

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgen receptor (Homo sapiens (Human)) | BDBM50204245 (CHEMBL3898284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204247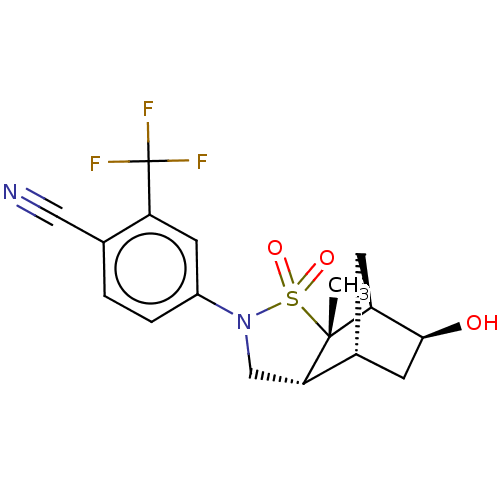 (CHEMBL3920247) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204246 (CHEMBL3893320) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204241 (CHEMBL3917372) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204251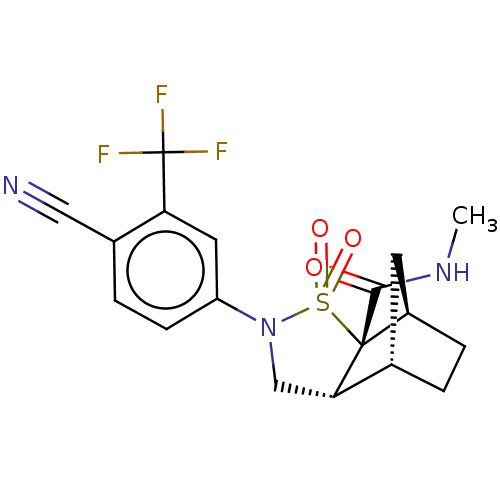 (CHEMBL3921315) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204243 (CHEMBL3926358) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204242 (CHEMBL3902310) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204249 (CHEMBL3935242) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204250 (CHEMBL3949176) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267028 (CHEMBL4073525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204248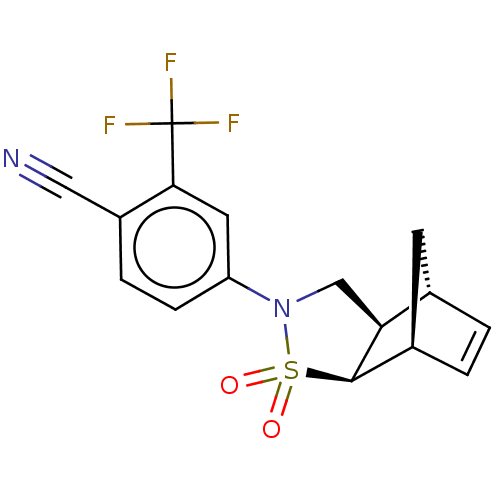 (CHEMBL3987109) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267031 (CHEMBL4079930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204240 (CHEMBL3939272) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267009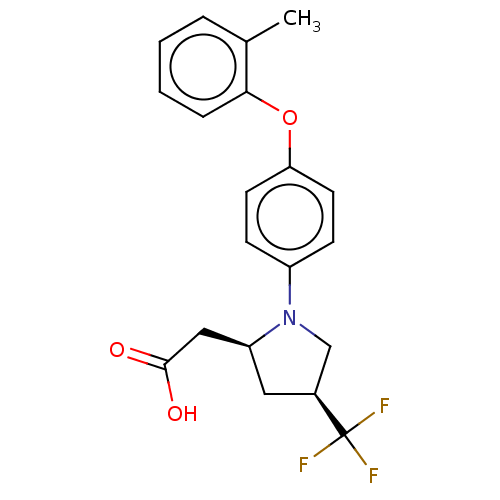 (CHEMBL4089171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267029 (CHEMBL4069191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267010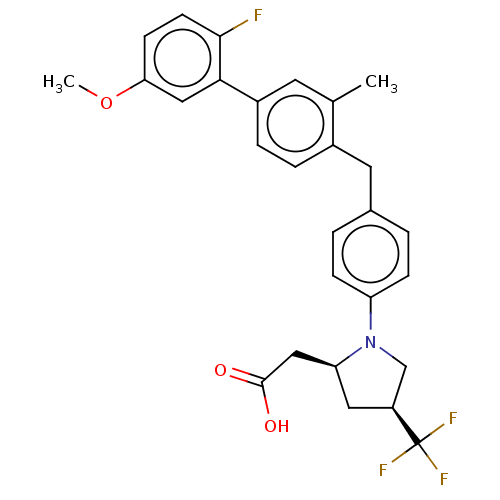 (CHEMBL4082395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267117 (CHEMBL4060499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267030 (CHEMBL4090240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267044 (CHEMBL4069764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204244 (CHEMBL3907304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50204244 (CHEMBL3907304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 106 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [3H]DHT from androgen receptor in human MDA-MB-453 cells | Bioorg Med Chem Lett 26: 5707-5711 (2016) Article DOI: 10.1016/j.bmcl.2016.10.059 BindingDB Entry DOI: 10.7270/Q2PK0J43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267039 (CHEMBL4090766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267035 (CHEMBL4071899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267049 (CHEMBL4095599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50089354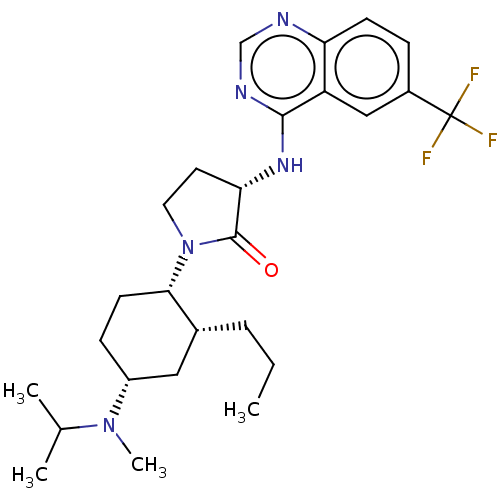 (CHEMBL3577945) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M1 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267038 (CHEMBL4061093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 865 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M4 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267046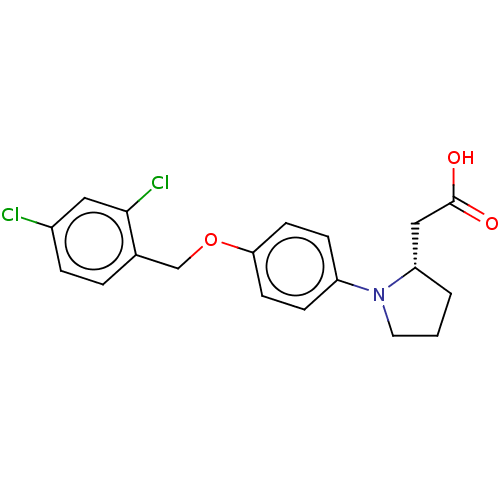 (CHEMBL4103729) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267118 (CHEMBL4096887) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267066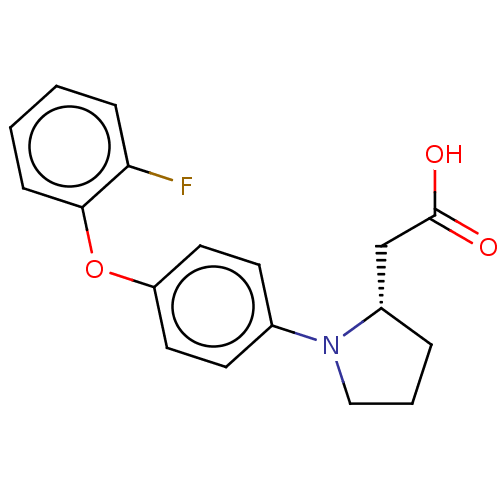 (CHEMBL4067052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267059 (CHEMBL4091552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50089354 (CHEMBL3577945) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 4.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of muscarinic acetylcholine receptor M2 (unknown origin) | ACS Med Chem Lett 6: 439-44 (2015) Article DOI: 10.1021/ml500505q BindingDB Entry DOI: 10.7270/Q2668FWM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267047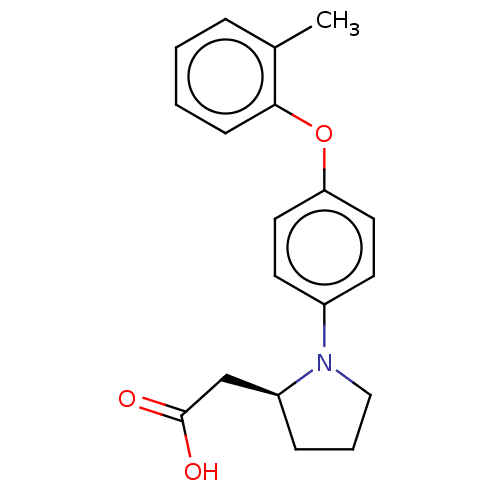 (CHEMBL4078852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267064 (CHEMBL4070703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267045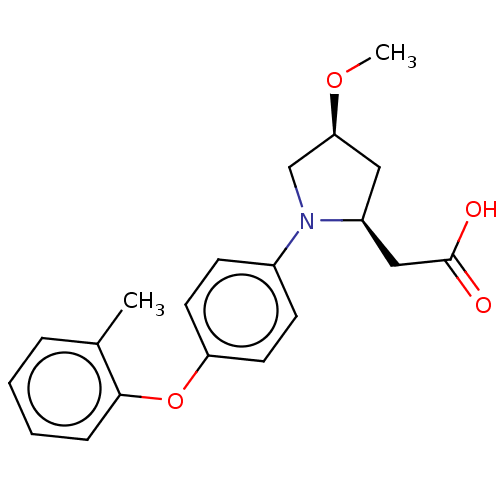 (CHEMBL4063889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267048 (CHEMBL4075190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267021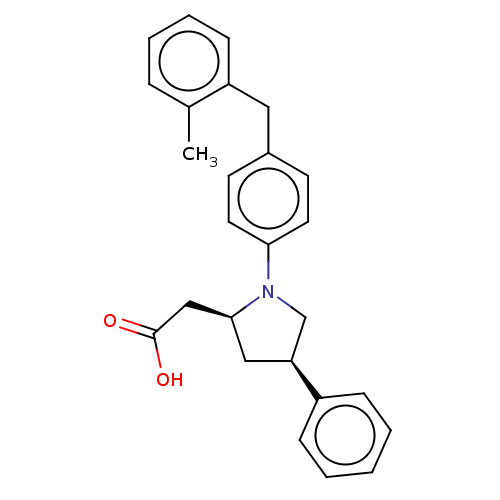 (CHEMBL4091669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267113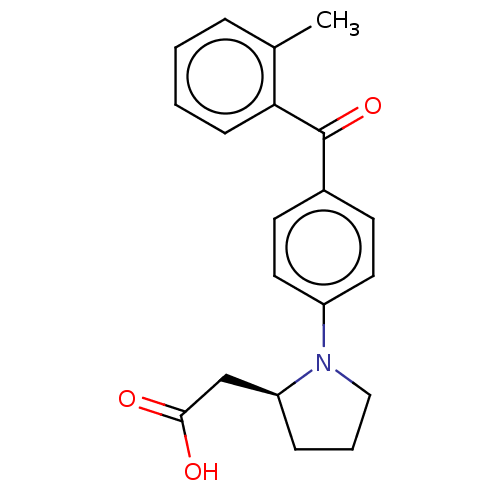 (CHEMBL4105565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23165 (CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting | ACS Med Chem Lett 7: 283-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00448 BindingDB Entry DOI: 10.7270/Q2D79D95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50169441 (CHEMBL3806205) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM258470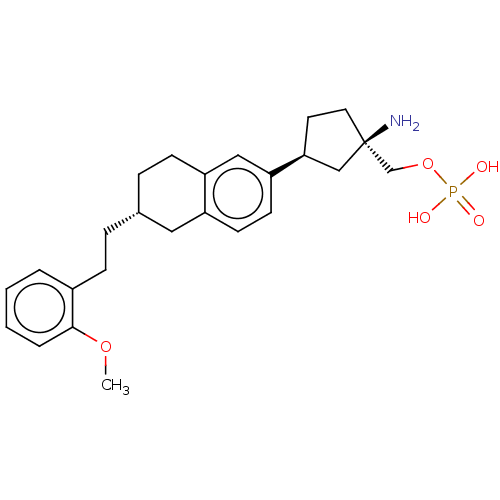 (US9522888, 697) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by radioligand competitive binding analy... | Citation and Details Article DOI: 10.1021/acs.jmedchem.8b01695 BindingDB Entry DOI: 10.7270/Q2TQ659P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM23163 (CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Displacement of 33-P-S1P from from human S1P receptor expressed in CHO cell membranes after 50 mins by scintillation counting | ACS Med Chem Lett 7: 283-8 (2016) Article DOI: 10.1021/acsmedchemlett.5b00448 BindingDB Entry DOI: 10.7270/Q2D79D95 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM258466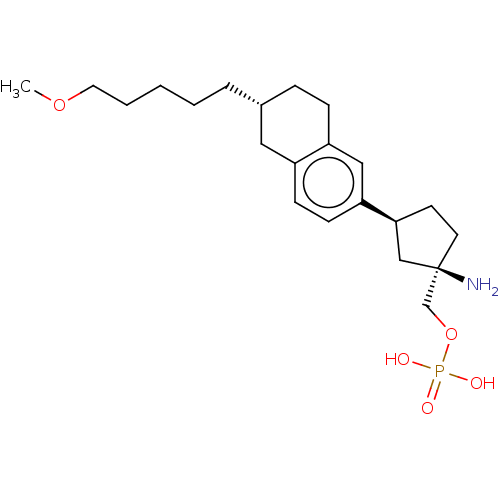 (US9522888, 689) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50562873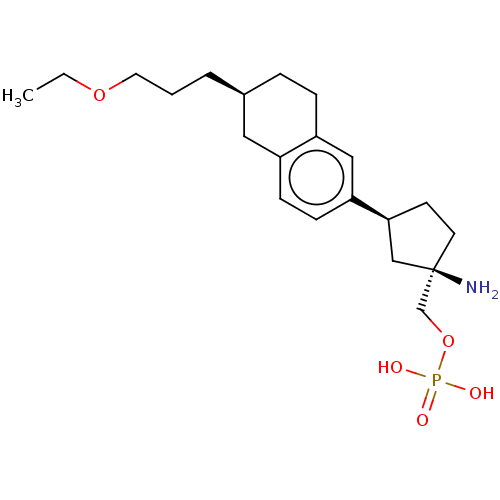 (CHEMBL4786296) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [33P]-SIP from human recombinant S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount scintillation counting metho... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01109 BindingDB Entry DOI: 10.7270/Q28919KX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform (Homo sapiens (Human)) | BDBM50239718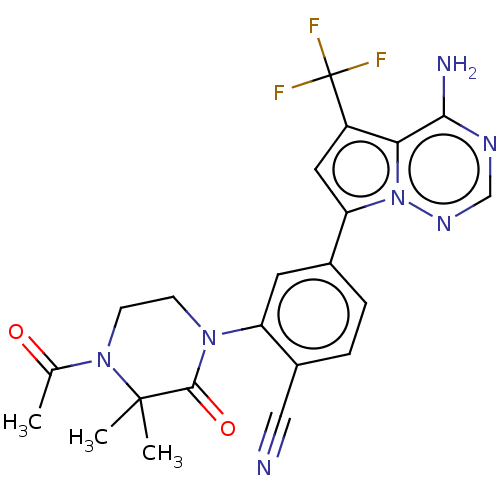 (CHEMBL4064666 | US10214537, Example 639) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Antisecretory activity evaluated by the inhibition of 14C -AP uptake in isolated rabbit parietal cells stimulated by exogenous histamine | J Med Chem 60: 5193-5208 (2017) Article DOI: 10.1021/acs.jmedchem.7b00618 BindingDB Entry DOI: 10.7270/Q2WW7KVJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532543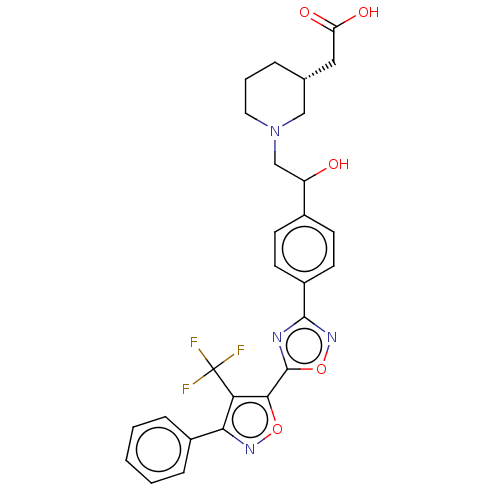 (CHEMBL4474984) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50532543 (CHEMBL4474984) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Displacement of [33P]S1P from human S1P1 expressed in CHO cell membranes measured after 45 mins by TopCount method | J Med Chem 59: 6248-64 (2016) Article DOI: 10.1021/acs.jmedchem.6b00373 BindingDB Entry DOI: 10.7270/Q2FJ2M8Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2687 total ) | Next | Last >> |