16-9-15(11-23)22(29-2)24-12-16/h3-4,9-10,12,18H,5-8,13-14H2,1-2H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204062
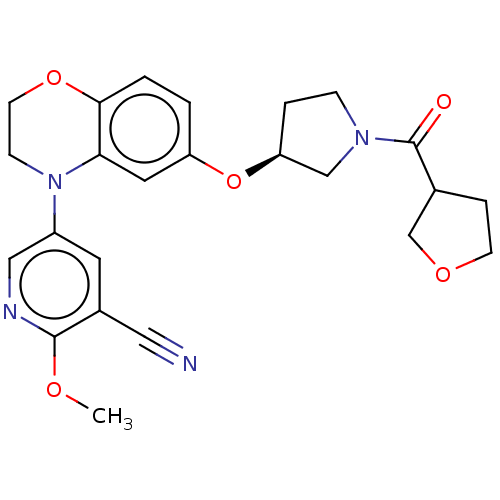
(US9539260, D28 | US9539260, D29 | US9763952, Examp...)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r| Show InChI InChI=1S/C24H26N4O5/c1-30-23-17(12-25)10-18(13-26-23)28-7-9-32-22-3-2-19(11-21(22)28)33-20-4-6-27(14-20)24(29)16-5-8-31-15-16/h2-3,10-11,13,16,20H,4-9,14-15H2,1H3/t16?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204070

(US9539260, D35 | US9539260, D36 | US9763952, Examp...)Show SMILES COC1CCC(CC1)C(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r,wD:13.16,(8.9,-5.23,;8.51,-3.74,;7.02,-3.35,;5.93,-4.43,;4.44,-4.04,;4.04,-2.55,;5.13,-1.46,;6.62,-1.86,;2.56,-2.15,;2.16,-.66,;1.47,-3.24,;.56,-4.48,;-.9,-4.01,;-.9,-2.47,;.56,-1.99,;-2.24,-1.7,;-3.57,-2.47,;-3.57,-4.01,;-4.9,-4.78,;-6.24,-4.01,;-7.57,-4.78,;-8.9,-4.01,;-8.9,-2.47,;-7.57,-1.7,;-7.57,-.16,;-8.9,.61,;-8.9,2.15,;-7.57,2.92,;-7.57,4.46,;-6.24,5.23,;-6.24,2.15,;-6.24,.61,;-4.9,2.92,;-3.57,3.69,;-6.24,-2.47,;-4.9,-1.7,)| Show InChI InChI=1S/C27H32N4O5/c1-33-21-5-3-18(4-6-21)27(32)30-10-9-23(17-30)36-22-7-8-25-24(14-22)31(11-12-35-25)20-13-19(15-28)26(34-2)29-16-20/h7-8,13-14,16,18,21,23H,3-6,9-12,17H2,1-2H3/t18?,21?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
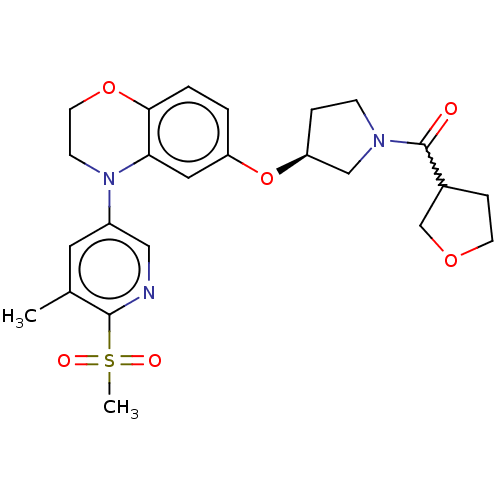
(Homo sapiens (Human)) | BDBM203396
(US9539260, B52 | US9539260, B53 | US9763952, Examp...)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r,w:25.28| Show InChI InChI=1S/C24H29N3O6S/c1-16-11-18(13-25-23(16)34(2,29)30)27-8-10-32-22-4-3-19(12-21(22)27)33-20-5-7-26(14-20)24(28)17-6-9-31-15-17/h3-4,11-13,17,20H,5-10,14-15H2,1-2H3/t17?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203414

(US9539260, B70 | US9763952, Example B70)Show SMILES CS(=O)(=O)c1ncc(cc1C(F)F)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C25H29F2N3O6S/c1-37(32,33)24-20(23(26)27)12-17(14-28-24)30-8-11-35-22-3-2-18(13-21(22)30)36-19-4-7-29(15-19)25(31)16-5-9-34-10-6-16/h2-3,12-14,16,19,23H,4-11,15H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203425
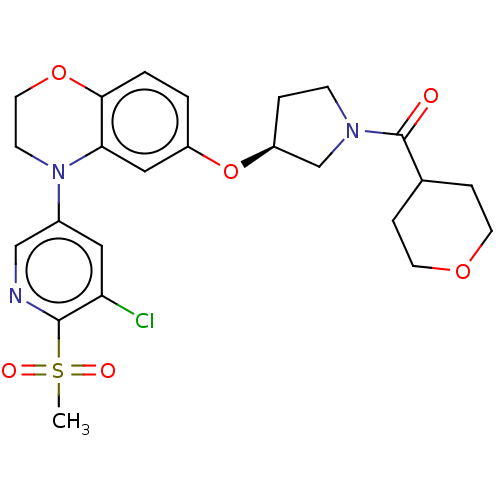
(US9539260, B79 | US9763952, Example B79)Show SMILES CS(=O)(=O)c1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H28ClN3O6S/c1-35(30,31)23-20(25)12-17(14-26-23)28-8-11-33-22-3-2-18(13-21(22)28)34-19-4-7-27(15-19)24(29)16-5-9-32-10-6-16/h2-3,12-14,16,19H,4-11,15H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
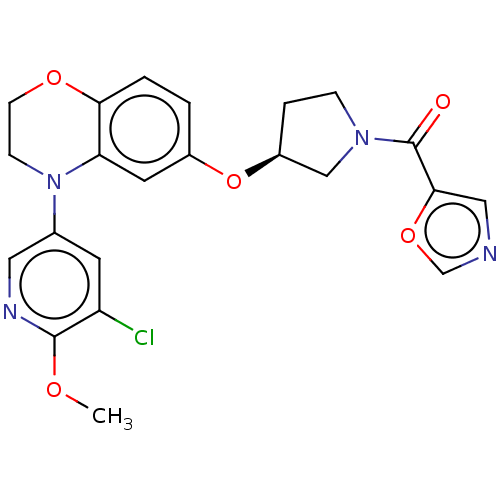
(Homo sapiens (Human)) | BDBM203661
(US9539260, B108 | US9763952, Example B108)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)c3cnco3)cc12 |r| Show InChI InChI=1S/C22H21ClN4O5/c1-29-21-17(23)8-14(10-25-21)27-6-7-30-19-3-2-15(9-18(19)27)32-16-4-5-26(12-16)22(28)20-11-24-13-31-20/h2-3,8-11,13,16H,4-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203662
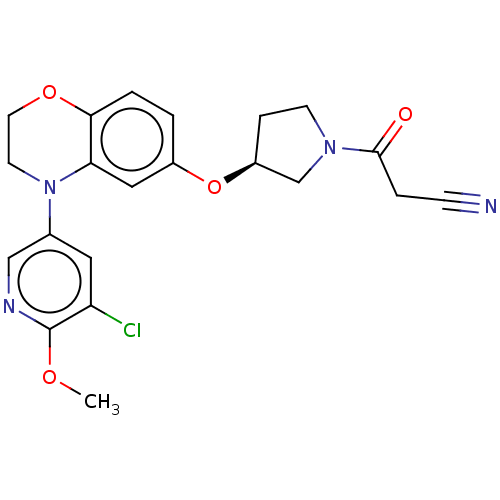
(US9539260, B109 | US9763952, Example B109)Show SMILES COc1ncc(cc1Cl)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)CC#N)cc12 |r| Show InChI InChI=1S/C21H21ClN4O4/c1-28-21-17(22)10-14(12-24-21)26-8-9-29-19-3-2-15(11-18(19)26)30-16-5-7-25(13-16)20(27)4-6-23/h2-3,10-12,16H,4-5,7-9,13H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204120

(US9539260, K | US9763952, Example K)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)[C@@H]3CCN(C3)C(C)=O)cc12 |r| Show InChI InChI=1S/C26H29N5O5/c1-17(32)29-7-5-18(15-29)26(33)30-8-6-22(16-30)36-21-3-4-24-23(12-21)31(9-10-35-24)20-11-19(13-27)25(34-2)28-14-20/h3-4,11-12,14,18,22H,5-10,15-16H2,1-2H3/t18-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203396

(US9539260, B52 | US9539260, B53 | US9763952, Examp...)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r,w:25.28| Show InChI InChI=1S/C24H29N3O6S/c1-16-11-18(13-25-23(16)34(2,29)30)27-8-10-32-22-4-3-19(12-21(22)27)33-20-5-7-26(14-20)24(28)17-6-9-31-15-17/h3-4,11-13,17,20H,5-10,14-15H2,1-2H3/t17?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204102
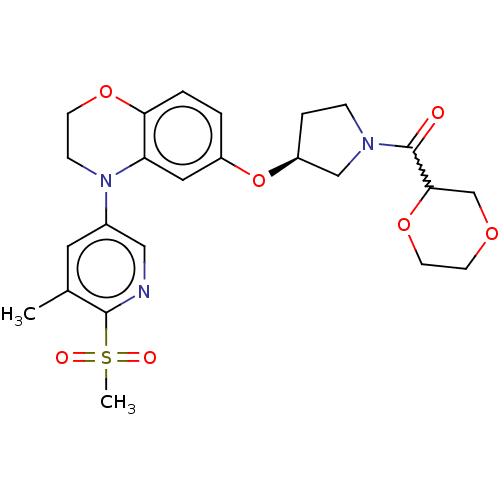
(US9539260, H3 | US9539260, H4 | US9763952, Example...)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3COCCO3)cc12 |r,w:25.28| Show InChI InChI=1S/C24H29N3O7S/c1-16-11-17(13-25-23(16)35(2,29)30)27-7-8-32-21-4-3-18(12-20(21)27)34-19-5-6-26(14-19)24(28)22-15-31-9-10-33-22/h3-4,11-13,19,22H,5-10,14-15H2,1-2H3/t19-,22?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM203381
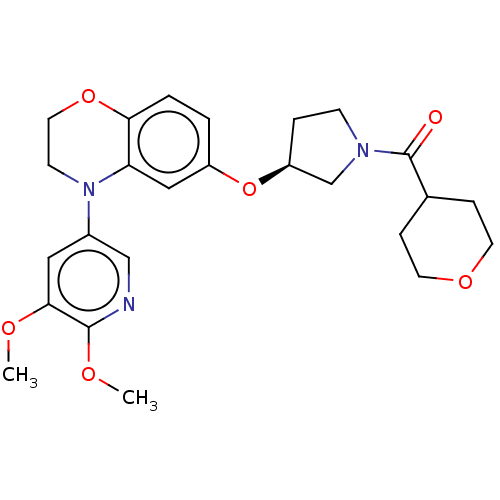
(US9539260, B38 | US9763952, Example B38)Show SMILES COc1cc(cnc1OC)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C25H31N3O6/c1-30-23-13-18(15-26-24(23)31-2)28-9-12-33-22-4-3-19(14-21(22)28)34-20-5-8-27(16-20)25(29)17-6-10-32-11-7-17/h3-4,13-15,17,20H,5-12,16H2,1-2H3/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
NOVARTIS AG
US Patent
| |
US Patent US9763952 (2017)
BindingDB Entry DOI: 10.7270/Q2V40X9Z |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204008

(US9539260, D12 | US9763952, Example D12)Show SMILES COCC(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r| Show InChI InChI=1S/C22H24N4O5/c1-28-14-21(27)25-6-5-18(13-25)31-17-3-4-20-19(10-17)26(7-8-30-20)16-9-15(11-23)22(29-2)24-12-16/h3-4,9-10,12,18H,5-8,13-14H2,1-2H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204062

(US9539260, D28 | US9539260, D29 | US9763952, Examp...)Show SMILES COc1ncc(cc1C#N)N1CCOc2ccc(O[C@H]3CCN(C3)C(=O)C3CCOC3)cc12 |r| Show InChI InChI=1S/C24H26N4O5/c1-30-23-17(12-25)10-18(13-26-23)28-7-9-32-22-3-2-19(11-21(22)28)33-20-4-6-27(14-20)24(29)16-5-8-31-15-16/h2-3,10-11,13,16,20H,4-9,14-15H2,1H3/t16?,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204070

(US9539260, D35 | US9539260, D36 | US9763952, Examp...)Show SMILES COC1CCC(CC1)C(=O)N1CC[C@@H](C1)Oc1ccc2OCCN(c3cnc(OC)c(c3)C#N)c2c1 |r,wD:13.16,(8.9,-5.23,;8.51,-3.74,;7.02,-3.35,;5.93,-4.43,;4.44,-4.04,;4.04,-2.55,;5.13,-1.46,;6.62,-1.86,;2.56,-2.15,;2.16,-.66,;1.47,-3.24,;.56,-4.48,;-.9,-4.01,;-.9,-2.47,;.56,-1.99,;-2.24,-1.7,;-3.57,-2.47,;-3.57,-4.01,;-4.9,-4.78,;-6.24,-4.01,;-7.57,-4.78,;-8.9,-4.01,;-8.9,-2.47,;-7.57,-1.7,;-7.57,-.16,;-8.9,.61,;-8.9,2.15,;-7.57,2.92,;-7.57,4.46,;-6.24,5.23,;-6.24,2.15,;-6.24,.61,;-4.9,2.92,;-3.57,3.69,;-6.24,-2.47,;-4.9,-1.7,)| Show InChI InChI=1S/C27H32N4O5/c1-33-21-5-3-18(4-6-21)27(32)30-10-9-23(17-30)36-22-7-8-25-24(14-22)31(11-12-35-25)20-13-19(15-28)26(34-2)29-16-20/h7-8,13-14,16,18,21,23H,3-6,9-12,17H2,1-2H3/t18?,21?,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204077

(US9539260, E3 | US9763952, Example E3)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H30N4O5/c1-16-11-18(13-26-23(16)30-2)28-7-10-32-21-14-25-22(12-20(21)28)33-19-3-6-27(15-19)24(29)17-4-8-31-9-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204083

(US9539260, E11 | US9763952, Example E11)Show SMILES Cc1cc(cnc1OC(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CC3)cc12 |r| Show InChI InChI=1S/C22H24F2N4O4/c1-13-8-15(10-26-20(13)32-22(23)24)28-6-7-30-18-11-25-19(9-17(18)28)31-16-4-5-27(12-16)21(29)14-2-3-14/h8-11,14,16,22H,2-7,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204084
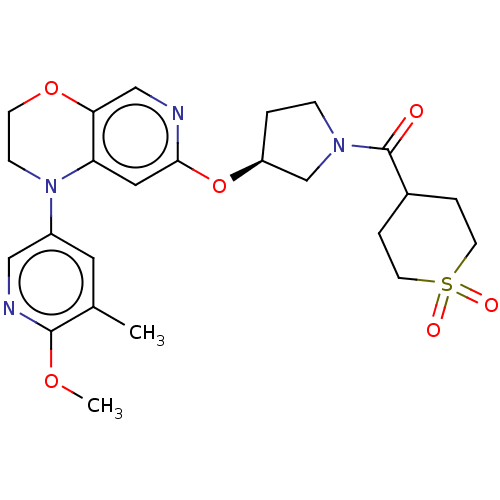
(US9539260, F1)Show SMILES COc1ncc(cc1C)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCS(=O)(=O)CC3)cc12 |r| Show InChI InChI=1S/C24H30N4O6S/c1-16-11-18(13-26-23(16)32-2)28-7-8-33-21-14-25-22(12-20(21)28)34-19-3-6-27(15-19)24(29)17-4-9-35(30,31)10-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204087

(US9539260, F4 | US9763952, Example F4)Show SMILES COc1ncc(cc1C#N)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H27N5O5/c1-31-23-17(12-25)10-18(13-27-23)29-6-9-33-21-14-26-22(11-20(21)29)34-19-2-5-28(15-19)24(30)16-3-7-32-8-4-16/h10-11,13-14,16,19H,2-9,15H2,1H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204089

(US9539260, F6 | US9763952, Example F6)Show SMILES Cc1cc(cnc1S(C)(=O)=O)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCOCC3)cc12 |r| Show InChI InChI=1S/C24H30N4O6S/c1-16-11-18(13-26-23(16)35(2,30)31)28-7-10-33-21-14-25-22(12-20(21)28)34-19-3-6-27(15-19)24(29)17-4-8-32-9-5-17/h11-14,17,19H,3-10,15H2,1-2H3/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM204090

(US9539260, F7 | US9763952, Example F7)Show SMILES COc1ncc(cc1C(F)F)N1CCOc2cnc(O[C@H]3CCN(C3)C(=O)C3CCS(=O)(=O)CC3)cc12 |r| Show InChI InChI=1S/C24H28F2N4O6S/c1-34-23-18(22(25)26)10-16(12-28-23)30-6-7-35-20-13-27-21(11-19(20)30)36-17-2-5-29(14-17)24(31)15-3-8-37(32,33)9-4-15/h10-13,15,17,22H,2-9,14H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <3 | n/a | n/a | n/a | n/a | n/a | 25 |
NOVARTIS AG
US Patent
| Assay Description
Determination of Enzymatic PI3K Alpha and PI3K Delta Isoform Inhibition1.1 Test of Lipid Kinase ActivityThe efficacy of the compounds of examples 1-1... |
US Patent US9539260 (2017)
BindingDB Entry DOI: 10.7270/Q2W37TH1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data