 Found 47 hits with Last Name = 'doman' and Initial = 'tn'
Found 47 hits with Last Name = 'doman' and Initial = 'tn' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse transcriptase/RNaseH
(Human immunodeficiency virus 1) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Therapeutic concentration on reverse transcriptase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113299
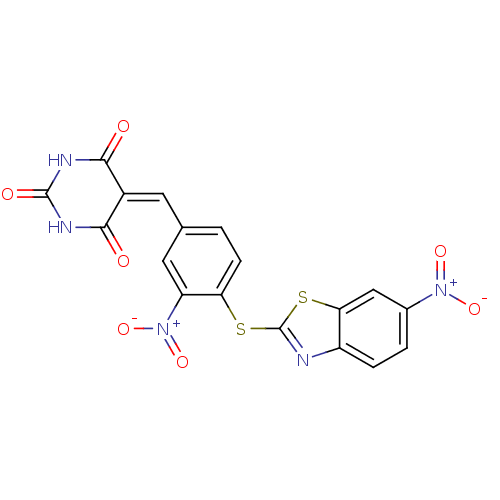
(5-[3-Nitro-4-(6-nitro-benzothiazol-2-ylsulfanyl)-b...)Show SMILES [#8-]-[#7+](=O)-c1ccc2nc(-[#16]-c3ccc(\[#6]=[#6]-4/[#6](=O)-[#7]-[#6](=O)-[#7]-[#6]-4=O)cc3-[#7+](-[#8-])=O)sc2c1 Show InChI InChI=1S/C18H9N5O7S2/c24-15-10(16(25)21-17(26)20-15)5-8-1-4-13(12(6-8)23(29)30)31-18-19-11-3-2-9(22(27)28)7-14(11)32-18/h1-7H,(H2,20,21,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113293
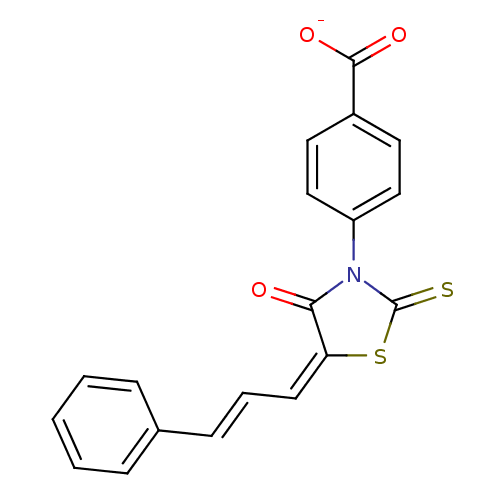
(4-[4-Oxo-5-(3-phenyl-allylidene)-2-thioxo-thiazoli...)Show SMILES [O-]C(=O)c1ccc(cc1)N1C(=S)S\C(=C\C=C\c2ccccc2)C1=O Show InChI InChI=1S/C19H13NO3S2/c21-17-16(8-4-7-13-5-2-1-3-6-13)25-19(24)20(17)15-11-9-14(10-12-15)18(22)23/h1-12H,(H,22,23)/p-1/b7-4+,16-8+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113297
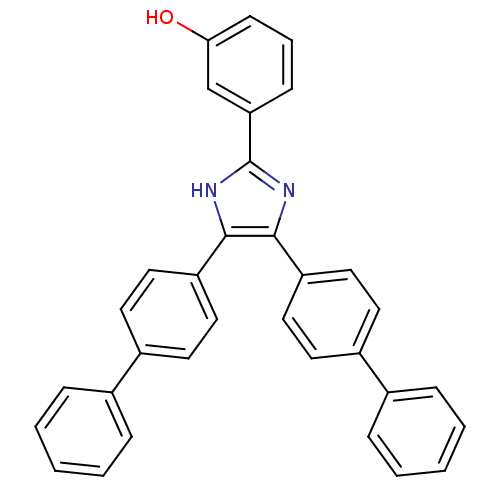
(3-(4,5-Bis-biphenyl-4-yl-1H-imidazol-2-yl)-phenol ...)Show SMILES Oc1cccc(c1)-c1nc(c([nH]1)-c1ccc(cc1)-c1ccccc1)-c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C33H24N2O/c36-30-13-7-12-29(22-30)33-34-31(27-18-14-25(15-19-27)23-8-3-1-4-9-23)32(35-33)28-20-16-26(17-21-28)24-10-5-2-6-11-24/h1-22,36H,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113296
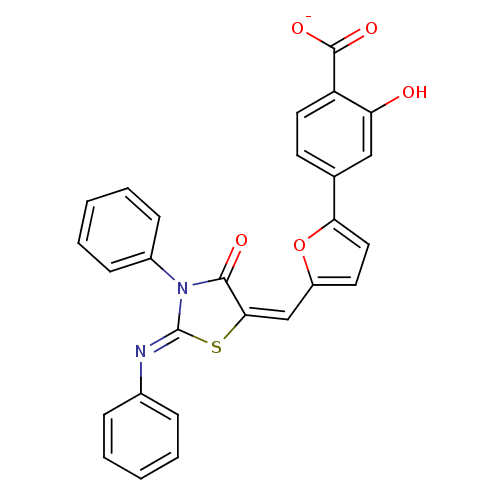
(2-Hydroxy-4-[5-(4-oxo-3-phenyl-2-phenylimino-thiaz...)Show SMILES Oc1cc(ccc1C([O-])=O)-c1ccc(\C=C2\S\C(=N/c3ccccc3)N(C2=O)c2ccccc2)o1 Show InChI InChI=1S/C27H18N2O5S/c30-22-15-17(11-13-21(22)26(32)33)23-14-12-20(34-23)16-24-25(31)29(19-9-5-2-6-10-19)27(35-24)28-18-7-3-1-4-8-18/h1-16,30H,(H,32,33)/p-1/b24-16+,28-27- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113295
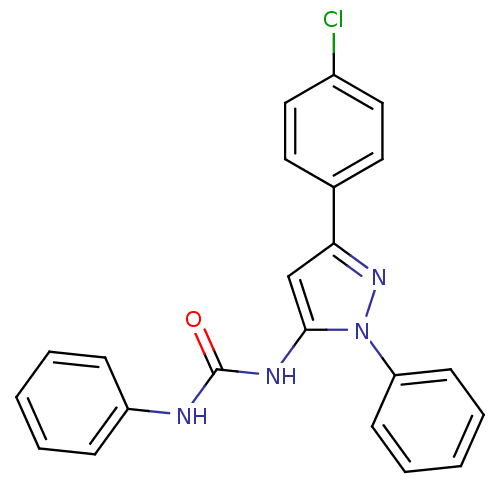
(1-[5-(4-Chloro-phenyl)-2-phenyl-2H-pyrazol-3-yl]-3...)Show SMILES Clc1ccc(cc1)-c1cc(NC(=O)Nc2ccccc2)n(n1)-c1ccccc1 Show InChI InChI=1S/C22H17ClN4O/c23-17-13-11-16(12-14-17)20-15-21(27(26-20)19-9-5-2-6-10-19)25-22(28)24-18-7-3-1-4-8-18/h1-15H,(H2,24,25,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM31770
(Exelderm | Sulconazole Nitrate | cid_65495 | sulco...)Show InChI InChI=1S/C18H15Cl3N2S/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113303
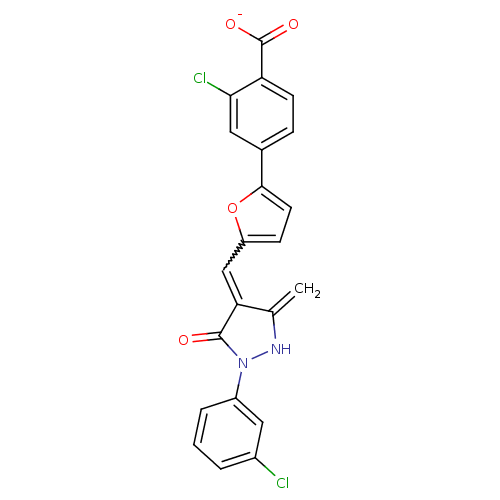
(2-Chloro-4-{5-[1-(3-chloro-phenyl)-3-methyl-5-oxo-...)Show SMILES [O-]C(=O)c1ccc(cc1Cl)-c1ccc(C=c2c(=C)[nH]n(-c3cccc(Cl)c3)c2=O)o1 |w:14.14| Show InChI InChI=1S/C22H14Cl2N2O4/c1-12-18(21(27)26(25-12)15-4-2-3-14(23)10-15)11-16-6-8-20(30-16)13-5-7-17(22(28)29)19(24)9-13/h2-11,25H,1H2,(H,28,29)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM31774
(CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...)Show InChI InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM31770

(Exelderm | Sulconazole Nitrate | cid_65495 | sulco...)Show InChI InChI=1S/C18H15Cl3N2S/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50367298

(Cardene | NICARDIPINE)Show SMILES COC(=O)C1C(C(C(=O)OCCN(C)Cc2ccccc2)=C(C)N=C1C)c1cccc(c1)[N+]([O-])=O |c:24,t:21| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,22,24H,13-14,16H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113302
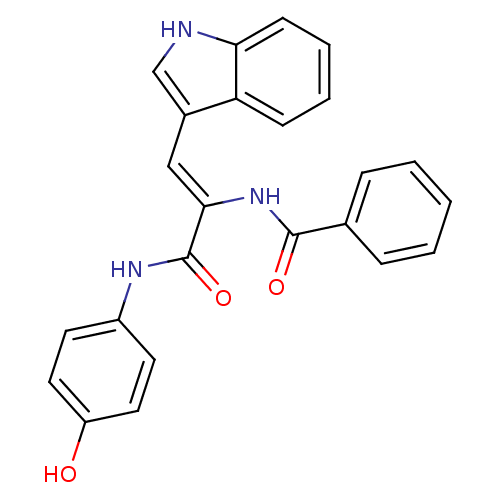
(CHEMBL420925 | N-[1-(4-Hydroxy-phenylcarbamoyl)-2-...)Show SMILES Oc1ccc(NC(=O)C(\NC(=O)c2ccccc2)=C\c2c[nH]c3ccccc23)cc1 Show InChI InChI=1S/C24H19N3O3/c28-19-12-10-18(11-13-19)26-24(30)22(27-23(29)16-6-2-1-3-7-16)14-17-15-25-21-9-5-4-8-20(17)21/h1-15,25,28H,(H,26,30)(H,27,29)/b22-14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113301

(4-(3-Benzyl-4-oxo-2-thioxo-thiazolidin-5-ylideneme...)Show SMILES [O-]C(=O)c1ccc(\C=C2\SC(=S)N(Cc3ccccc3)C2=O)cc1 Show InChI InChI=1S/C18H13NO3S2/c20-16-15(10-12-6-8-14(9-7-12)17(21)22)24-18(23)19(16)11-13-4-2-1-3-5-13/h1-10H,11H2,(H,21,22)/p-1/b15-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM31773
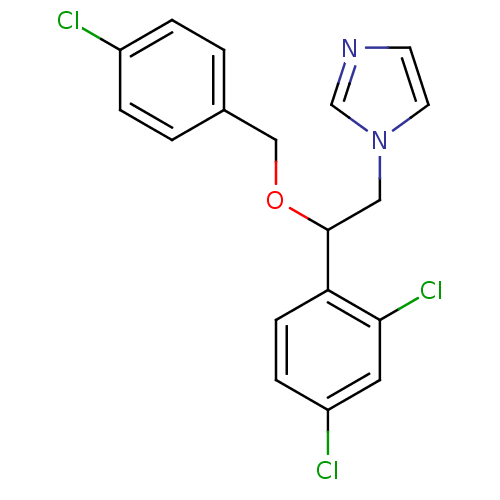
(ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...)Show InChI InChI=1S/C18H15Cl3N2O/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM31772
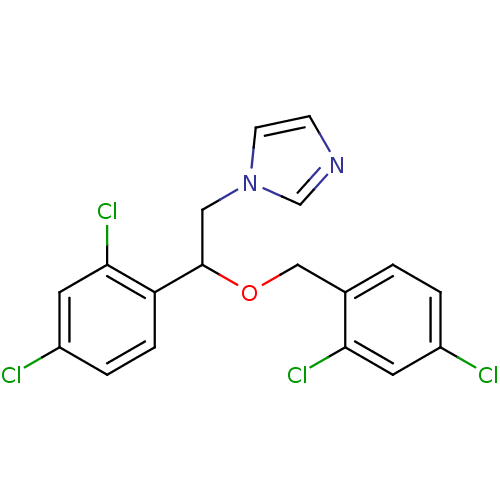
(1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...)Show SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM31773

(ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...)Show InChI InChI=1S/C18H15Cl3N2O/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113294

(2-[2-(5-Bromo-furan-2-carbonyloxy)-acetylamino]-be...)Show SMILES COC(=O)c1ccc2nc(NC(=O)COC(=O)c3ccc(Br)o3)sc2c1 Show InChI InChI=1S/C16H11BrN2O6S/c1-23-14(21)8-2-3-9-11(6-8)26-16(18-9)19-13(20)7-24-15(22)10-4-5-12(17)25-10/h2-6H,7H2,1H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113300

(Acetic acid 4-(2-benzo[1,3]dioxol-5-yl-5-oxo-oxazo...)Show SMILES CCOc1cc(\C=C2\N=C(OC2=O)c2ccc3OCOc3c2)cc(Cl)c1OC(C)=O |c:8| Show InChI InChI=1S/C21H16ClNO7/c1-3-26-18-8-12(6-14(22)19(18)29-11(2)24)7-15-21(25)30-20(23-15)13-4-5-16-17(9-13)28-10-27-16/h4-9H,3,10H2,1-2H3/b15-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM31774

(CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...)Show InChI InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM31772

(1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...)Show SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM50367298

(Cardene | NICARDIPINE)Show SMILES COC(=O)C1C(C(C(=O)OCCN(C)Cc2ccccc2)=C(C)N=C1C)c1cccc(c1)[N+]([O-])=O |c:24,t:21| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,22,24H,13-14,16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50113298
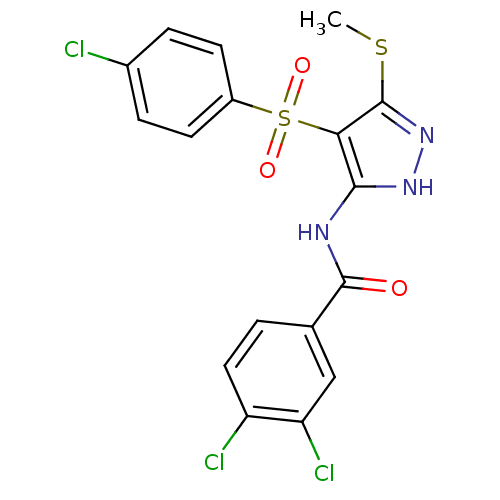
(3,4-Dichloro-N-[4-(4-chloro-benzenesulfonyl)-5-met...)Show SMILES CSc1n[nH]c(NC(=O)c2ccc(Cl)c(Cl)c2)c1S(=O)(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C17H12Cl3N3O3S2/c1-27-17-14(28(25,26)11-5-3-10(18)4-6-11)15(22-23-17)21-16(24)9-2-7-12(19)13(20)8-9/h2-8H,1H3,(H2,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human Protein-tyrosine phosphatase 1B (PTP1B) dephosphorylation of insulin receptor peptide |
J Med Chem 45: 2213-21 (2002)
BindingDB Entry DOI: 10.7270/Q2SB46F1 |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM31774

(CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...)Show InChI InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50134035
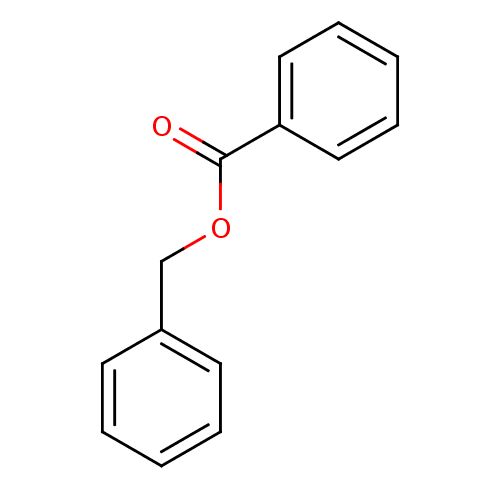
(BENZYL BENZOATE | CHEMBL1239)Show InChI InChI=1S/C14H12O2/c15-14(13-9-5-2-6-10-13)16-11-12-7-3-1-4-8-12/h1-10H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM31770

(Exelderm | Sulconazole Nitrate | cid_65495 | sulco...)Show InChI InChI=1S/C18H15Cl3N2S/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM31772

(1-[2-(2,4-dichlorobenzyl)oxy-2-(2,4-dichlorophenyl...)Show SMILES Clc1ccc(COC(Cn2ccnc2)c2ccc(Cl)cc2Cl)c(Cl)c1 Show InChI InChI=1S/C18H14Cl4N2O/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22/h1-8,11,18H,9-10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM50134035

(BENZYL BENZOATE | CHEMBL1239)Show InChI InChI=1S/C14H12O2/c15-14(13-9-5-2-6-10-13)16-11-12-7-3-1-4-8-12/h1-10H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM31768

(CHEMBL295698 | Ketoconazole | Nizoral | Panfungol)Show SMILES CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r| Show InChI InChI=1S/C26H28Cl2N4O4/c1-19(33)31-10-12-32(13-11-31)21-3-5-22(6-4-21)34-15-23-16-35-26(36-23,17-30-9-8-29-18-30)24-7-2-20(27)14-25(24)28/h2-9,14,18,23H,10-13,15-17H2,1H3/t23-,26-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM31773

(ECONAZOLE | Econazole nitrate | Gyno-pevaryl | Pev...)Show InChI InChI=1S/C18H15Cl3N2O/c19-14-3-1-13(2-4-14)11-24-18(10-23-8-7-22-12-23)16-6-5-15(20)9-17(16)21/h1-9,12,18H,10-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50367298

(Cardene | NICARDIPINE)Show SMILES COC(=O)C1C(C(C(=O)OCCN(C)Cc2ccccc2)=C(C)N=C1C)c1cccc(c1)[N+]([O-])=O |c:24,t:21| Show InChI InChI=1S/C26H29N3O6/c1-17-22(25(30)34-4)24(20-11-8-12-21(15-20)29(32)33)23(18(2)27-17)26(31)35-14-13-28(3)16-19-9-6-5-7-10-19/h5-12,15,22,24H,13-14,16H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM50002861
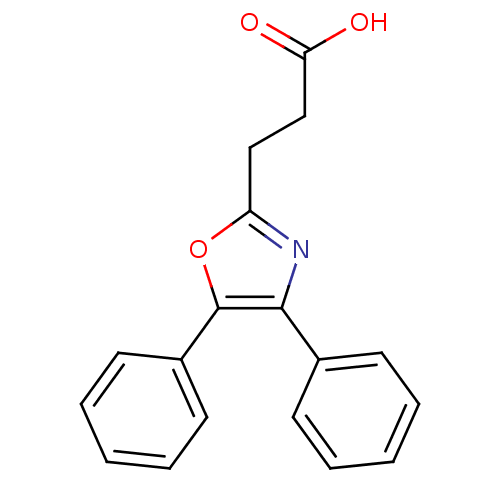
(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50002861

(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM50134036
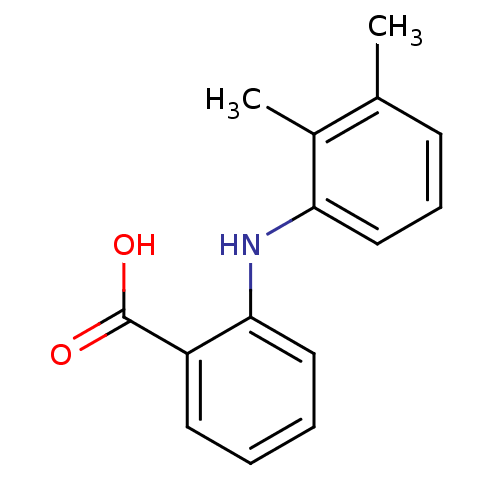
(2-(2,3-Dimethyl-phenylamino)-benzoic acid | 2-(2,3...)Show InChI InChI=1S/C15H15NO2/c1-10-6-5-9-13(11(10)2)16-14-8-4-3-7-12(14)15(17)18/h3-9,16H,1-2H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM1944

(BHAP deriv. | CHEMBL593 | DELAVIRDINE MESYLATE | D...)Show SMILES CC(C)Nc1cccnc1N1CCN(CC1)C(=O)c1cc2cc(NS(C)(=O)=O)ccc2[nH]1 Show InChI InChI=1S/C22H28N6O3S/c1-15(2)24-19-5-4-8-23-21(19)27-9-11-28(12-10-27)22(29)20-14-16-13-17(26-32(3,30)31)6-7-18(16)25-20/h4-8,13-15,24-26H,9-12H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50134035

(BENZYL BENZOATE | CHEMBL1239)Show InChI InChI=1S/C14H12O2/c15-14(13-9-5-2-6-10-13)16-11-12-7-3-1-4-8-12/h1-10H,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50012957
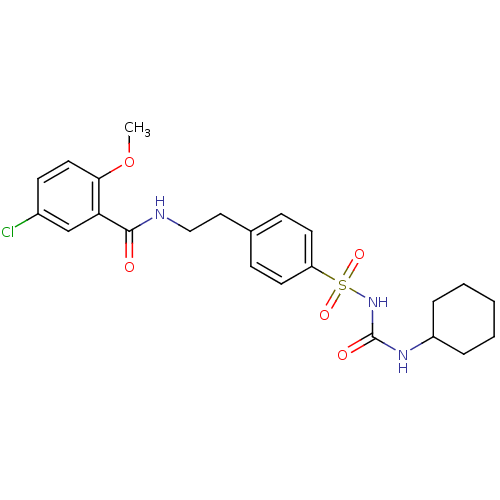
(1-((p-(2-(5-chloro-o-anisamido)ethyl)phenyl)sulfon...)Show SMILES COc1ccc(Cl)cc1C(=O)NCCc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50134036

(2-(2,3-Dimethyl-phenylamino)-benzoic acid | 2-(2,3...)Show InChI InChI=1S/C15H15NO2/c1-10-6-5-9-13(11(10)2)16-14-8-4-3-7-12(14)15(17)18/h3-9,16H,1-2H3,(H,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM50012957

(1-((p-(2-(5-chloro-o-anisamido)ethyl)phenyl)sulfon...)Show SMILES COc1ccc(Cl)cc1C(=O)NCCc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM50012957

(1-((p-(2-(5-chloro-o-anisamido)ethyl)phenyl)sulfon...)Show SMILES COc1ccc(Cl)cc1C(=O)NCCc1ccc(cc1)S(=O)(=O)NC(=O)NC1CCCCC1 Show InChI InChI=1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen A
(Bos taurus (bovine)) | BDBM25817
(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of Chymotrypsinogen |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM50002861

(3-(4,5-Diphenyl-oxazol-2-yl)-propionic acid | CHEM...)Show InChI InChI=1S/C18H15NO3/c20-16(21)12-11-15-19-17(13-7-3-1-4-8-13)18(22-15)14-9-5-2-6-10-14/h1-10H,11-12H2,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Malate dehydrogenase, cytoplasmic
(Homo sapiens (Human)) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Inhibition of malate dehydrogenase (MDH) |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |
Beta-lactamase
(Escherichia coli) | BDBM25817

(2-(2,4-difluorophenyl)-1,3-bis(1H-1,2,4-triazol-1-...)Show InChI InChI=1S/C13H12F2N6O/c14-10-1-2-11(12(15)3-10)13(22,4-20-8-16-6-18-20)5-21-9-17-7-19-21/h1-3,6-9,22H,4-5H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northwestern University
Curated by ChEMBL
| Assay Description
Compound was tested for the inhibition of beta-lactamase |
J Med Chem 46: 4477-86 (2003)
Article DOI: 10.1021/jm030191r
BindingDB Entry DOI: 10.7270/Q2X34Z5C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data