 Found 232 hits with Last Name = 'ellsworth' and Initial = 'kp'
Found 232 hits with Last Name = 'ellsworth' and Initial = 'kp' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125977
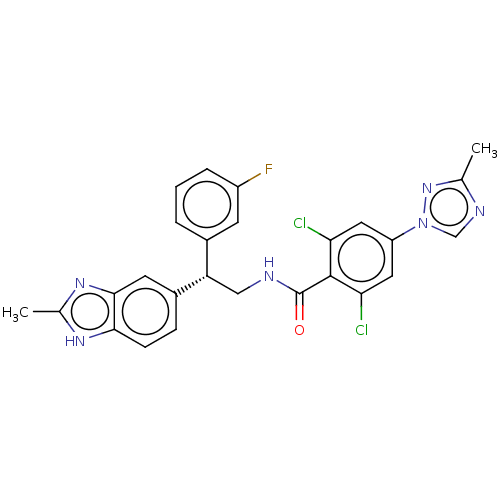
(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50151405

(CHEMBL3775211 | US10189819, Example 79)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2cc(F)c3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H20Cl2F2N6O/c1-13-32-12-36(35-13)18-9-20(27)24(21(28)10-18)26(37)31-11-19(15-4-3-5-17(29)6-15)16-7-22(30)25-23(8-16)33-14(2)34-25/h3-10,12,19H,11H2,1-2H3,(H,31,37)(H,33,34)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50125977

(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a using n-Acetyl-KPR-AFC as substrate preinubated for 30 mins followed by substrate addition measured after 1 hr by fluo... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328246
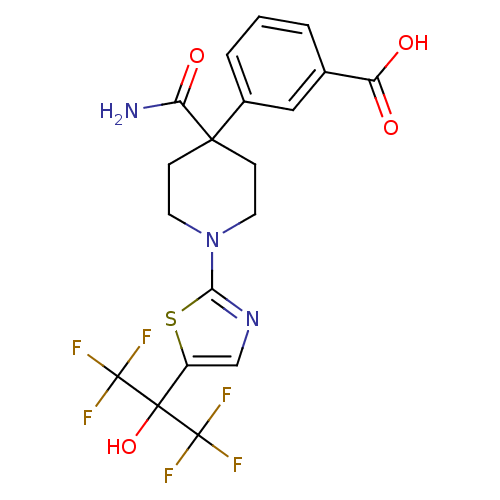
(3-(4-carbamoyl-1-(5-(1,1,1,3,3,3-hexafluoro-2-hydr...)Show SMILES NC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1cccc(c1)C(O)=O Show InChI InChI=1S/C19H17F6N3O4S/c20-18(21,22)17(32,19(23,24)25)12-9-27-15(33-12)28-6-4-16(5-7-28,14(26)31)11-3-1-2-10(8-11)13(29)30/h1-3,8-9,32H,4-7H2,(H2,26,31)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328247
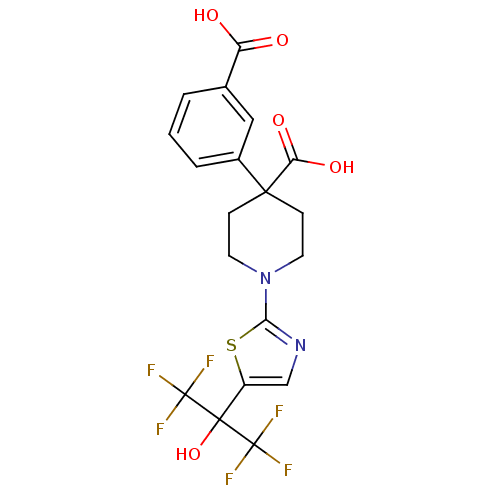
(4-(3-carboxyphenyl)-1-(5-(1,1,1,3,3,3-hexafluoro-2...)Show SMILES OC(=O)c1cccc(c1)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)C(O)=O Show InChI InChI=1S/C19H16F6N2O5S/c20-18(21,22)17(32,19(23,24)25)12-9-26-15(33-12)27-6-4-16(5-7-27,14(30)31)11-3-1-2-10(8-11)13(28)29/h1-3,8-9,32H,4-7H2,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328235
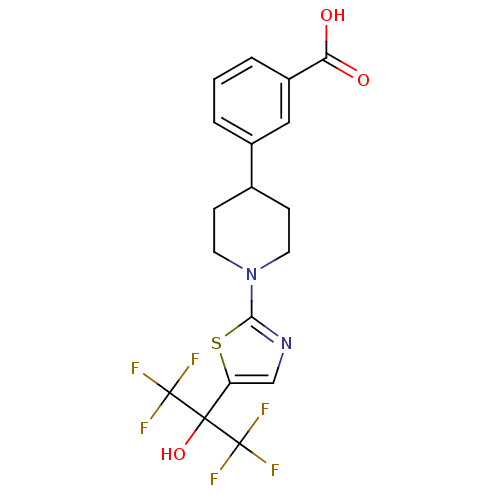
(3-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1cccc(c1)C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H16F6N2O3S/c19-17(20,21)16(29,18(22,23)24)13-9-25-15(30-13)26-6-4-10(5-7-26)11-2-1-3-12(8-11)14(27)28/h1-3,8-10,29H,4-7H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328248
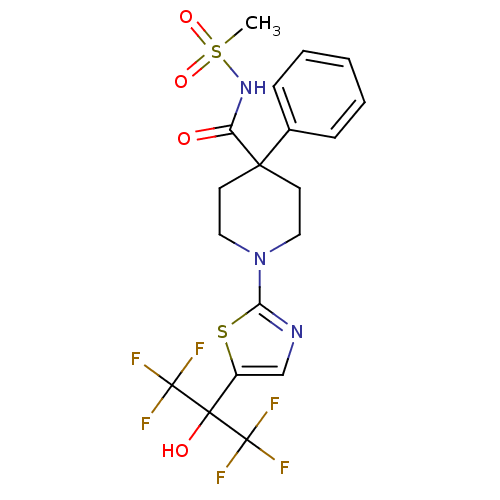
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES CS(=O)(=O)NC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C19H19F6N3O4S2/c1-34(31,32)27-14(29)16(12-5-3-2-4-6-12)7-9-28(10-8-16)15-26-11-13(33-15)17(30,18(20,21)22)19(23,24)25/h2-6,11,30H,7-10H2,1H3,(H,27,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135558
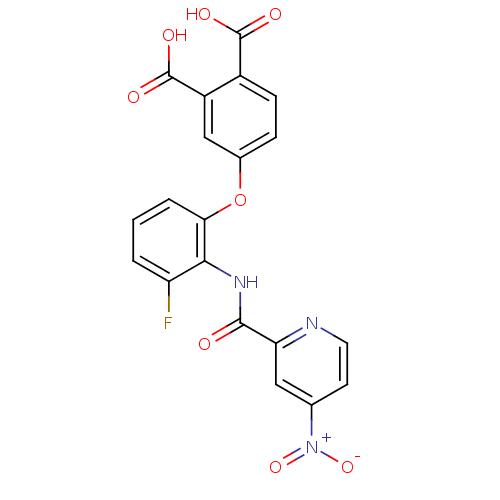
(4-{3-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-14-2-1-3-16(32-11-4-5-12(19(26)27)13(9-11)20(28)29)17(14)23-18(25)15-8-10(24(30)31)6-7-22-15/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135554

(4-{3-[(4-Nitro-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C24H15N3O8/c28-22(20-11-15(27(33)34)7-8-25-20)26-19-9-13-3-1-2-4-14(13)10-21(19)35-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328241
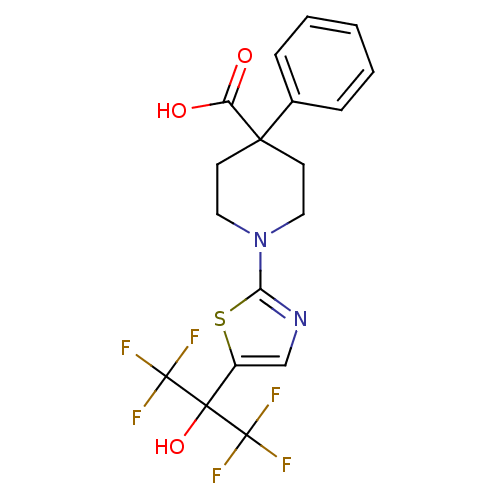
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES OC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C18H16F6N2O3S/c19-17(20,21)16(29,18(22,23)24)12-10-25-14(30-12)26-8-6-15(7-9-26,13(27)28)11-4-2-1-3-5-11/h1-5,10,29H,6-9H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328222
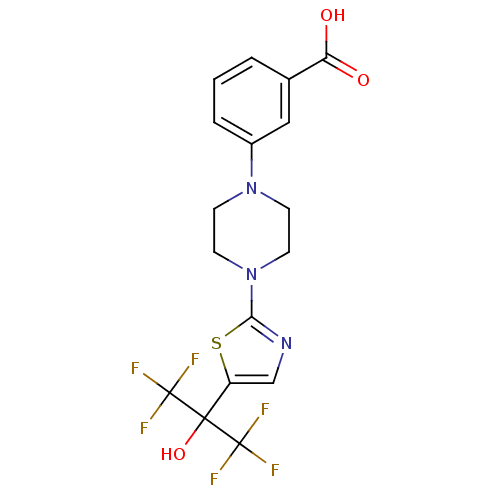
(3-(4-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1cccc(c1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O3S/c18-16(19,20)15(29,17(21,22)23)12-9-24-14(30-12)26-6-4-25(5-7-26)11-3-1-2-10(8-11)13(27)28/h1-3,8-9,29H,4-7H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328244
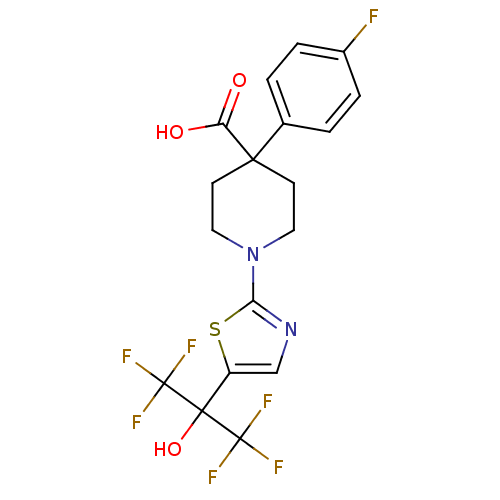
(4-(4-fluorophenyl)-1-(5-(1,1,1,3,3,3-hexafluoro-2-...)Show SMILES OC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccc(F)cc1 Show InChI InChI=1S/C18H15F7N2O3S/c19-11-3-1-10(2-4-11)15(13(28)29)5-7-27(8-6-15)14-26-9-12(31-14)16(30,17(20,21)22)18(23,24)25/h1-4,9,30H,5-8H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328226

(2-(2-(4-(3-(1H-tetrazol-5-yl)phenyl)piperazin-1-yl...)Show SMILES OC(c1cnc(s1)N1CCN(CC1)c1cccc(c1)-c1nnn[nH]1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H15F6N7OS/c18-16(19,20)15(31,17(21,22)23)12-9-24-14(32-12)30-6-4-29(5-7-30)11-3-1-2-10(8-11)13-25-27-28-26-13/h1-3,8-9,31H,4-7H2,(H,25,26,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328245
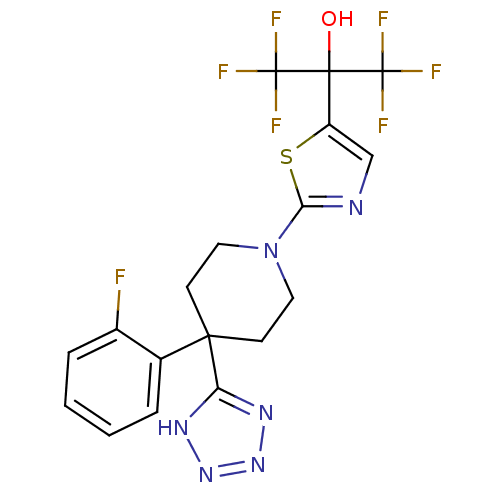
(1,1,1,3,3,3-hexafluoro-2-(2-(4-(2-fluorophenyl)-4-...)Show SMILES OC(c1cnc(s1)N1CCC(CC1)(c1nnn[nH]1)c1ccccc1F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H15F7N6OS/c19-11-4-2-1-3-10(11)15(13-27-29-30-28-13)5-7-31(8-6-15)14-26-9-12(33-14)16(32,17(20,21)22)18(23,24)25/h1-4,9,32H,5-8H2,(H,27,28,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328234
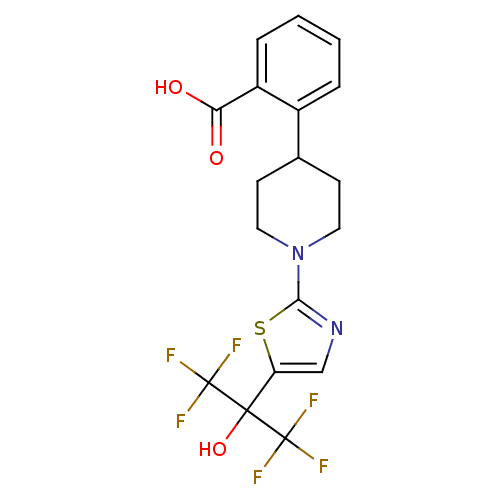
(2-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1ccccc1C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H16F6N2O3S/c19-17(20,21)16(29,18(22,23)24)13-9-25-15(30-13)26-7-5-10(6-8-26)11-3-1-2-4-12(11)14(27)28/h1-4,9-10,29H,5-8H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328237
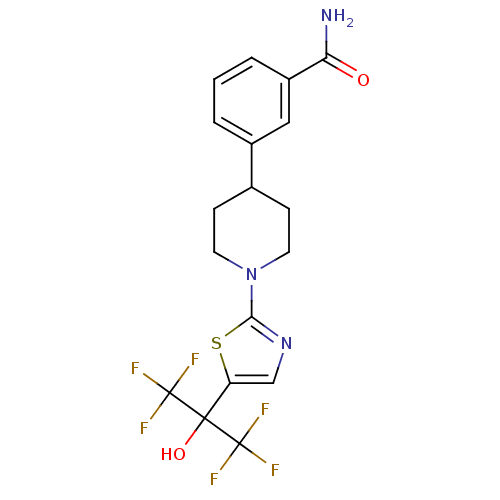
(3-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES NC(=O)c1cccc(c1)C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H17F6N3O2S/c19-17(20,21)16(29,18(22,23)24)13-9-26-15(30-13)27-6-4-10(5-7-27)11-2-1-3-12(8-11)14(25)28/h1-3,8-10,29H,4-7H2,(H2,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50151406
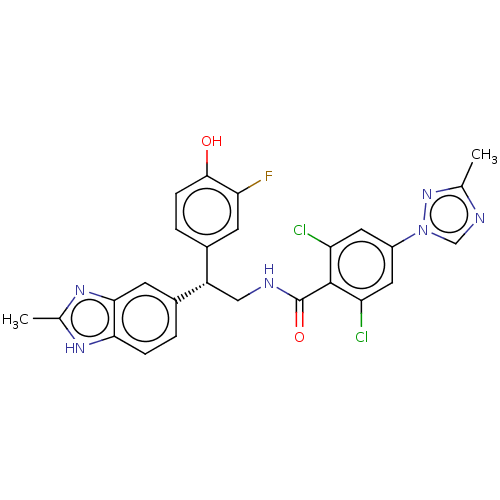
(CHEMBL3774670)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2ccc(O)c(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O2/c1-13-31-12-35(34-13)17-9-19(27)25(20(28)10-17)26(37)30-11-18(15-4-6-24(36)21(29)7-15)16-3-5-22-23(8-16)33-14(2)32-22/h3-10,12,18,36H,11H2,1-2H3,(H,30,37)(H,32,33)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135565
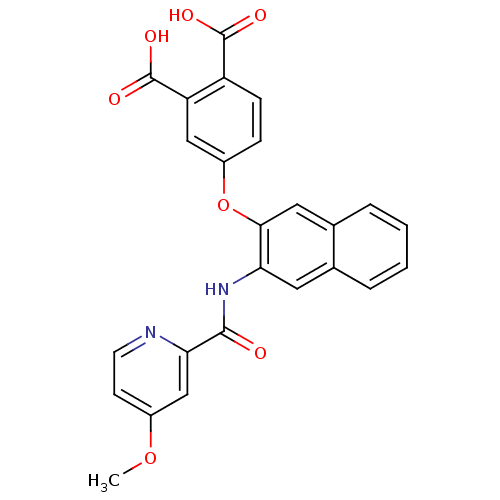
(4-{3-[(4-Methoxy-pyridine-2-carbonyl)-amino]-napht...)Show SMILES COc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C25H18N2O7/c1-33-16-8-9-26-21(13-16)23(28)27-20-10-14-4-2-3-5-15(14)11-22(20)34-17-6-7-18(24(29)30)19(12-17)25(31)32/h2-13H,1H3,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135553
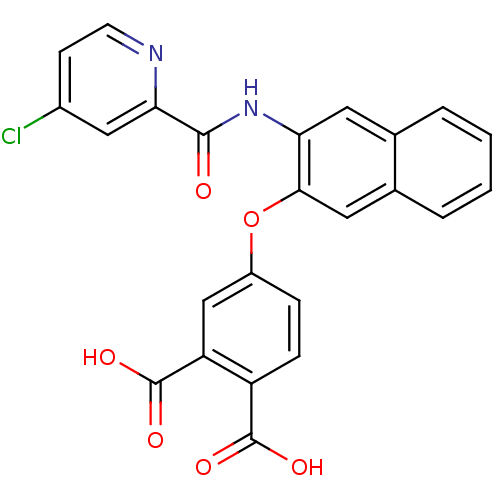
(4-{3-[(4-Chloro-pyridine-2-carbonyl)-amino]-naphth...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C24H15ClN2O6/c25-15-7-8-26-20(11-15)22(28)27-19-9-13-3-1-2-4-14(13)10-21(19)33-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,27,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50133440
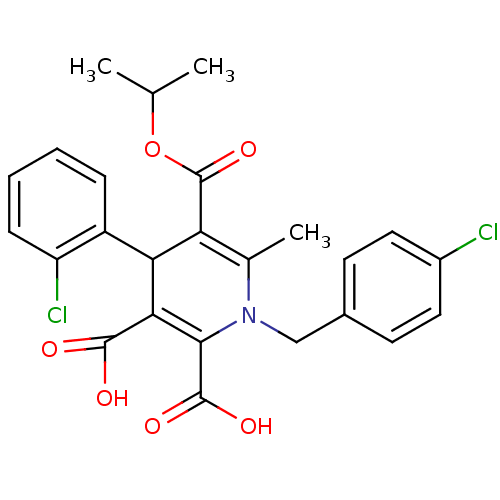
(1-(4-Chloro-benzyl)-4-(2-chloro-phenyl)-6-methyl-1...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2ccc(Cl)cc2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,22| Show InChI InChI=1S/C25H23Cl2NO6/c1-13(2)34-25(33)19-14(3)28(12-15-8-10-16(26)11-9-15)22(24(31)32)21(23(29)30)20(19)17-6-4-5-7-18(17)27/h4-11,13,20H,12H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328225
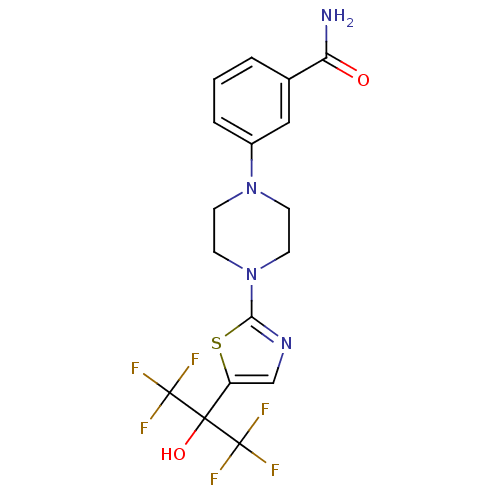
(3-(4-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES NC(=O)c1cccc(c1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H16F6N4O2S/c18-16(19,20)15(29,17(21,22)23)12-9-25-14(30-12)27-6-4-26(5-7-27)11-3-1-2-10(8-11)13(24)28/h1-3,8-9,29H,4-7H2,(H2,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328227
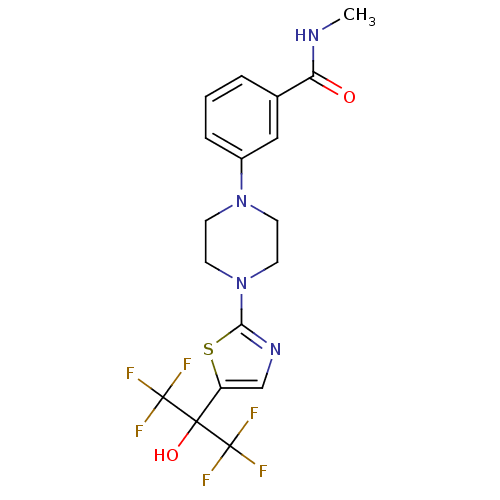
(3-(4-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES CNC(=O)c1cccc(c1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H18F6N4O2S/c1-25-14(29)11-3-2-4-12(9-11)27-5-7-28(8-6-27)15-26-10-13(31-15)16(30,17(19,20)21)18(22,23)24/h2-4,9-10,30H,5-8H2,1H3,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328221
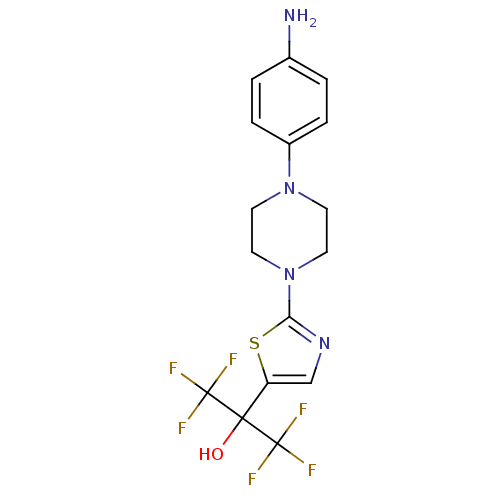
(2-(2-(4-(4-aminophenyl)piperazin-1-yl)thiazol-5-yl...)Show SMILES Nc1ccc(cc1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H16F6N4OS/c17-15(18,19)14(27,16(20,21)22)12-9-24-13(28-12)26-7-5-25(6-8-26)11-3-1-10(23)2-4-11/h1-4,9,27H,5-8,23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135562
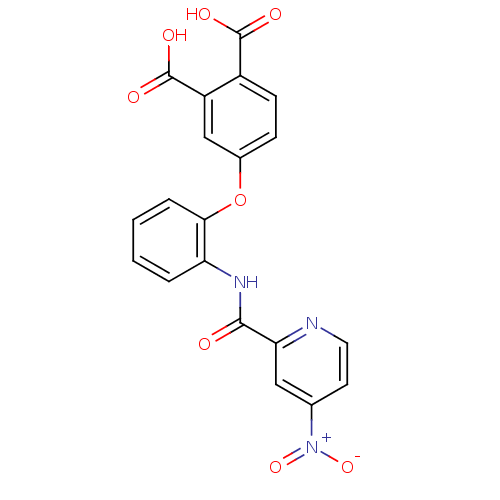
(4-{2-[(4-Nitro-pyridine-2-carbonyl)-amino]-phenoxy...)Show SMILES OC(=O)c1ccc(Oc2ccccc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H13N3O8/c24-18(16-9-11(23(29)30)7-8-21-16)22-15-3-1-2-4-17(15)31-12-5-6-13(19(25)26)14(10-12)20(27)28/h1-10H,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135554

(4-{3-[(4-Nitro-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES OC(=O)c1ccc(Oc2cc3ccccc3cc2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C24H15N3O8/c28-22(20-11-15(27(33)34)7-8-25-20)26-19-9-13-3-1-2-4-14(13)10-21(19)35-16-5-6-17(23(29)30)18(12-16)24(31)32/h1-12H,(H,26,28)(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328238
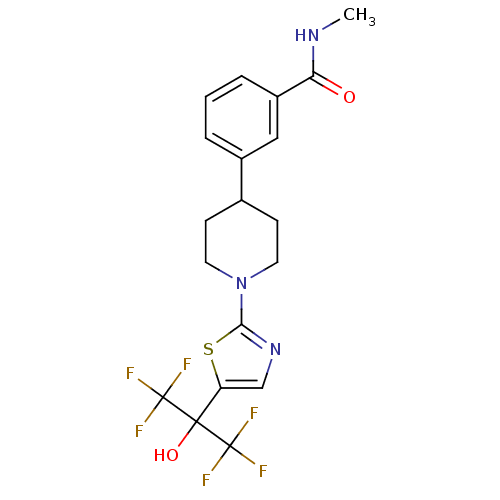
(3-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES CNC(=O)c1cccc(c1)C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C19H19F6N3O2S/c1-26-15(29)13-4-2-3-12(9-13)11-5-7-28(8-6-11)16-27-10-14(31-16)17(30,18(20,21)22)19(23,24)25/h2-4,9-11,30H,5-8H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328220
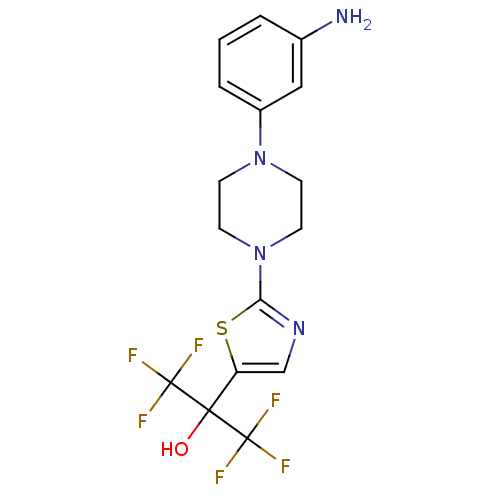
(2-(2-(4-(3-aminophenyl)piperazin-1-yl)thiazol-5-yl...)Show SMILES Nc1cccc(c1)N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H16F6N4OS/c17-15(18,19)14(27,16(20,21)22)12-9-24-13(28-12)26-6-4-25(5-7-26)11-3-1-2-10(23)8-11/h1-3,8-9,27H,4-7,23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328249
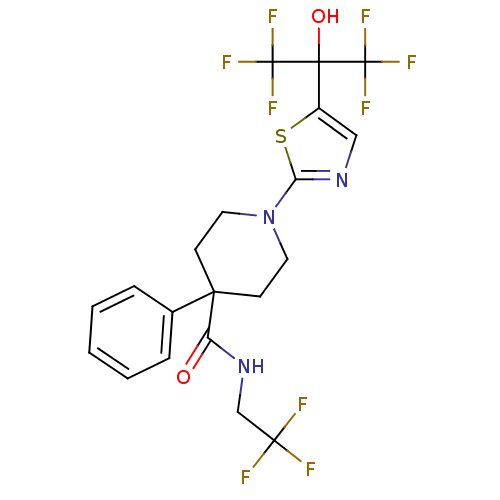
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES OC(c1cnc(s1)N1CCC(CC1)(C(=O)NCC(F)(F)F)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H18F9N3O2S/c21-17(22,23)11-31-14(33)16(12-4-2-1-3-5-12)6-8-32(9-7-16)15-30-10-13(35-15)18(34,19(24,25)26)20(27,28)29/h1-5,10,34H,6-9,11H2,(H,31,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50133438

(4-(2-Chloro-phenyl)-1-(3,4-dimethoxy-benzyl)-6-met...)Show SMILES COc1ccc(CN2C(C)=C(C(C(C(O)=O)=C2C(O)=O)c2ccccc2Cl)C(=O)OC(C)C)cc1OC |c:9,15| Show InChI InChI=1S/C27H28ClNO8/c1-14(2)37-27(34)21-15(3)29(13-16-10-11-19(35-4)20(12-16)36-5)24(26(32)33)23(25(30)31)22(21)17-8-6-7-9-18(17)28/h6-12,14,22H,13H2,1-5H3,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50135557
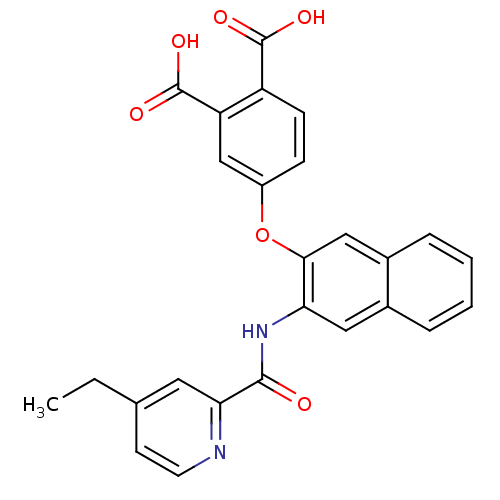
(4-{3-[(4-Ethyl-pyridine-2-carbonyl)-amino]-naphtha...)Show SMILES CCc1ccnc(c1)C(=O)Nc1cc2ccccc2cc1Oc1ccc(C(O)=O)c(c1)C(O)=O Show InChI InChI=1S/C26H20N2O6/c1-2-15-9-10-27-22(11-15)24(29)28-21-12-16-5-3-4-6-17(16)13-23(21)34-18-7-8-19(25(30)31)20(14-18)26(32)33/h3-14H,2H2,1H3,(H,28,29)(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HLGP(human liver glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328236
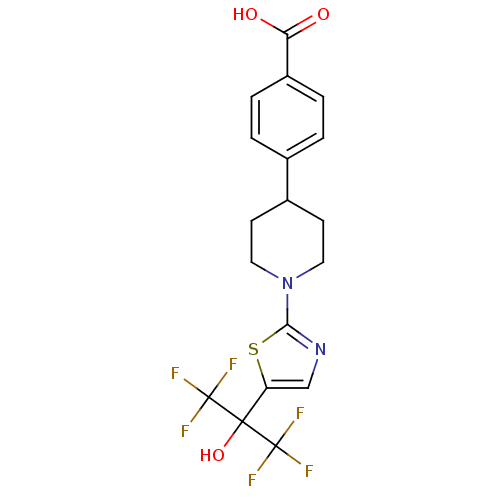
(4-(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1ccc(cc1)C1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H16F6N2O3S/c19-17(20,21)16(29,18(22,23)24)13-9-25-15(30-13)26-7-5-11(6-8-26)10-1-3-12(4-2-10)14(27)28/h1-4,9,11,29H,5-8H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328239
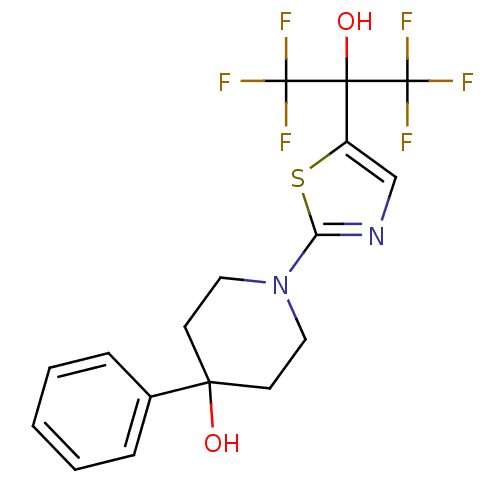
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES OC(c1cnc(s1)N1CCC(O)(CC1)c1ccccc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H16F6N2O2S/c18-16(19,20)15(27,17(21,22)23)12-10-24-13(28-12)25-8-6-14(26,7-9-25)11-4-2-1-3-5-11/h1-5,10,26-27H,6-9H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328243
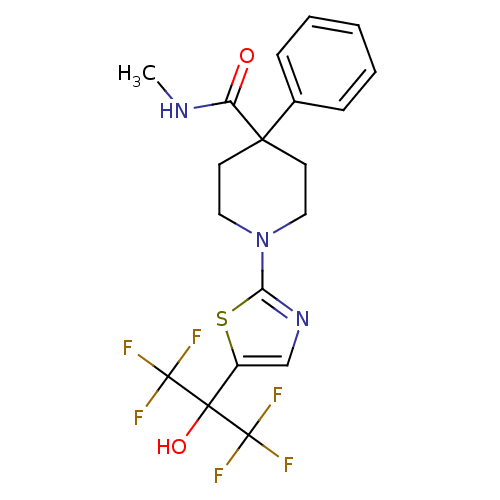
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES CNC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C19H19F6N3O2S/c1-26-14(29)16(12-5-3-2-4-6-12)7-9-28(10-8-16)15-27-11-13(31-15)17(30,18(20,21)22)19(23,24)25/h2-6,11,30H,7-10H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328242
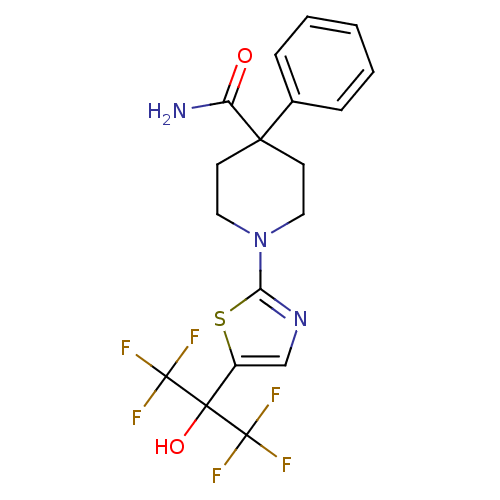
(1-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)...)Show SMILES NC(=O)C1(CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F)c1ccccc1 Show InChI InChI=1S/C18H17F6N3O2S/c19-17(20,21)16(29,18(22,23)24)12-10-26-14(30-12)27-8-6-15(7-9-27,13(25)28)11-4-2-1-3-5-11/h1-5,10,29H,6-9H2,(H2,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50133442

(4-(2-Chloro-phenyl)-6-methyl-1-(3-nitro-benzyl)-1,...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2cccc(c2)[N+]([O-])=O)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,24| Show InChI InChI=1S/C25H23ClN2O8/c1-13(2)36-25(33)19-14(3)27(12-15-7-6-8-16(11-15)28(34)35)22(24(31)32)21(23(29)30)20(19)17-9-4-5-10-18(17)26/h4-11,13,20H,12H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328223
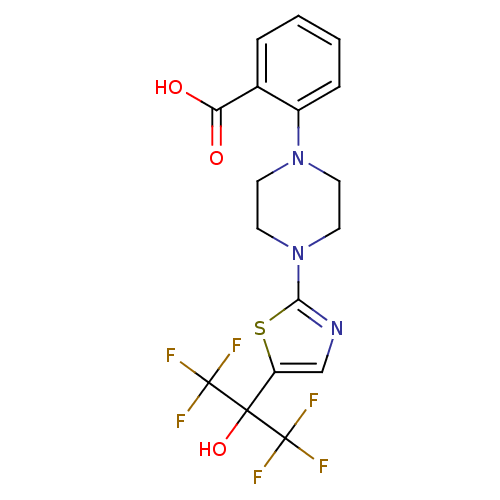
(2-(4-(5-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-...)Show SMILES OC(=O)c1ccccc1N1CCN(CC1)c1ncc(s1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C17H15F6N3O3S/c18-16(19,20)15(29,17(21,22)23)12-9-24-14(30-12)26-7-5-25(6-8-26)11-4-2-1-3-10(11)13(27)28/h1-4,9,29H,5-8H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328254
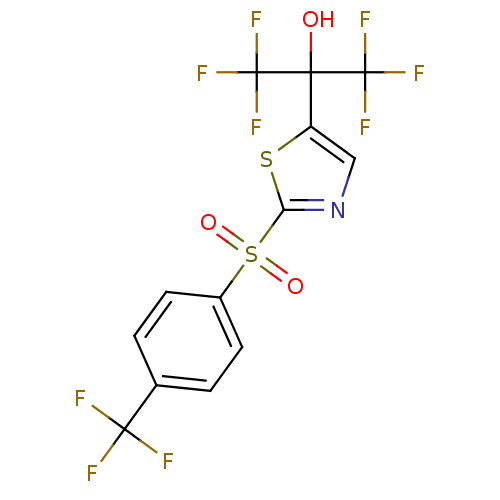
(1,1,1,3,3,3-hexafluoro-2-(2-(4-(trifluoromethyl)ph...)Show SMILES OC(c1cnc(s1)S(=O)(=O)c1ccc(cc1)C(F)(F)F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C13H6F9NO3S2/c14-11(15,16)6-1-3-7(4-2-6)28(25,26)9-23-5-8(27-9)10(24,12(17,18)19)13(20,21)22/h1-5,24H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133440

(1-(4-Chloro-benzyl)-4-(2-chloro-phenyl)-6-methyl-1...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2ccc(Cl)cc2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,22| Show InChI InChI=1S/C25H23Cl2NO6/c1-13(2)34-25(33)19-14(3)28(12-15-8-10-16(26)11-9-15)22(24(31)32)21(23(29)30)20(19)17-6-4-5-7-18(17)27/h4-11,13,20H,12H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328250
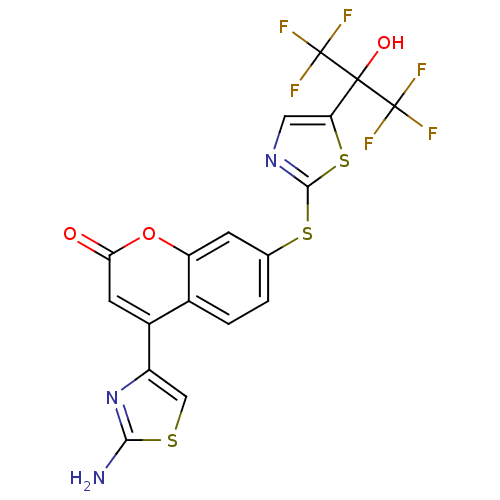
(4-(2-aminothiazol-4-yl)-7-(5-(1,1,1,3,3,3-hexafluo...)Show SMILES Nc1nc(cs1)-c1cc(=O)oc2cc(Sc3ncc(s3)C(O)(C(F)(F)F)C(F)(F)F)ccc12 Show InChI InChI=1S/C18H9F6N3O3S3/c19-17(20,21)16(29,18(22,23)24)12-5-26-15(33-12)32-7-1-2-8-9(10-6-31-14(25)27-10)4-13(28)30-11(8)3-7/h1-6,29H,(H2,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50133443

(4-(2-Chloro-phenyl)-1-ethyl-5-isobutylcarbamoyl-6-...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)NCC(C)C |c:4,10| Show InChI InChI=1S/C21H25ClN2O5/c1-5-24-12(4)15(19(25)23-10-11(2)3)16(13-8-6-7-9-14(13)22)17(20(26)27)18(24)21(28)29/h6-9,11,16H,5,10H2,1-4H3,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.88 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50133431
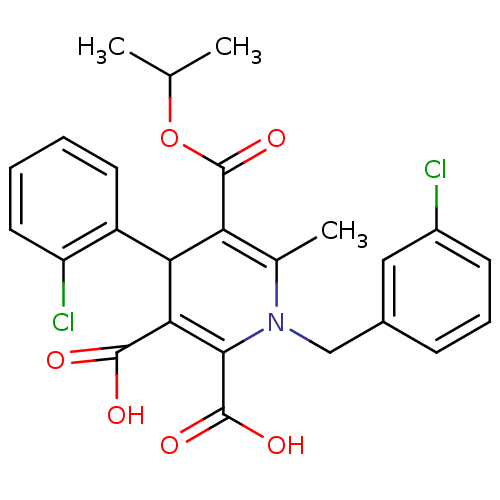
(1-(3-Chloro-benzyl)-4-(2-chloro-phenyl)-6-methyl-1...)Show SMILES CC(C)OC(=O)C1=C(C)N(Cc2cccc(Cl)c2)C(C(O)=O)=C(C1c1ccccc1Cl)C(O)=O |c:6,22| Show InChI InChI=1S/C25H23Cl2NO6/c1-13(2)34-25(33)19-14(3)28(12-15-7-6-8-16(26)11-15)22(24(31)32)21(23(29)30)20(19)17-9-4-5-10-18(17)27/h4-11,13,20H,12H2,1-3H3,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328212
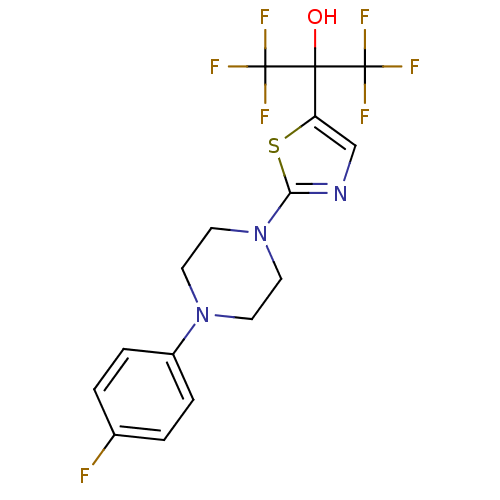
(1,1,1,3,3,3-hexafluoro-2-(2-(4-(4-fluorophenyl)pip...)Show SMILES OC(c1cnc(s1)N1CCN(CC1)c1ccc(F)cc1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H14F7N3OS/c17-10-1-3-11(4-2-10)25-5-7-26(8-6-25)13-24-9-12(28-13)14(27,15(18,19)20)16(21,22)23/h1-4,9,27H,5-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50133443

(4-(2-Chloro-phenyl)-1-ethyl-5-isobutylcarbamoyl-6-...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)NCC(C)C |c:4,10| Show InChI InChI=1S/C21H25ClN2O5/c1-5-24-12(4)15(19(25)23-10-11(2)3)16(13-8-6-7-9-14(13)22)17(20(26)27)18(24)21(28)29/h6-9,11,16H,5,10H2,1-4H3,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.28 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human muscle glycogen phosphorylase a (HMGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328211
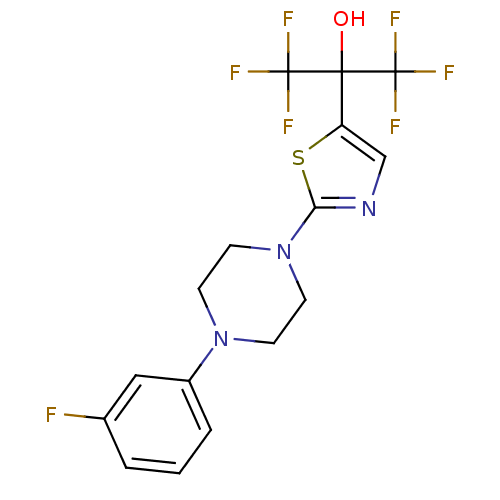
(1,1,1,3,3,3-hexafluoro-2-(2-(4-(3-fluorophenyl)pip...)Show SMILES OC(c1cnc(s1)N1CCN(CC1)c1cccc(F)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H14F7N3OS/c17-10-2-1-3-11(8-10)25-4-6-26(7-5-25)13-24-9-12(28-13)14(27,15(18,19)20)16(21,22)23/h1-3,8-9,27H,4-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Malonyl-CoA decarboxylase, mitochondrial
(Homo sapiens (Human)) | BDBM50328217
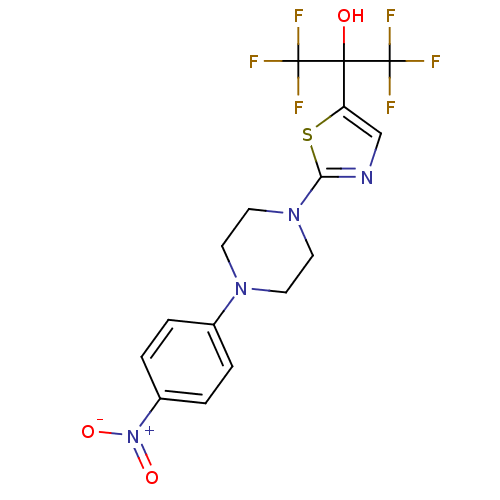
(1,1,1,3,3,3-hexafluoro-2-(2-(4-(4-nitrophenyl)pipe...)Show SMILES OC(c1cnc(s1)N1CCN(CC1)c1ccc(cc1)[N+]([O-])=O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C16H14F6N4O3S/c17-15(18,19)14(27,16(20,21)22)12-9-23-13(30-12)25-7-5-24(6-8-25)10-1-3-11(4-2-10)26(28)29/h1-4,9,27H,5-8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human MCD |
Bioorg Med Chem Lett 20: 6088-92 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.047
BindingDB Entry DOI: 10.7270/Q2542NTX |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, muscle form
(Homo sapiens (Human)) | BDBM50135558

(4-{3-Fluoro-2-[(4-nitro-pyridine-2-carbonyl)-amino...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(ccn2)[N+]([O-])=O)cc1C(O)=O Show InChI InChI=1S/C20H12FN3O8/c21-14-2-1-3-16(32-11-4-5-12(19(26)27)13(9-11)20(28)29)17(14)23-18(25)15-8-10(24(30)31)6-7-22-15/h1-9H,(H,23,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMGP(human muscle glycogen phosphorylase) |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Mus musculus) | BDBM50135550
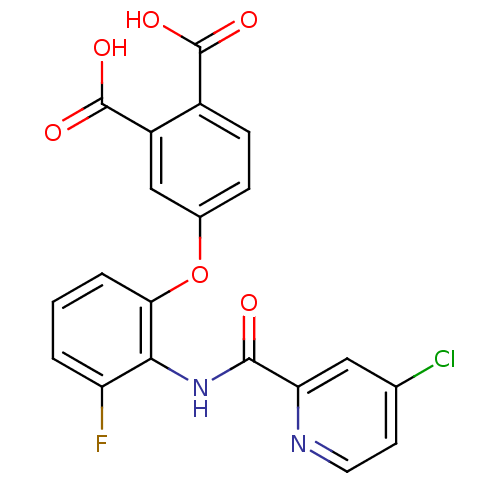
(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Rattus norvegicus) | BDBM50135550

(4-{2-[(4-Chloro-pyridine-2-carbonyl)-amino]-3-fluo...)Show SMILES OC(=O)c1ccc(Oc2cccc(F)c2NC(=O)c2cc(Cl)ccn2)cc1C(O)=O Show InChI InChI=1S/C20H12ClFN2O6/c21-10-6-7-23-15(8-10)18(25)24-17-14(22)2-1-3-16(17)30-11-4-5-12(19(26)27)13(9-11)20(28)29/h1-9H,(H,24,25)(H,26,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | <9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat liver glycogen phosphorylase |
Bioorg Med Chem Lett 13: 4125-8 (2003)
BindingDB Entry DOI: 10.7270/Q26M367F |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50133444
(4-(2-Chloro-phenyl)-1-ethyl-5-isopropylcarbamoyl-6...)Show SMILES CCN1C(C)=C(C(C(C(O)=O)=C1C(O)=O)c1ccccc1Cl)C(=O)NC(C)C |c:4,10| Show InChI InChI=1S/C20H23ClN2O5/c1-5-23-11(4)14(18(24)22-10(2)3)15(12-8-6-7-9-13(12)21)16(19(25)26)17(23)20(27)28/h6-10,15H,5H2,1-4H3,(H,22,24)(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against human liver glycogen phosphorylase a (HLGPa) |
Bioorg Med Chem Lett 13: 3405-8 (2003)
BindingDB Entry DOI: 10.7270/Q2TQ60XP |
More data for this
Ligand-Target Pair | |
Coagulation factor IX
(Homo sapiens (Human)) | BDBM50125977

(CHEMBL3627897)Show SMILES Cc1ncn(n1)-c1cc(Cl)c(C(=O)NC[C@@H](c2cccc(F)c2)c2ccc3[nH]c(C)nc3c2)c(Cl)c1 |r| Show InChI InChI=1S/C26H21Cl2FN6O/c1-14-31-13-35(34-14)19-10-21(27)25(22(28)11-19)26(36)30-12-20(16-4-3-5-18(29)8-16)17-6-7-23-24(9-17)33-15(2)32-23/h3-11,13,20H,12H2,1-2H3,(H,30,36)(H,32,33)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human factor 9a using CH3SO2-DCHG-Gly-Arg-AFC.AcOH as substrate preinubated for 30 mins followed by substrate addition measured after 1... |
J Med Chem 59: 1818-29 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01293
BindingDB Entry DOI: 10.7270/Q29Z96S8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data