 Found 362 hits with Last Name = 'cunningham' and Initial = 'k'
Found 362 hits with Last Name = 'cunningham' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50503346

(CHEMBL4560254)Show SMILES [H][C@@]1(O[C@H](SC)[C@H](O)[C@@H](O)[C@H]1O)[C@H](NC(=O)[C@@H]1C[C@H](CCCCCCCCCCC)CCN1)[C@H](C)Cl |r| Show InChI InChI=1S/C26H49ClN2O5S/c1-4-5-6-7-8-9-10-11-12-13-18-14-15-28-19(16-18)25(33)29-20(17(2)27)24-22(31)21(30)23(32)26(34-24)35-3/h17-24,26,28,30-32H,4-16H2,1-3H3,(H,29,33)/t17-,18+,19-,20+,21-,22+,23+,24+,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Positive allosteric modulation of 5-HT2C receptor (unknown origin) assessed as increase in 5-HT-induced displacement of [3H]mesulergine by measuring ... |
J Med Chem 62: 88-127 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00875
BindingDB Entry DOI: 10.7270/Q2PR8081 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50161646
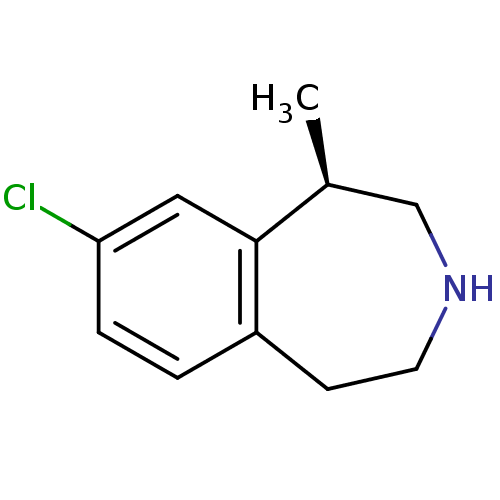
((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...)Show InChI InChI=1S/C11H14ClN/c1-8-7-13-5-4-9-2-3-10(12)6-11(8)9/h2-3,6,8,13H,4-5,7H2,1H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2CR (unknown origin) |
J Med Chem 62: 288-305 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00401
BindingDB Entry DOI: 10.7270/Q20868KK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50161646

((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...)Show InChI InChI=1S/C11H14ClN/c1-8-7-13-5-4-9-2-3-10(12)6-11(8)9/h2-3,6,8,13H,4-5,7H2,1H3/t8-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2AR (unknown origin) |
J Med Chem 62: 288-305 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00401
BindingDB Entry DOI: 10.7270/Q20868KK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2B
(Homo sapiens (Human)) | BDBM50161646

((1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-ben...)Show InChI InChI=1S/C11H14ClN/c1-8-7-13-5-4-9-2-3-10(12)6-11(8)9/h2-3,6,8,13H,4-5,7H2,1H3/t8-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at 5-HT2BR (unknown origin) |
J Med Chem 62: 288-305 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00401
BindingDB Entry DOI: 10.7270/Q20868KK |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50503222
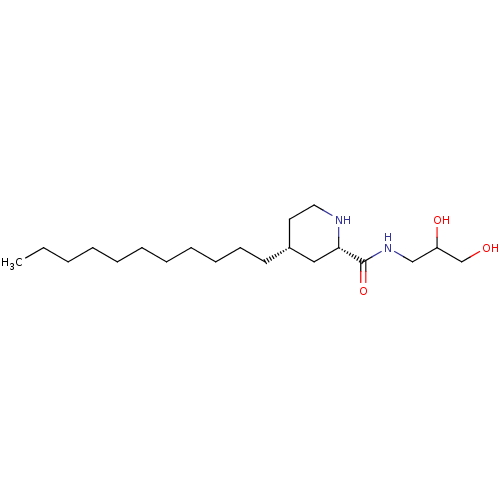
(CHEMBL4454100)Show SMILES CCCCCCCCCCC[C@@H]1CCN[C@@H](C1)C(=O)NCC(O)CO |r| Show InChI InChI=1S/C20H40N2O3/c1-2-3-4-5-6-7-8-9-10-11-17-12-13-21-19(14-17)20(25)22-15-18(24)16-23/h17-19,21,23-24H,2-16H2,1H3,(H,22,25)/t17-,18?,19+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-WIN35428 from recombinant human DAT expressed in HEK cell membranes after 90 mins by scintillation counting method |
J Med Chem 62: 288-305 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00401
BindingDB Entry DOI: 10.7270/Q20868KK |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50544002
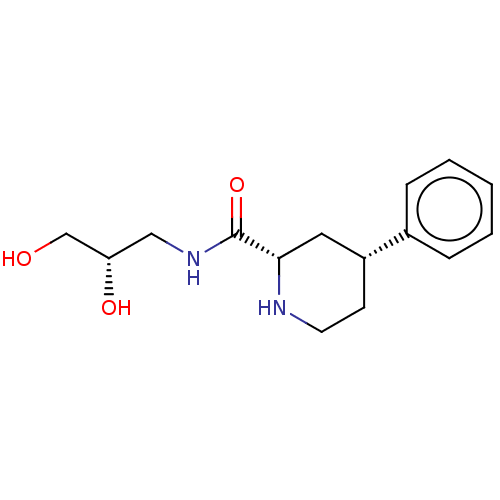
(CHEMBL4633717)Show SMILES OC[C@@H](O)CNC(=O)[C@@H]1C[C@@H](CCN1)c1ccccc1 |r| Show InChI InChI=1S/C15H22N2O3/c18-10-13(19)9-17-15(20)14-8-12(6-7-16-14)11-4-2-1-3-5-11/h1-5,12-14,16,18-19H,6-10H2,(H,17,20)/t12-,13+,14+/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas Medical Branch
Curated by ChEMBL
| Assay Description
Displacement of [3H]-nisoxetine from human NET stably expressed in HEK cell membranes incubated for 90 mins under dark condition by scintillation cou... |
J Med Chem 63: 7529-7544 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01953
BindingDB Entry DOI: 10.7270/Q280565Q |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50503222

(CHEMBL4454100)Show SMILES CCCCCCCCCCC[C@@H]1CCN[C@@H](C1)C(=O)NCC(O)CO |r| Show InChI InChI=1S/C20H40N2O3/c1-2-3-4-5-6-7-8-9-10-11-17-12-13-21-19(14-17)20(25)22-15-18(24)16-23/h17-19,21,23-24H,2-16H2,1H3,(H,22,25)/t17-,18?,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-methylspiperone from recombinant human D3R expressed in HEK cell membranes after 90 mins by scintillation counting method |
J Med Chem 62: 288-305 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00401
BindingDB Entry DOI: 10.7270/Q20868KK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50554937
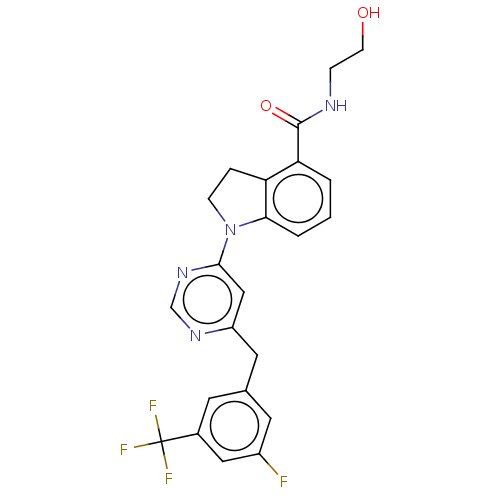
(CHEMBL4780654 | US20240059655, Compound PW0787)Show SMILES OCCNC(=O)c1cccc2N(CCc12)c1cc(Cc2cc(F)cc(c2)C(F)(F)F)ncn1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity to 5-HT2C (unknown origin) assessed as inhibition of ligand binding |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01498
BindingDB Entry DOI: 10.7270/Q22N55XV |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360964

(CHEMBL1935285)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1ccc(C)o1)C(O)=O |r| Show InChI InChI=1S/C22H21NO6S/c1-12(2)21(22(24)25)23-30(26,27)15-6-7-16-17-10-14(18-8-4-13(3)28-18)5-9-19(17)29-20(16)11-15/h4-12,21,23H,1-3H3,(H,24,25)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360965

(CHEMBL1935286)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1ccc(Cl)o1)C(O)=O |r| Show InChI InChI=1S/C21H18ClNO6S/c1-11(2)20(21(24)25)23-30(26,27)13-4-5-14-15-9-12(16-7-8-19(22)29-16)3-6-17(15)28-18(14)10-13/h3-11,20,23H,1-2H3,(H,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 1
(Homo sapiens (Human)) | BDBM50503347

(CHEMBL4442431)Show SMILES [Na;v0+].[#6]-[#6@@H](-[#6](=O)-[#7-]S([#6])(=O)=O)-c1ccc(-[#8]S(=O)(=O)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C11H12F3NO6S2.Na/c1-7(10(16)15-22(2,17)18)8-3-5-9(6-4-8)21-23(19,20)11(12,13)14;/h3-7H,1-2H3,(H,15,16);/q;+1/p-1/t7-;/m1./s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CXCR1 (unknown origin) |
J Med Chem 62: 88-127 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00875
BindingDB Entry DOI: 10.7270/Q2PR8081 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50503347

(CHEMBL4442431)Show SMILES [Na;v0+].[#6]-[#6@@H](-[#6](=O)-[#7-]S([#6])(=O)=O)-c1ccc(-[#8]S(=O)(=O)C(F)(F)F)cc1 |r| Show InChI InChI=1S/C11H12F3NO6S2.Na/c1-7(10(16)15-22(2,17)18)8-3-5-9(6-4-8)21-23(19,20)11(12,13)14;/h3-7H,1-2H3,(H,15,16);/q;+1/p-1/t7-;/m1./s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of CXCR2 (unknown origin) |
J Med Chem 62: 88-127 (2019)
Article DOI: 10.1021/acs.jmedchem.8b00875
BindingDB Entry DOI: 10.7270/Q2PR8081 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360967
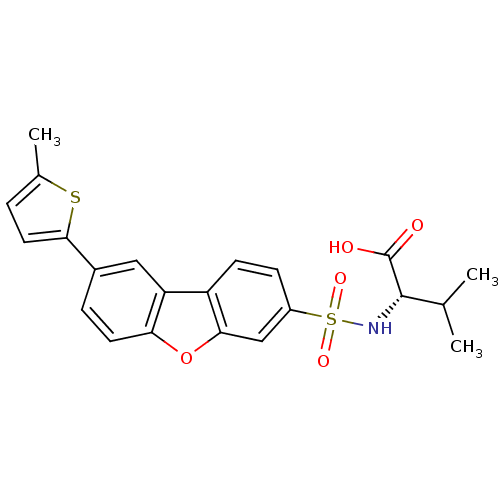
(CHEMBL1935288)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1ccc(C)s1)C(O)=O |r| Show InChI InChI=1S/C22H21NO5S2/c1-12(2)21(22(24)25)23-30(26,27)15-6-7-16-17-10-14(20-9-4-13(3)29-20)5-8-18(17)28-19(16)11-15/h4-12,21,23H,1-3H3,(H,24,25)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360966
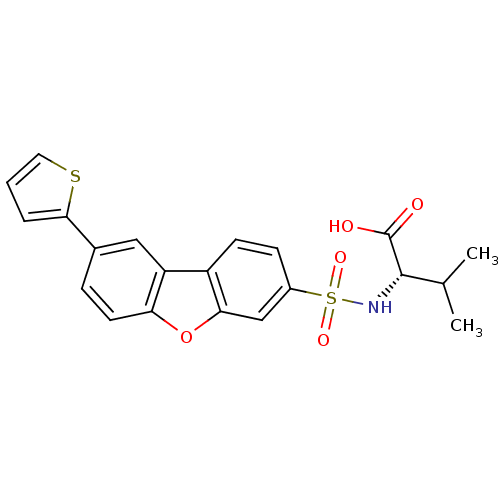
(CHEMBL1935287)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1cccs1)C(O)=O |r| Show InChI InChI=1S/C21H19NO5S2/c1-12(2)20(21(23)24)22-29(25,26)14-6-7-15-16-10-13(19-4-3-9-28-19)5-8-17(16)27-18(15)11-14/h3-12,20,22H,1-2H3,(H,23,24)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360956

(CHEMBL1935304)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1noc(n1)C1CCOC1)C(O)=O |r| Show InChI InChI=1S/C23H23N3O7S/c1-12(2)20(23(27)28)26-34(29,30)15-4-5-16-17-9-13(3-6-18(17)32-19(16)10-15)21-24-22(33-25-21)14-7-8-31-11-14/h3-6,9-10,12,14,20,26H,7-8,11H2,1-2H3,(H,27,28)/t14?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360963
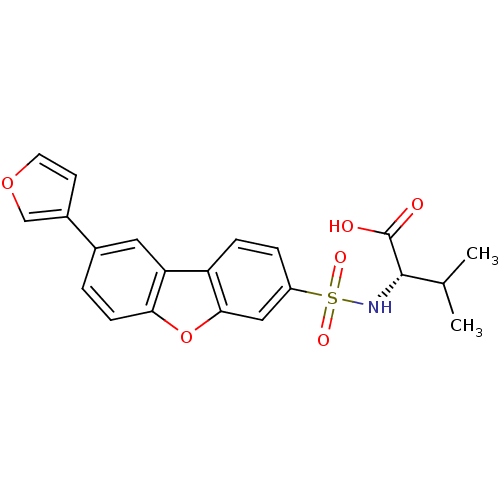
(CHEMBL1935284)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1ccoc1)C(O)=O |r| Show InChI InChI=1S/C21H19NO6S/c1-12(2)20(21(23)24)22-29(25,26)15-4-5-16-17-9-13(14-7-8-27-11-14)3-6-18(17)28-19(16)10-15/h3-12,20,22H,1-2H3,(H,23,24)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173235

((S)-2-{4'-[(5-Bromo-4-methoxy-benzofuran-2-carbony...)Show SMILES COc1c(Br)ccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H25BrN2O7S/c1-15(2)24(27(32)33)30-38(34,35)19-10-6-17(7-11-19)16-4-8-18(9-5-16)29-26(31)23-14-20-22(37-23)13-12-21(28)25(20)36-3/h4-15,24,30H,1-3H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360968

(CHEMBL1935290)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1ccc[nH]1)C(O)=O |r| Show InChI InChI=1S/C21H20N2O5S/c1-12(2)20(21(24)25)23-29(26,27)14-6-7-15-16-10-13(17-4-3-9-22-17)5-8-18(16)28-19(15)11-14/h3-12,20,22-23H,1-2H3,(H,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50295489
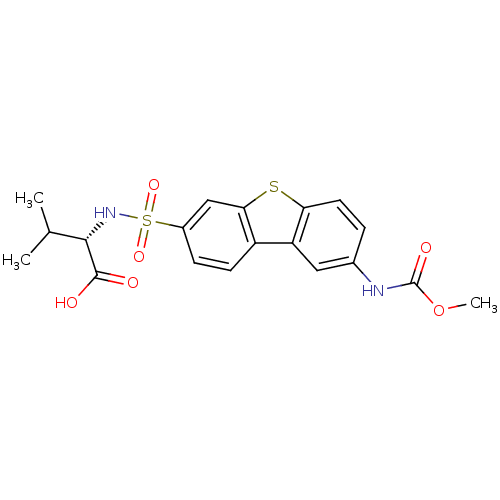
((S)-2-(8-(Methoxycarbonylamino)dibenzo[b,d]thiophe...)Show SMILES COC(=O)Nc1ccc2sc3cc(ccc3c2c1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H20N2O6S2/c1-10(2)17(18(22)23)21-29(25,26)12-5-6-13-14-8-11(20-19(24)27-3)4-7-15(14)28-16(13)9-12/h4-10,17,21H,1-3H3,(H,20,24)(H,22,23)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP12 |
J Med Chem 52: 5408-19 (2009)
Article DOI: 10.1021/jm900809r
BindingDB Entry DOI: 10.7270/Q21J99SW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173236

((S)-2-{4'-[(5-Bromo-benzofuran-2-carbonyl)-amino]-...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3cc(Br)ccc3o2)cc1)C(O)=O Show InChI InChI=1S/C26H23BrN2O6S/c1-15(2)24(26(31)32)29-36(33,34)21-10-5-17(6-11-21)16-3-8-20(9-4-16)28-25(30)23-14-18-13-19(27)7-12-22(18)35-23/h3-15,24,29H,1-2H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360959
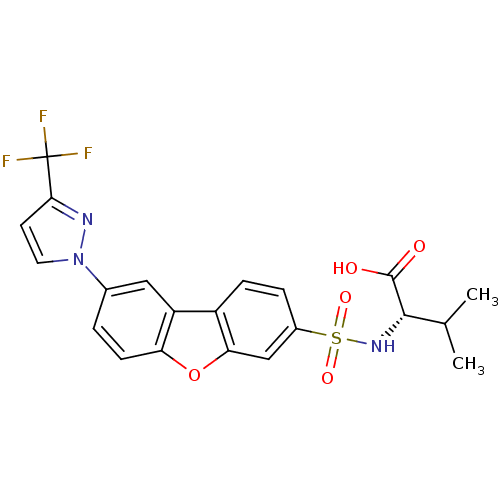
(CHEMBL1935280)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-n1ccc(n1)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C21H18F3N3O5S/c1-11(2)19(20(28)29)26-33(30,31)13-4-5-14-15-9-12(3-6-16(15)32-17(14)10-13)27-8-7-18(25-27)21(22,23)24/h3-11,19,26H,1-2H3,(H,28,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM28480
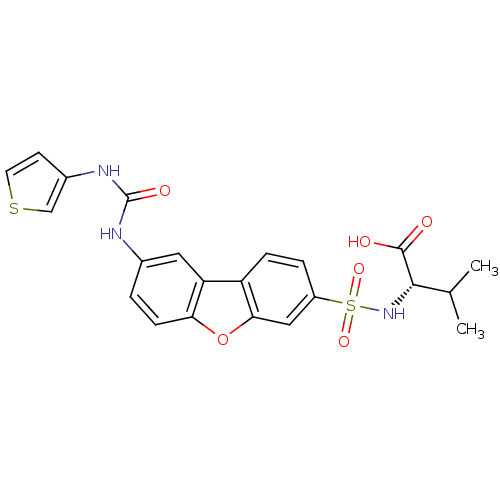
((2S)-3-methyl-2-({12-[(thiophen-3-ylcarbamoyl)amin...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(NC(=O)Nc3ccsc3)cc21)C(O)=O |r| Show InChI InChI=1S/C22H21N3O6S2/c1-12(2)20(21(26)27)25-33(29,30)15-4-5-16-17-9-13(3-6-18(17)31-19(16)10-15)23-22(28)24-14-7-8-32-11-14/h3-12,20,25H,1-2H3,(H,26,27)(H2,23,24,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research
| Assay Description
The assays for human MMP activity were performed by incubating fluorogenic peptide substrate with recombinant human MMP catalytic domain along with v... |
J Med Chem 52: 1799-802 (2009)
Article DOI: 10.1021/jm900093d
BindingDB Entry DOI: 10.7270/Q2X928NP |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50173236

((S)-2-{4'-[(5-Bromo-benzofuran-2-carbonyl)-amino]-...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3cc(Br)ccc3o2)cc1)C(O)=O Show InChI InChI=1S/C26H23BrN2O6S/c1-15(2)24(26(31)32)29-36(33,34)21-10-5-17(6-11-21)16-3-8-20(9-4-16)28-25(30)23-14-18-13-19(27)7-12-22(18)35-23/h3-15,24,29H,1-2H3,(H,28,30)(H,31,32)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-2 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360969

(CHEMBL1935291)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1nccs1)C(O)=O |r| Show InChI InChI=1S/C20H18N2O5S2/c1-11(2)18(20(23)24)22-29(25,26)13-4-5-14-15-9-12(19-21-7-8-28-19)3-6-16(15)27-17(14)10-13/h3-11,18,22H,1-2H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50026624
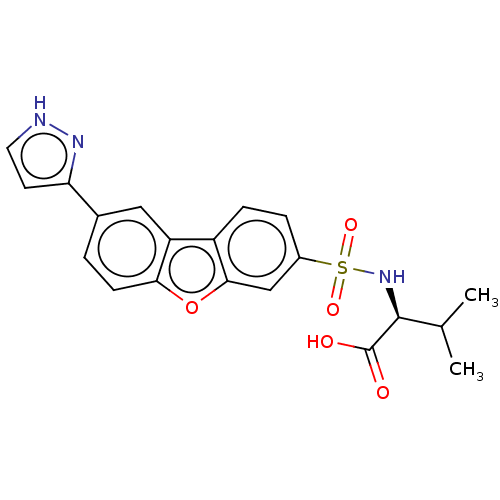
(CHEMBL1935289)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1cc[nH]n1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM28481
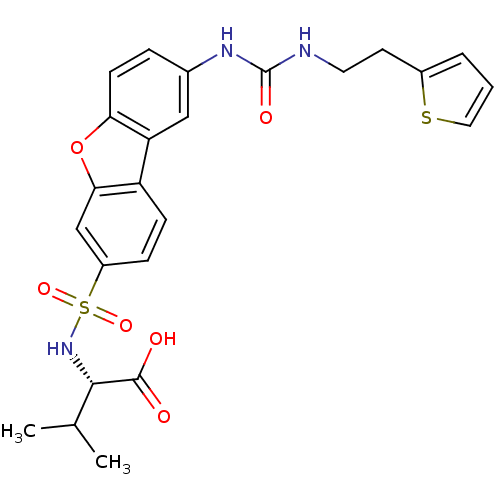
((2S)-3-methyl-2-{[12-({[2-(thiophen-2-yl)ethyl]car...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(NC(=O)NCCc3cccs3)cc21)C(O)=O |r| Show InChI InChI=1S/C24H25N3O6S2/c1-14(2)22(23(28)29)27-35(31,32)17-6-7-18-19-12-15(5-8-20(19)33-21(18)13-17)26-24(30)25-10-9-16-4-3-11-34-16/h3-8,11-14,22,27H,9-10H2,1-2H3,(H,28,29)(H2,25,26,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research
| Assay Description
The assays for human MMP activity were performed by incubating fluorogenic peptide substrate with recombinant human MMP catalytic domain along with v... |
J Med Chem 52: 1799-802 (2009)
Article DOI: 10.1021/jm900093d
BindingDB Entry DOI: 10.7270/Q2X928NP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM28481

((2S)-3-methyl-2-{[12-({[2-(thiophen-2-yl)ethyl]car...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(NC(=O)NCCc3cccs3)cc21)C(O)=O |r| Show InChI InChI=1S/C24H25N3O6S2/c1-14(2)22(23(28)29)27-35(31,32)17-6-7-18-19-12-15(5-8-20(19)33-21(18)13-17)26-24(30)25-10-9-16-4-3-11-34-16/h3-8,11-14,22,27H,9-10H2,1-2H3,(H,28,29)(H2,25,26,30)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
The assays for human MMP activity were performed by incubating fluorogenic peptide substrate with recombinant human MMP catalytic domain along with v... |
J Med Chem 52: 1799-802 (2009)
Article DOI: 10.1021/jm900093d
BindingDB Entry DOI: 10.7270/Q2X928NP |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50004169
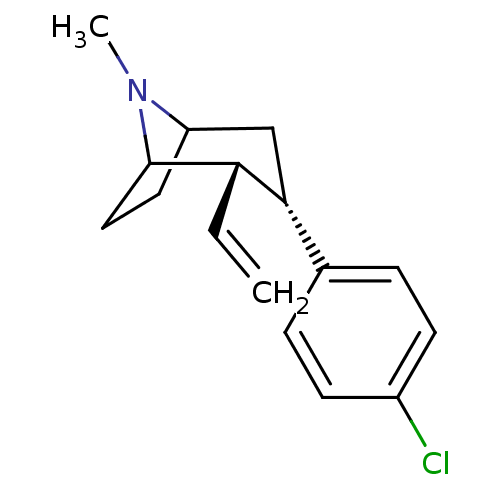
((2S,3S)-3-(4-Chloro-phenyl)-8-methyl-2-vinyl-8-aza...)Show SMILES CN1C2CCC1[C@@H](C=C)[C@H](C2)c1ccc(Cl)cc1 |TLB:7:6:1:4.3,THB:11:9:1:4.3,0:1:6.9.10:4.3| Show InChI InChI=1S/C16H20ClN/c1-3-14-15(11-4-6-12(17)7-5-11)10-13-8-9-16(14)18(13)2/h3-7,13-16H,1,8-10H2,2H3/t13?,14-,15+,16?/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Education and Research
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [3H]mazindol binding from rat striatal membranes |
J Med Chem 35: 4764-6 (1993)
BindingDB Entry DOI: 10.7270/Q2QR4XQT |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360970
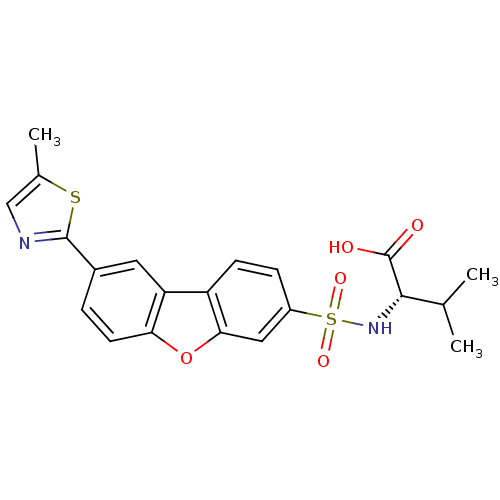
(CHEMBL1935292)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1ncc(C)s1)C(O)=O |r| Show InChI InChI=1S/C21H20N2O5S2/c1-11(2)19(21(24)25)23-30(26,27)14-5-6-15-16-8-13(20-22-10-12(3)29-20)4-7-17(16)28-18(15)9-14/h4-11,19,23H,1-3H3,(H,24,25)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50295488

((R)-2-(8-(Methoxycarbonylamino)dibenzo[b,d]thiophe...)Show SMILES COC(=O)Nc1ccc2sc3cc(ccc3c2c1)S(=O)(=O)N[C@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H20N2O6S2/c1-10(2)17(18(22)23)21-29(25,26)12-5-6-13-14-8-11(20-19(24)27-3)4-7-15(14)28-16(13)9-12/h4-10,17,21H,1-3H3,(H,20,24)(H,22,23)/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 |
J Med Chem 52: 5408-19 (2009)
Article DOI: 10.1021/jm900809r
BindingDB Entry DOI: 10.7270/Q21J99SW |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM28485
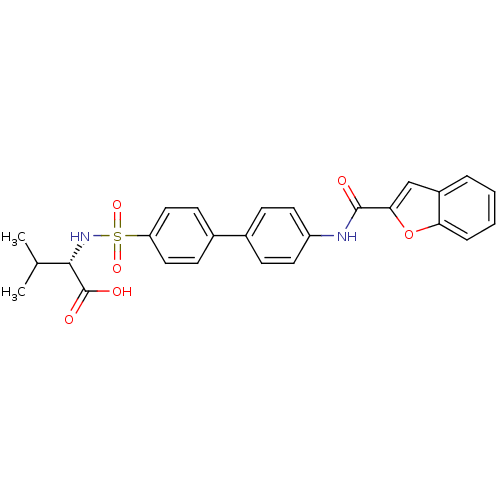
((2S)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
The assays for human MMP activity were performed by incubating fluorogenic peptide substrate with recombinant human MMP catalytic domain along with v... |
J Med Chem 52: 1799-802 (2009)
Article DOI: 10.1021/jm900093d
BindingDB Entry DOI: 10.7270/Q2X928NP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Collagenase 3
(Homo sapiens (Human)) | BDBM28485

((2S)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP13 |
J Med Chem 52: 5408-19 (2009)
Article DOI: 10.1021/jm900809r
BindingDB Entry DOI: 10.7270/Q21J99SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50295479
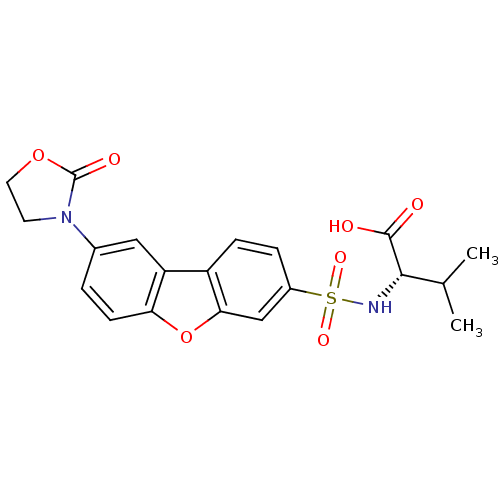
((S)-3-Methyl-2-(8-(2-oxooxazolidin-3-yl)dibenzo[b,...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)N1CCOC1=O)C(O)=O |r| Show InChI InChI=1S/C20H20N2O7S/c1-11(2)18(19(23)24)21-30(26,27)13-4-5-14-15-9-12(22-7-8-28-20(22)25)3-6-16(15)29-17(14)10-13/h3-6,9-11,18,21H,7-8H2,1-2H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP12 |
J Med Chem 52: 5408-19 (2009)
Article DOI: 10.1021/jm900809r
BindingDB Entry DOI: 10.7270/Q21J99SW |
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50035738

((R)-3-(4-Chloro-phenyl)-8-methyl-8-aza-bicyclo[3.2...)Show SMILES COC(=O)C1C2CCC(CC1c1ccc(Cl)cc1)N2C |TLB:11:10:18:6.7,THB:2:4:18:6.7| Show InChI InChI=1S/C16H20ClNO2/c1-18-12-7-8-14(18)15(16(19)20-2)13(9-12)10-3-5-11(17)6-4-10/h3-6,12-15H,7-9H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Mayo Foundation for Education and Research
Curated by ChEMBL
| Assay Description
Compound was evaluated for its ability to displace [3H]mazindol binding from rat striatal membranes |
J Med Chem 35: 4764-6 (1993)
BindingDB Entry DOI: 10.7270/Q2QR4XQT |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360952
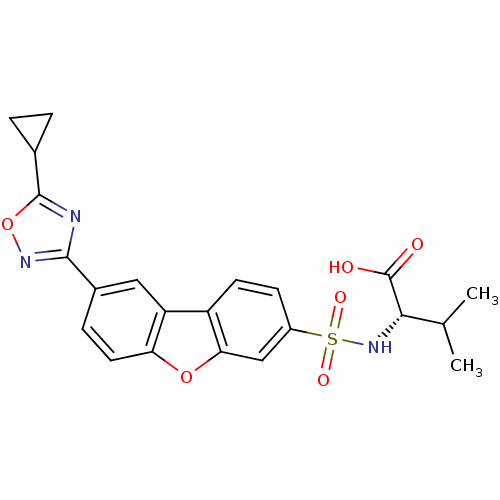
(CHEMBL1935300)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1noc(n1)C1CC1)C(O)=O |r| Show InChI InChI=1S/C22H21N3O6S/c1-11(2)19(22(26)27)25-32(28,29)14-6-7-15-16-9-13(5-8-17(16)30-18(15)10-14)20-23-21(31-24-20)12-3-4-12/h5-12,19,25H,3-4H2,1-2H3,(H,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50295488

((R)-2-(8-(Methoxycarbonylamino)dibenzo[b,d]thiophe...)Show SMILES COC(=O)Nc1ccc2sc3cc(ccc3c2c1)S(=O)(=O)N[C@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H20N2O6S2/c1-10(2)17(18(22)23)21-29(25,26)12-5-6-13-14-8-11(20-19(24)27-3)4-7-15(14)28-16(13)9-12/h4-10,17,21H,1-3H3,(H,20,24)(H,22,23)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP12 |
J Med Chem 52: 5408-19 (2009)
Article DOI: 10.1021/jm900809r
BindingDB Entry DOI: 10.7270/Q21J99SW |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360950

(CHEMBL1935298)Show SMILES COCc1nc(no1)-c1ccc2oc3cc(ccc3c2c1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C21H21N3O7S/c1-11(2)19(21(25)26)24-32(27,28)13-5-6-14-15-8-12(4-7-16(15)30-17(14)9-13)20-22-18(10-29-3)31-23-20/h4-9,11,19,24H,10H2,1-3H3,(H,25,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173220

((S)-2-{4'-[(5-Chloro-4-methoxy-3-methyl-benzofuran...)Show SMILES COc1c(Cl)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27ClN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50295479

((S)-3-Methyl-2-(8-(2-oxooxazolidin-3-yl)dibenzo[b,...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)N1CCOC1=O)C(O)=O |r| Show InChI InChI=1S/C20H20N2O7S/c1-11(2)18(19(23)24)21-30(26,27)13-4-5-14-15-9-12(22-7-8-28-20(22)25)3-6-16(15)29-17(14)10-13/h3-6,9-11,18,21H,7-8H2,1-2H3,(H,23,24)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM28469
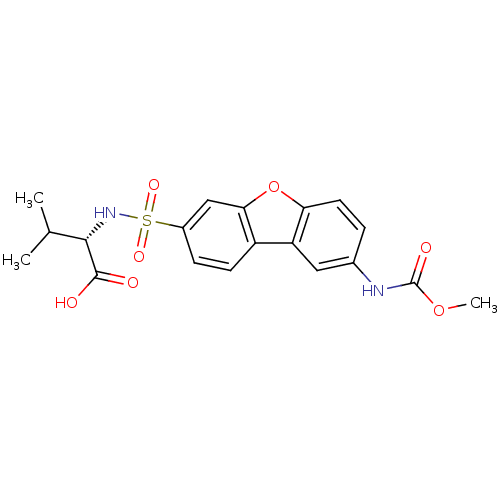
((2S)-2-({12-[(methoxycarbonyl)amino]-8-oxatricyclo...)Show SMILES COC(=O)Nc1ccc2oc3cc(ccc3c2c1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H20N2O7S/c1-10(2)17(18(22)23)21-29(25,26)12-5-6-13-14-8-11(20-19(24)27-3)4-7-15(14)28-16(13)9-12/h4-10,17,21H,1-3H3,(H,20,24)(H,22,23)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MMP12 |
J Med Chem 52: 5408-19 (2009)
Article DOI: 10.1021/jm900809r
BindingDB Entry DOI: 10.7270/Q21J99SW |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM28469

((2S)-2-({12-[(methoxycarbonyl)amino]-8-oxatricyclo...)Show SMILES COC(=O)Nc1ccc2oc3cc(ccc3c2c1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O |r| Show InChI InChI=1S/C19H20N2O7S/c1-10(2)17(18(22)23)21-29(25,26)12-5-6-13-14-8-11(20-19(24)27-3)4-7-15(14)28-16(13)9-12/h4-10,17,21H,1-3H3,(H,20,24)(H,22,23)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research
| Assay Description
The assays for human MMP activity were performed by incubating fluorogenic peptide substrate with recombinant human MMP catalytic domain along with v... |
J Med Chem 52: 1799-802 (2009)
Article DOI: 10.1021/jm900093d
BindingDB Entry DOI: 10.7270/Q2X928NP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM28479
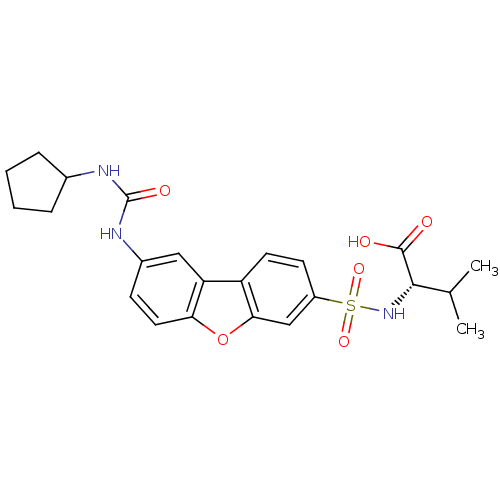
((2S)-2-({12-[(cyclopentylcarbamoyl)amino]-8-oxatri...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(NC(=O)NC3CCCC3)cc21)C(O)=O |r| Show InChI InChI=1S/C23H27N3O6S/c1-13(2)21(22(27)28)26-33(30,31)16-8-9-17-18-11-15(7-10-19(18)32-20(17)12-16)25-23(29)24-14-5-3-4-6-14/h7-14,21,26H,3-6H2,1-2H3,(H,27,28)(H2,24,25,29)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
The assays for human MMP activity were performed by incubating fluorogenic peptide substrate with recombinant human MMP catalytic domain along with v... |
J Med Chem 52: 1799-802 (2009)
Article DOI: 10.1021/jm900093d
BindingDB Entry DOI: 10.7270/Q2X928NP |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173218

((S)-2-{4'-[(5-Iodo-4-methoxy-3-methyl-benzofuran-2...)Show SMILES COc1c(I)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27IN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM28473
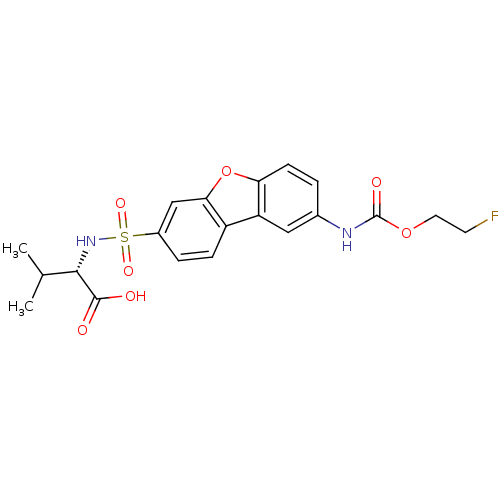
((2S)-2-[(12-{[(2-fluoroethoxy)carbonyl]amino}-8-ox...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(NC(=O)OCCF)cc21)C(O)=O |r| Show InChI InChI=1S/C20H21FN2O7S/c1-11(2)18(19(24)25)23-31(27,28)13-4-5-14-15-9-12(22-20(26)29-8-7-21)3-6-16(15)30-17(14)10-13/h3-6,9-11,18,23H,7-8H2,1-2H3,(H,22,26)(H,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Wyeth Research
| Assay Description
The assays for human MMP activity were performed by incubating fluorogenic peptide substrate with recombinant human MMP catalytic domain along with v... |
J Med Chem 52: 1799-802 (2009)
Article DOI: 10.1021/jm900093d
BindingDB Entry DOI: 10.7270/Q2X928NP |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360951
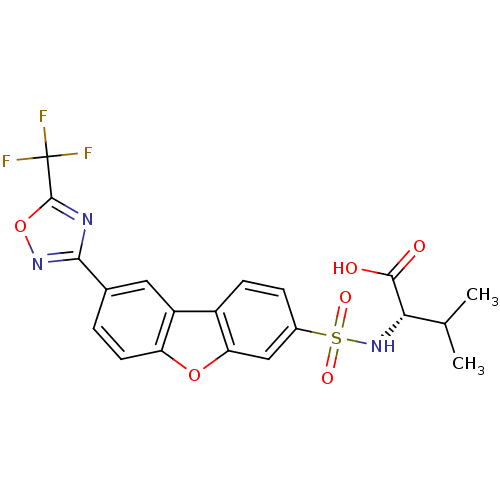
(CHEMBL1935299)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1noc(n1)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C20H16F3N3O6S/c1-9(2)16(18(27)28)26-33(29,30)11-4-5-12-13-7-10(3-6-14(13)31-15(12)8-11)17-24-19(32-25-17)20(21,22)23/h3-9,16,26H,1-2H3,(H,27,28)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360962

(CHEMBL1935283)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)N1CCOCC1)C(O)=O |r| Show InChI InChI=1S/C21H24N2O6S/c1-13(2)20(21(24)25)22-30(26,27)15-4-5-16-17-11-14(23-7-9-28-10-8-23)3-6-18(17)29-19(16)12-15/h3-6,11-13,20,22H,7-10H2,1-2H3,(H,24,25)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50170298
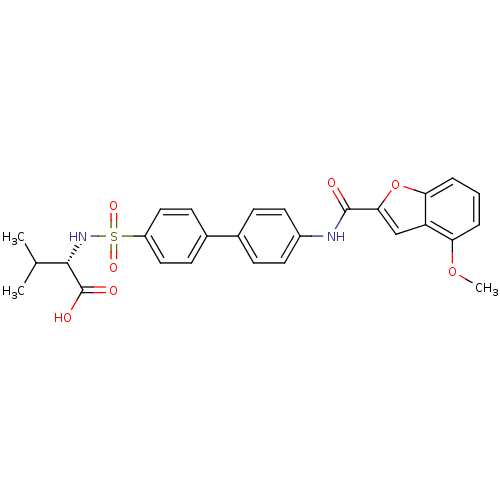
((S)-2-{4'-[(4-Methoxy-benzofuran-2-carbonyl)-amino...)Show SMILES COc1cccc2oc(cc12)C(=O)Nc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N[C@@H](C(C)C)C(O)=O Show InChI InChI=1S/C27H26N2O7S/c1-16(2)25(27(31)32)29-37(33,34)20-13-9-18(10-14-20)17-7-11-19(12-8-17)28-26(30)24-15-21-22(35-3)5-4-6-23(21)36-24/h4-16,25,29H,1-3H3,(H,28,30)(H,31,32)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Macrophage metalloelastase
(Homo sapiens (Human)) | BDBM50360953

(CHEMBL1935301)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc2c(c1)oc1ccc(cc21)-c1noc(n1)C1CCC1)C(O)=O |r| Show InChI InChI=1S/C23H23N3O6S/c1-12(2)20(23(27)28)26-33(29,30)15-7-8-16-17-10-14(6-9-18(17)31-19(16)11-15)21-24-22(32-25-21)13-4-3-5-13/h6-13,20,26H,3-5H2,1-2H3,(H,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human MMP-12 |
Bioorg Med Chem Lett 22: 138-43 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.046
BindingDB Entry DOI: 10.7270/Q2SJ1M20 |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50173223

((S)-2-(4'-(5-bromo-4-methoxy-3-methylbenzofuran-2-...)Show SMILES COc1c(Br)ccc2oc(C(=O)Nc3ccc(cc3)-c3ccc(cc3)S(=O)(=O)N[C@@H](C(C)C)C(O)=O)c(C)c12 Show InChI InChI=1S/C28H27BrN2O7S/c1-15(2)24(28(33)34)31-39(35,36)20-11-7-18(8-12-20)17-5-9-19(10-6-17)30-27(32)25-16(3)23-22(38-25)14-13-21(29)26(23)37-4/h5-15,24,31H,1-4H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM28485

((2S)-2-({4-[4-(1-benzofuran-2-amido)phenyl]benzene...)Show SMILES CC(C)[C@H](NS(=O)(=O)c1ccc(cc1)-c1ccc(NC(=O)c2cc3ccccc3o2)cc1)C(O)=O |r| Show InChI InChI=1S/C26H24N2O6S/c1-16(2)24(26(30)31)28-35(32,33)21-13-9-18(10-14-21)17-7-11-20(12-8-17)27-25(29)23-15-19-5-3-4-6-22(19)34-23/h3-16,24,28H,1-2H3,(H,27,29)(H,30,31)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MMP-13 |
Bioorg Med Chem Lett 15: 4961-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.001
BindingDB Entry DOI: 10.7270/Q20Z72TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data