

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897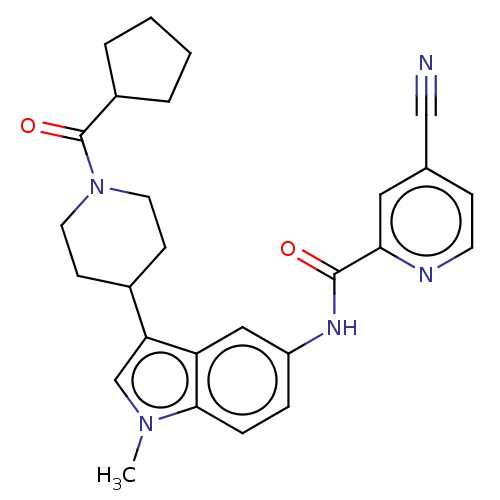 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891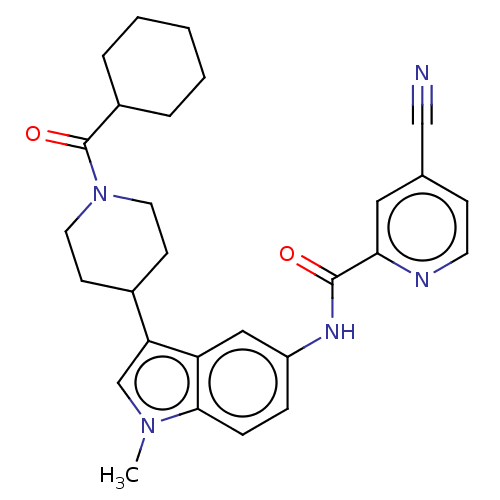 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893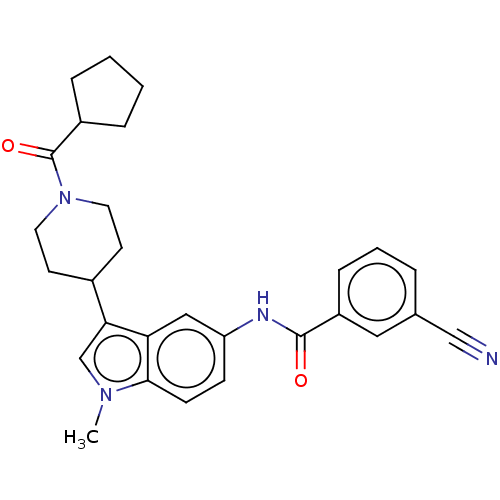 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466892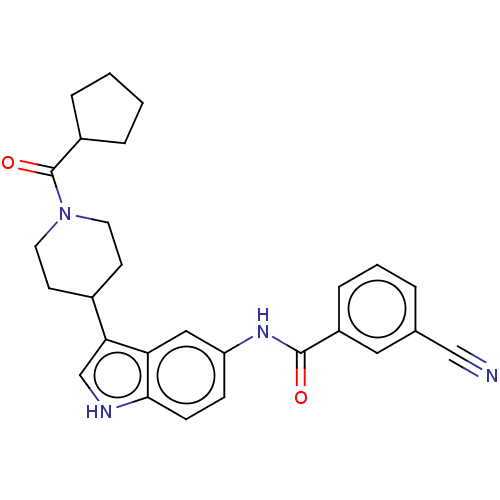 (CHEMBL4283871) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466913 (CHEMBL4289304) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250852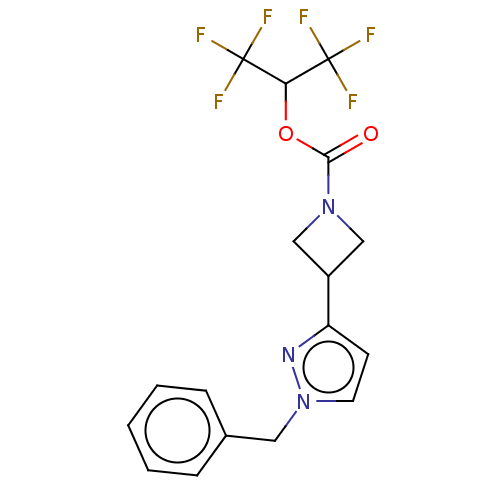 (CHEMBL4078217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250848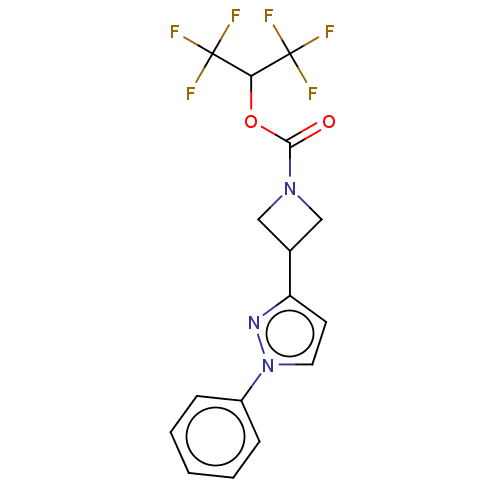 (CHEMBL4059676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250859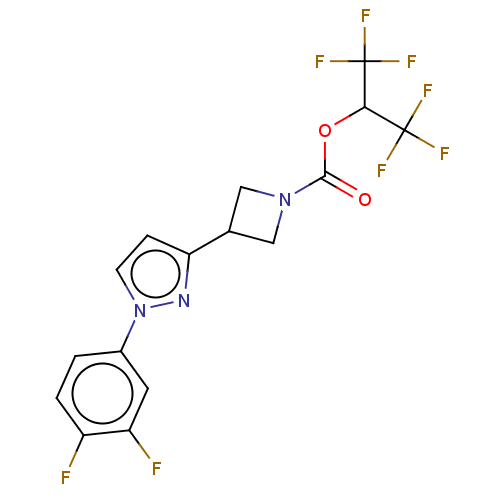 (CHEMBL4097203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250855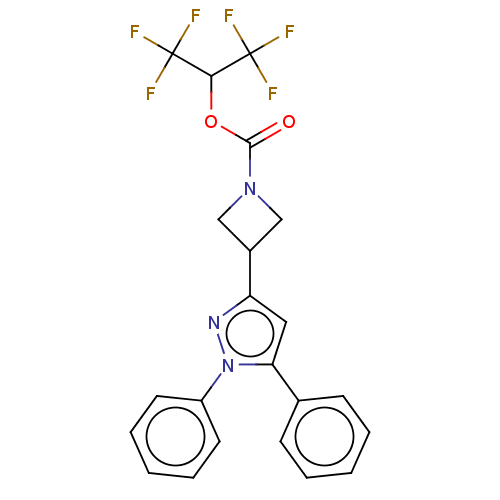 (CHEMBL4077745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250850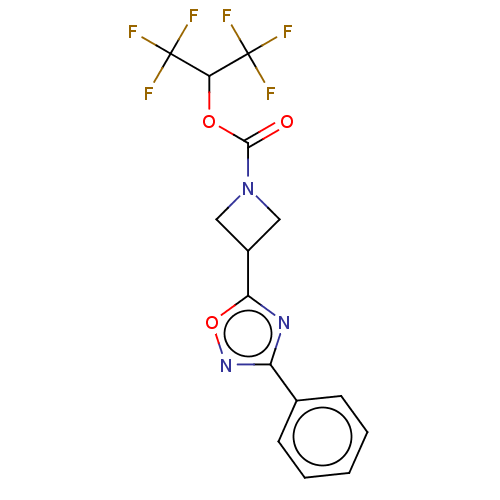 (CHEMBL4089505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250852 (CHEMBL4078217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250855 (CHEMBL4077745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893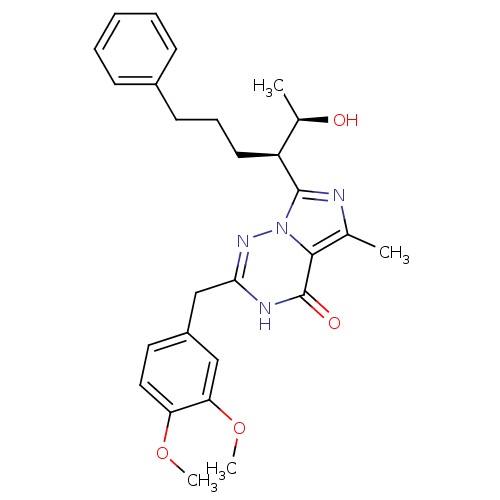 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250859 (CHEMBL4097203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250854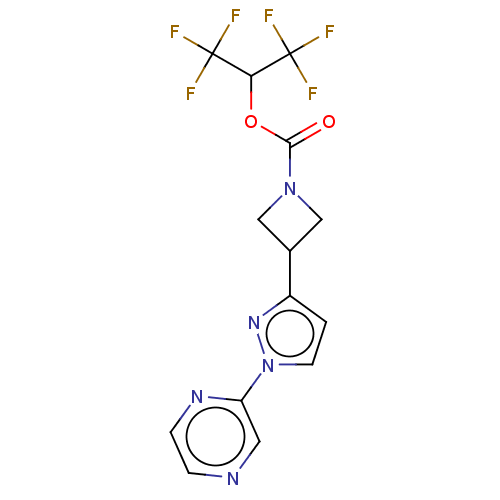 (CHEMBL4079190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250854 (CHEMBL4079190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM9049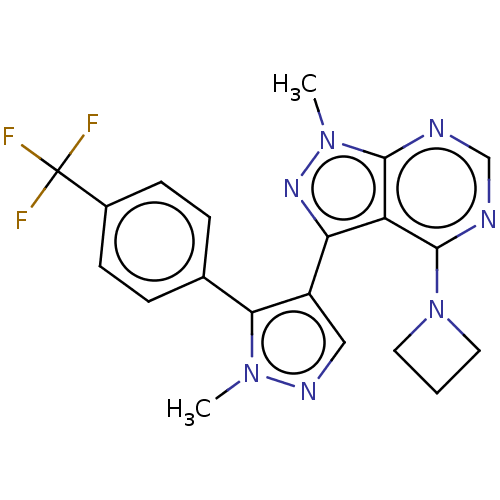 (US11419874, Example 15 | US8829010, 35 | US8829010...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250853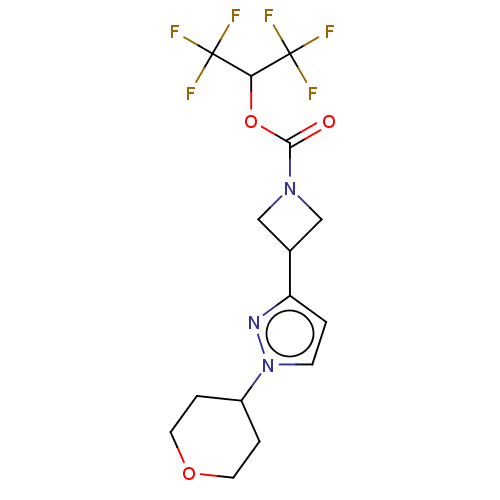 (CHEMBL4102496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250849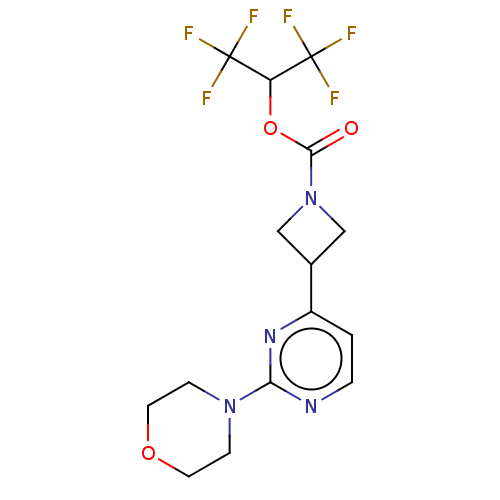 (CHEMBL4068332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250851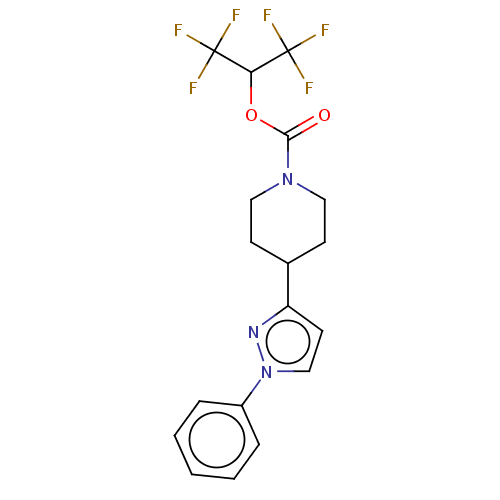 (CHEMBL4096459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM130958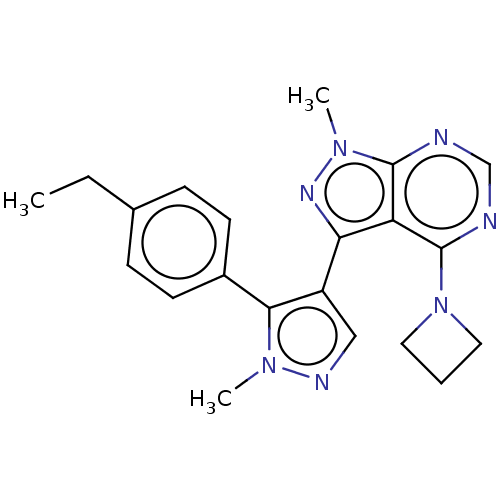 (US8829010, 9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250858 (CHEMBL4078417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250857 (CHEMBL4081625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890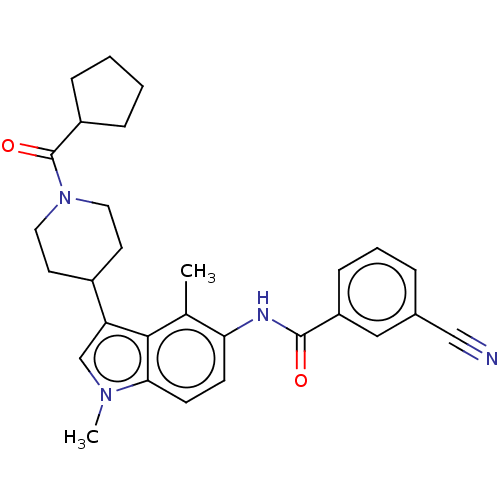 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM130982 (US8829010, 33) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM130966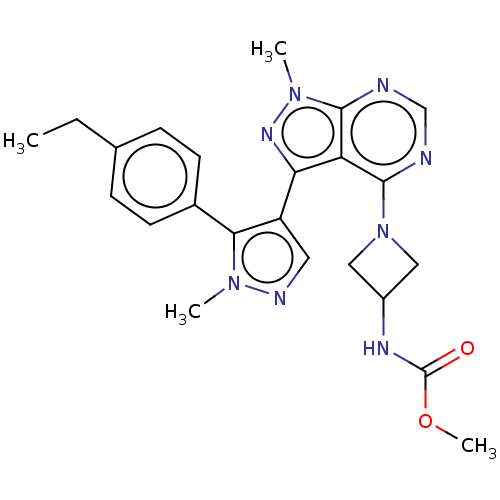 (US8829010, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description The inhibition activity of 5-HT1A at 1 uM | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM189877 (US10227346, Example 5 | US10426135, Example 5 | US...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50166893 (2-(3,4-Dimethoxy-benzyl)-7-[(R)-1-((R)-1-hydroxy-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDE2A (unknown origin) | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466912 (CHEMBL4283051) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM130956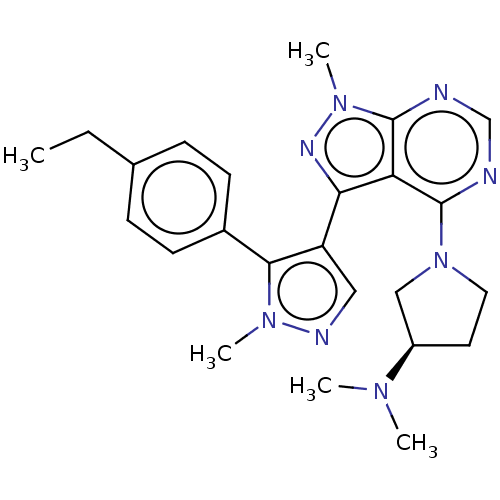 (US8829010, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466890 (CHEMBL4290728) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195581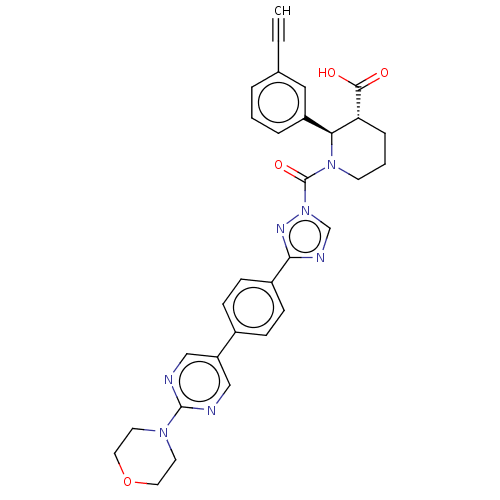 ((2R,3R)-2-(3-ethynylphenyl)-1-(3-(4-(2-morpholinop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195570 ((S)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195571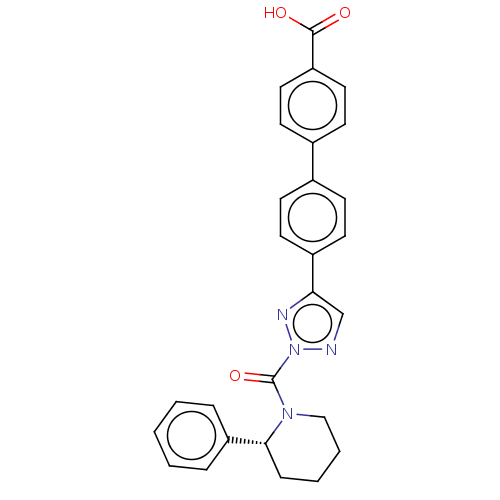 ((R)-4'-(2-(2-phenylpiperidine-1-carbonyl)-2H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195572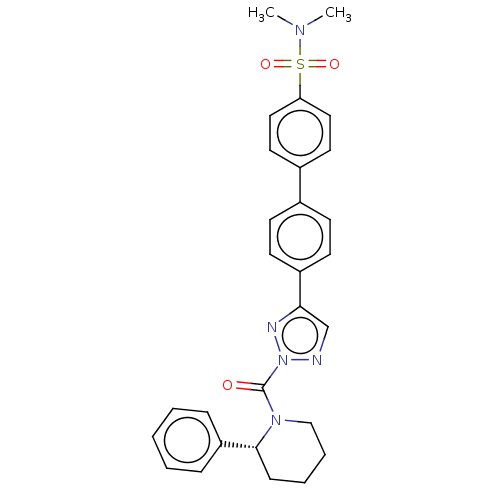 ((R)-N,N-dimethyl-4'-(2-(2-phenylpiperidine-1-c...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195574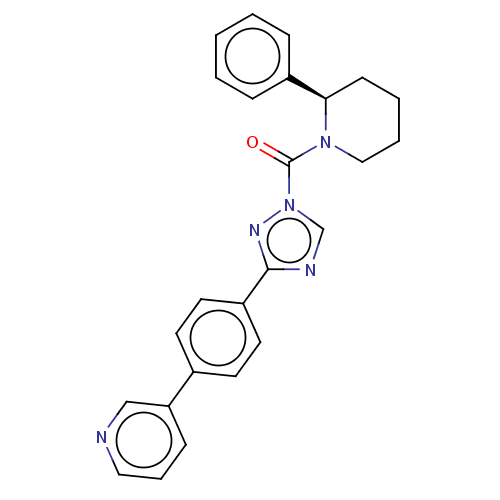 ((R)-(2-phenylpiperidin-1-yl)(3-(4-(pyridin-3-yl)ph...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195575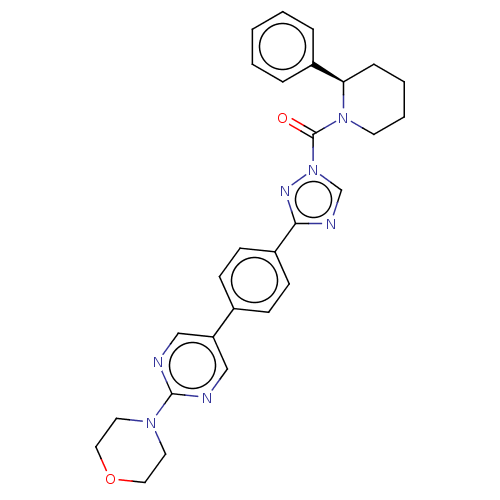 ((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Rattus norvegicus (rat)) | BDBM50350800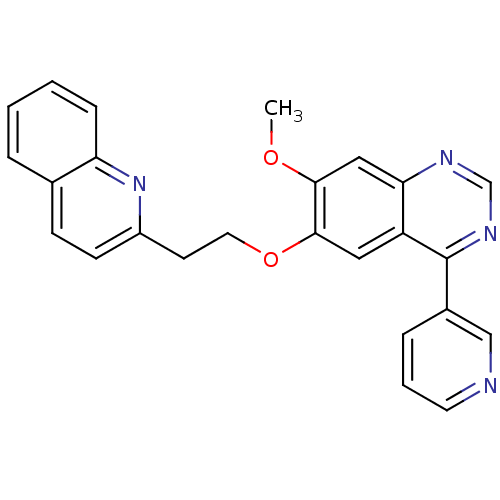 (CHEMBL1819135) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant rat PDE10A expressed in Sf9 cells using [3H]cAMP after 30 mins by scintillation proximity assay | J Med Chem 54: 4536-47 (2011) Article DOI: 10.1021/jm2001508 BindingDB Entry DOI: 10.7270/Q2PN961Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466897 (CHEMBL4287715) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466891 (CHEMBL4281109) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466893 (CHEMBL4291727) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM130989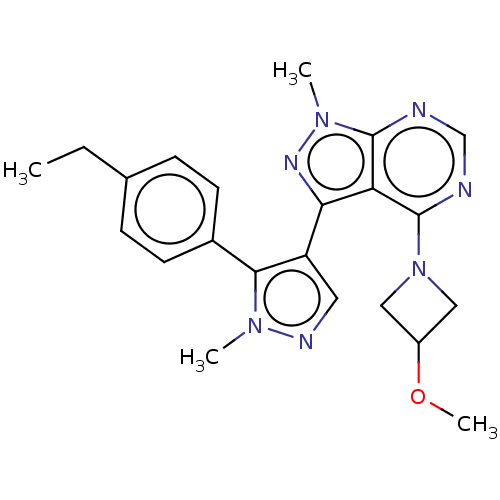 (US8829010, 40) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195580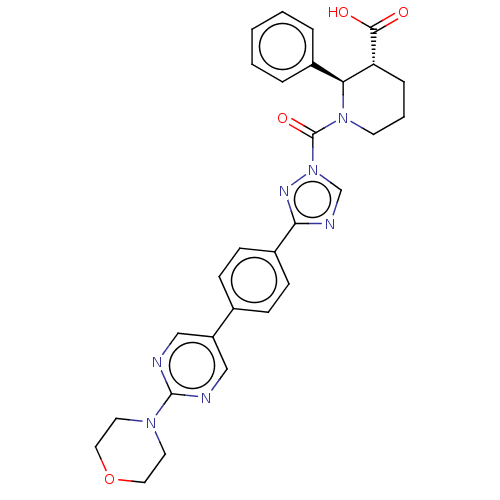 ((2R,3R)-1-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophospholipase-like protein 1 (Homo sapiens (Human)) | BDBM195575 ((R)-(3-(4-(2-morpholinopyrimidin-5-yl)phenyl)-1H-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for LYPLAL1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Liver carboxylesterase 1 (Homo sapiens (Human)) | BDBM195573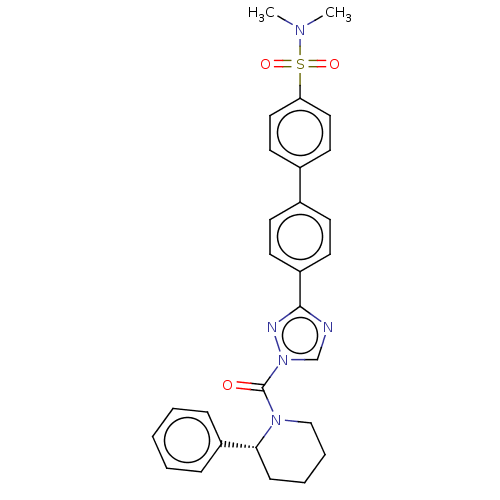 ((R)-4'-(1-(2-phenylpiperidine-1-carbonyl)-1H-1...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Pfizer Inc. | Assay Description For determination of IC50 values for CES1 inhibitors, the reactions were carried out in 1.5 mL microcentrifuge tubes in a total reaction volume of 25... | ACS Chem Biol 11: 2529-40 (2016) Article DOI: 10.1021/acschembio.6b00266 BindingDB Entry DOI: 10.7270/Q24X56K5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50239842 (CHEMBL4085594) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466914 (CHEMBL4283312) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50239841 (CHEMBL4101083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of full length recombinant human FLAG-tagged PDE2A3 expressed in sf21 cells using [3H]cGMP as substrate by scintillation proximity assay | J Med Chem 60: 5673-5698 (2017) Article DOI: 10.1021/acs.jmedchem.7b00397 BindingDB Entry DOI: 10.7270/Q2VX0JNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (Homo sapiens (Human)) | BDBM50466915 (CHEMBL4282848) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-inhibited 3',5'-cyclic phosphodiesterase 3A (Homo sapiens (Human)) | BDBM50350802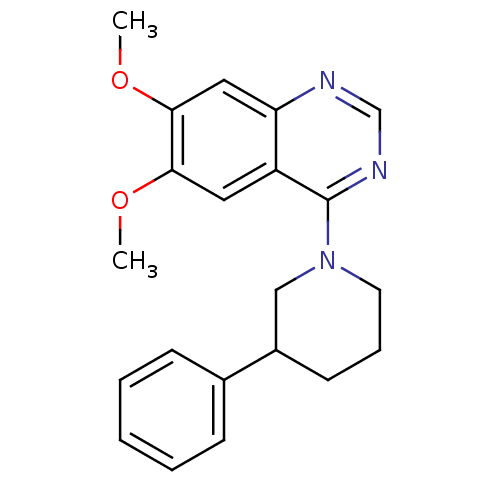 (CHEMBL1819121) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of PDE3A | J Med Chem 54: 4536-47 (2011) Article DOI: 10.1021/jm2001508 BindingDB Entry DOI: 10.7270/Q2PN961Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 186 total ) | Next | Last >> |