 Found 127 hits with Last Name = 'gibbs' and Initial = 'em'
Found 127 hits with Last Name = 'gibbs' and Initial = 'em' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM39807

(CP-526,423)Show SMILES Cc1ccc2[nH]c(cc2c1)C(=O)NCCOCCOCCNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C25H27ClN4O4/c1-16-2-4-20-17(12-16)14-22(29-20)24(31)27-6-8-33-10-11-34-9-7-28-25(32)23-15-18-13-19(26)3-5-21(18)30-23/h2-5,12-15,29-30H,6-11H2,1H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pfizer Inc
| Assay Description
Human liver glycogen phsphorylase (HLGP) activity was measured in the direction of glycogen synthesis by the release of phosphate from glucose-1-phos... |
Chem Biol 7: 677-82 (2000)
Article DOI: 10.1016/S1074-5521(00)00004-1
BindingDB Entry DOI: 10.7270/Q2H41PT2 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50355142
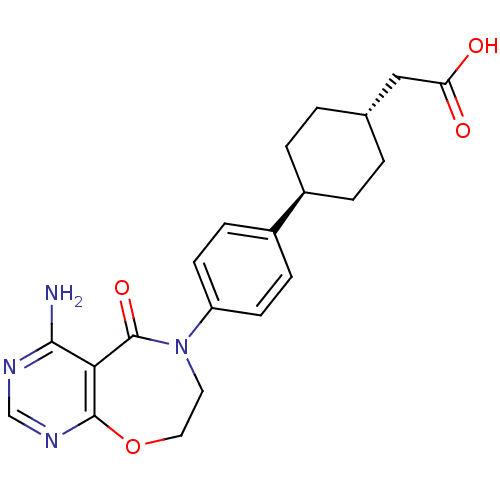
(CHEMBL1835919)Show SMILES Nc1ncnc2OCCN(c3ccc(cc3)[C@H]3CC[C@H](CC(O)=O)CC3)C(=O)c12 |r,wU:16.16,wD:19.20,(-8.41,-1.48,;-8.4,-3.02,;-9.73,-3.79,;-9.73,-5.34,;-8.4,-6.11,;-7.06,-5.34,;-5.73,-6.38,;-4.15,-6.01,;-3.48,-4.52,;-4.27,-3.04,;-3.51,-1.7,;-1.98,-1.68,;-1.22,-.34,;-2.01,.98,;-3.56,.96,;-4.3,-.39,;-1.26,2.32,;-2.05,3.64,;-1.29,4.98,;.25,5,;1.01,6.34,;2.55,6.36,;3.33,5.03,;3.3,7.7,;1.03,3.67,;.28,2.34,;-5.83,-2.78,;-6.22,-1.29,;-7.07,-3.79,)| Show InChI InChI=1S/C21H24N4O4/c22-19-18-20(24-12-23-19)29-10-9-25(21(18)28)16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-17(26)27/h5-8,12-14H,1-4,9-11H2,(H,26,27)(H2,22,23,24)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1-mediated triglyceride synthesis in human HT-29 cells using [3H]glycerol as substrate after 6 hrs by beta counting |
ACS Med Chem Lett 2: 407-412 (2011)
Article DOI: 10.1021/ml200051p
BindingDB Entry DOI: 10.7270/Q2MW2HM6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158278
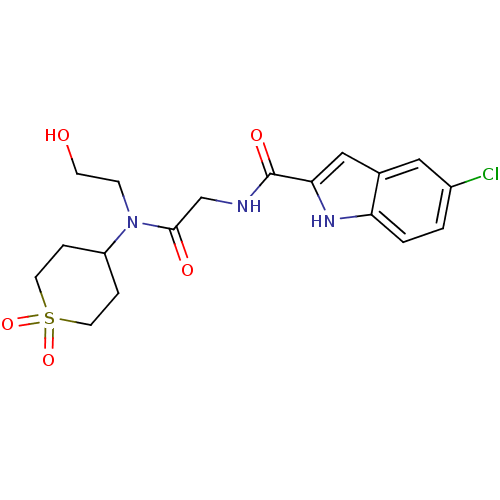
(5-Chloro-1H-indole-2-carboxylic acid {[(1,1-dioxo-...)Show SMILES OCCN(C1CCS(=O)(=O)CC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C18H22ClN3O5S/c19-13-1-2-15-12(9-13)10-16(21-15)18(25)20-11-17(24)22(5-6-23)14-3-7-28(26,27)8-4-14/h1-2,9-10,14,21,23H,3-8,11H2,(H,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158249
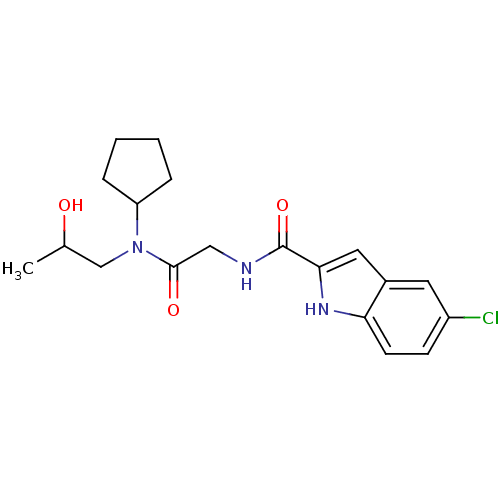
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES CC(O)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O3/c1-12(24)11-23(15-4-2-3-5-15)18(25)10-21-19(26)17-9-13-8-14(20)6-7-16(13)22-17/h6-9,12,15,22,24H,2-5,10-11H2,1H3,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM27947
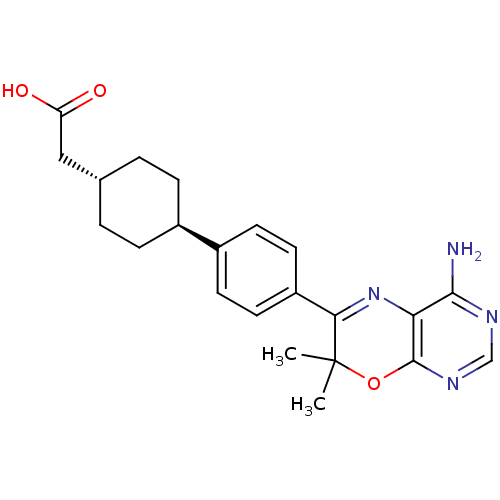
(2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(1.74,.99,;.97,2.32,;2.46,2.72,;-.37,1.55,;-1.7,2.32,;-3.03,1.55,;-4.37,2.32,;-4.37,3.86,;-3.03,4.63,;-3.03,6.17,;-1.7,3.86,;-.37,4.63,;.97,3.86,;2.3,4.63,;2.3,6.17,;3.63,6.94,;4.97,6.17,;4.97,4.63,;3.63,3.86,;6.19,6.75,;6.19,8.3,;7.53,9.07,;8.86,8.3,;10.19,9.07,;11.53,8.3,;12.86,9.07,;11.53,6.76,;8.86,6.75,;7.53,5.98,)| Show InChI InChI=1S/C22H26N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H,27,28)(H2,23,24,25)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1-mediated triglyceride synthesis in human HT-29 cells using [3H]glycerol as substrate after 6 hrs by beta counting |
ACS Med Chem Lett 2: 407-412 (2011)
Article DOI: 10.1021/ml200051p
BindingDB Entry DOI: 10.7270/Q2MW2HM6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50355142

(CHEMBL1835919)Show SMILES Nc1ncnc2OCCN(c3ccc(cc3)[C@H]3CC[C@H](CC(O)=O)CC3)C(=O)c12 |r,wU:16.16,wD:19.20,(-8.41,-1.48,;-8.4,-3.02,;-9.73,-3.79,;-9.73,-5.34,;-8.4,-6.11,;-7.06,-5.34,;-5.73,-6.38,;-4.15,-6.01,;-3.48,-4.52,;-4.27,-3.04,;-3.51,-1.7,;-1.98,-1.68,;-1.22,-.34,;-2.01,.98,;-3.56,.96,;-4.3,-.39,;-1.26,2.32,;-2.05,3.64,;-1.29,4.98,;.25,5,;1.01,6.34,;2.55,6.36,;3.33,5.03,;3.3,7.7,;1.03,3.67,;.28,2.34,;-5.83,-2.78,;-6.22,-1.29,;-7.07,-3.79,)| Show InChI InChI=1S/C21H24N4O4/c22-19-18-20(24-12-23-19)29-10-9-25(21(18)28)16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-17(26)27/h5-8,12-14H,1-4,9-11H2,(H,26,27)(H2,22,23,24)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human microsomal DGAT-1 expressed in baculovirus infected insect Sf9 cells using [14C]decanoylCoA as substrate after 1.5 hr... |
ACS Med Chem Lett 2: 407-412 (2011)
Article DOI: 10.1021/ml200051p
BindingDB Entry DOI: 10.7270/Q2MW2HM6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158254
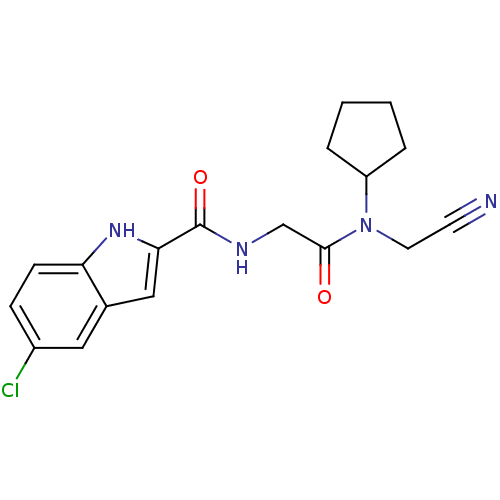
(5-Chloro-1H-indole-2-carboxylic acid [(cyanomethyl...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NCC(=O)N(CC#N)C1CCCC1 Show InChI InChI=1S/C18H19ClN4O2/c19-13-5-6-15-12(9-13)10-16(22-15)18(25)21-11-17(24)23(8-7-20)14-3-1-2-4-14/h5-6,9-10,14,22H,1-4,8,11H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158255
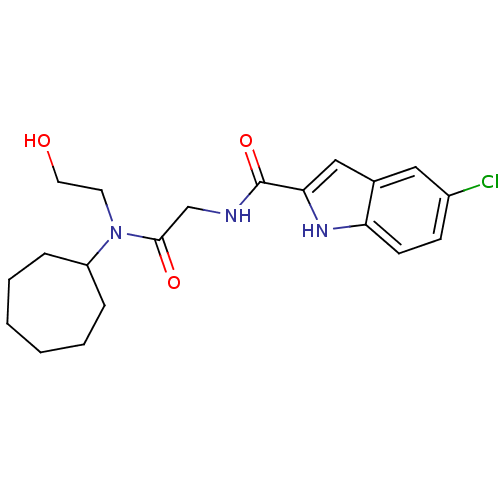
(5-Chloro-1H-indole-2-carboxylic acid {[cycloheptyl...)Show SMILES OCCN(C1CCCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O3/c21-15-7-8-17-14(11-15)12-18(23-17)20(27)22-13-19(26)24(9-10-25)16-5-3-1-2-4-6-16/h7-8,11-12,16,23,25H,1-6,9-10,13H2,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158283
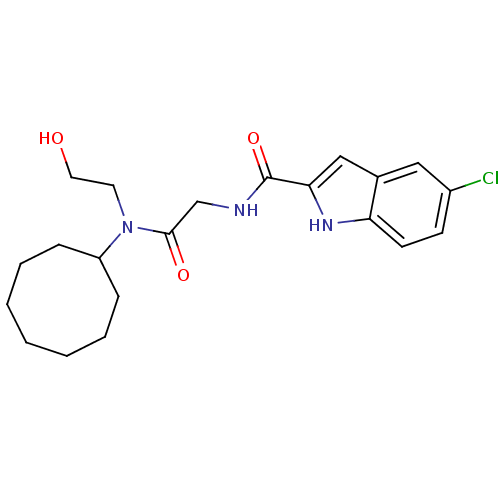
(5-Chloro-1H-indole-2-carboxylic acid {[cyclooctyl-...)Show SMILES OCCN(C1CCCCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H28ClN3O3/c22-16-8-9-18-15(12-16)13-19(24-18)21(28)23-14-20(27)25(10-11-26)17-6-4-2-1-3-5-7-17/h8-9,12-13,17,24,26H,1-7,10-11,14H2,(H,23,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158308
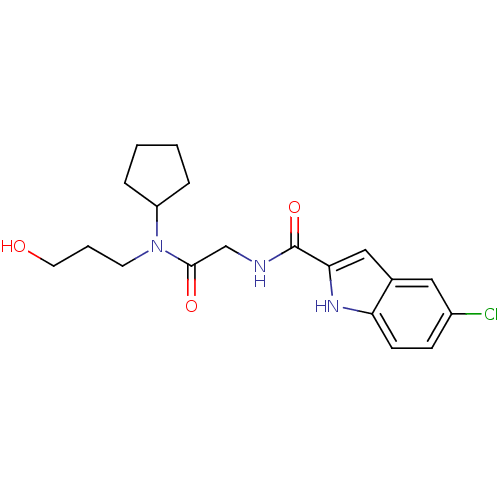
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES OCCCN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O3/c20-14-6-7-16-13(10-14)11-17(22-16)19(26)21-12-18(25)23(8-3-9-24)15-4-1-2-5-15/h6-7,10-11,15,22,24H,1-5,8-9,12H2,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50136446

(1-{(S)-2-[(5-Chloro-1H-indole-2-carbonyl)-amino]-3...)Show SMILES OC(=O)C1CN(C1)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H20ClN3O4/c23-16-6-7-17-14(9-16)10-18(24-17)20(27)25-19(8-13-4-2-1-3-5-13)21(28)26-11-15(12-26)22(29)30/h1-7,9-10,15,19,24H,8,11-12H2,(H,25,27)(H,29,30)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | 7.2 | 22 |
Pfizer Inc
| Assay Description
Human liver glycogen phsphorylase (HLGP) activity was measured in the direction of glycogen synthesis by the release of phosphate from glucose-1-phos... |
Chem Biol 7: 677-82 (2000)
Article DOI: 10.1016/S1074-5521(00)00004-1
BindingDB Entry DOI: 10.7270/Q2H41PT2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158303
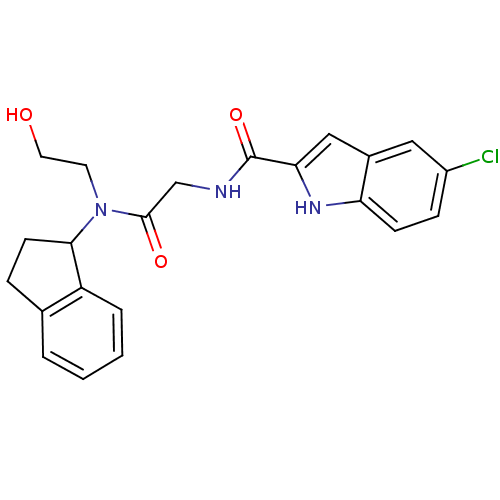
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show SMILES OCCN(C1CCc2ccccc12)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H22ClN3O3/c23-16-6-7-18-15(11-16)12-19(25-18)22(29)24-13-21(28)26(9-10-27)20-8-5-14-3-1-2-4-17(14)20/h1-4,6-7,11-12,20,25,27H,5,8-10,13H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158295
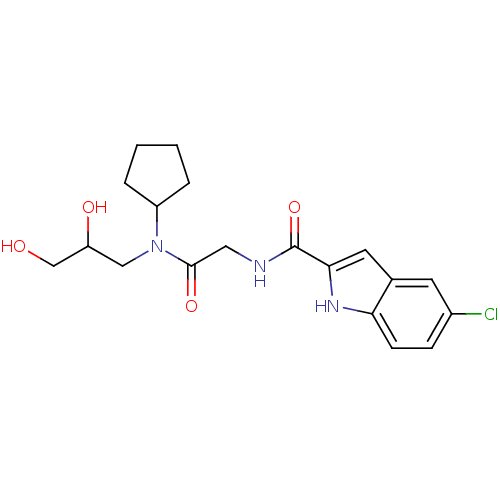
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES OCC(O)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O4/c20-13-5-6-16-12(7-13)8-17(22-16)19(27)21-9-18(26)23(10-15(25)11-24)14-3-1-2-4-14/h5-8,14-15,22,24-25H,1-4,9-11H2,(H,21,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158245
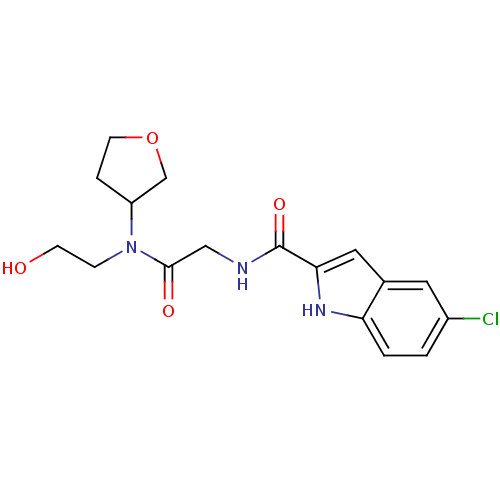
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show SMILES OCCN(C1CCOC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C17H20ClN3O4/c18-12-1-2-14-11(7-12)8-15(20-14)17(24)19-9-16(23)21(4-5-22)13-3-6-25-10-13/h1-2,7-8,13,20,22H,3-6,9-10H2,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158304
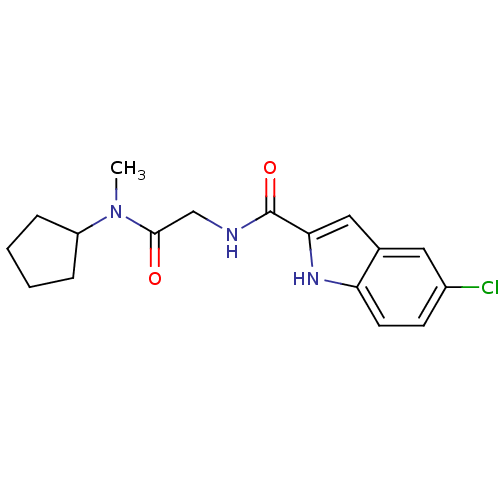
(5-Chloro-1H-indole-2-carboxylic acid [(cyclopentyl...)Show SMILES CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C17H20ClN3O2/c1-21(13-4-2-3-5-13)16(22)10-19-17(23)15-9-11-8-12(18)6-7-14(11)20-15/h6-9,13,20H,2-5,10H2,1H3,(H,19,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50363956
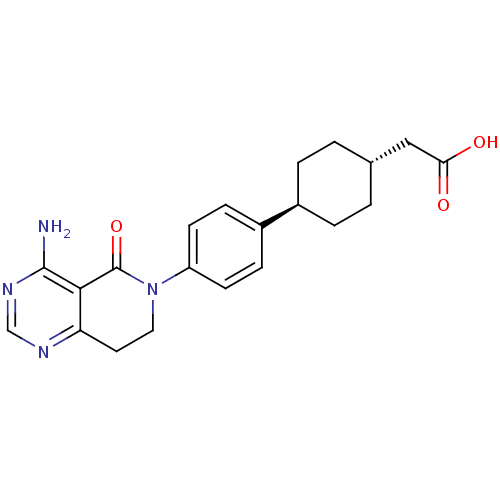
(CHEMBL1952025)Show SMILES Nc1ncnc2CCN(C(=O)c12)c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:18.20,wD:21.24,(26.2,1.37,;26.2,-.17,;24.87,-.94,;24.87,-2.48,;26.21,-3.25,;27.53,-2.49,;28.87,-3.26,;30.21,-2.49,;30.22,-.94,;28.87,-.15,;28.87,1.38,;27.54,-.93,;31.55,-.18,;32.88,-.95,;34.21,-.19,;34.22,1.35,;32.88,2.13,;31.55,1.36,;35.56,2.12,;35.56,3.65,;36.9,4.42,;38.23,3.64,;39.57,4.4,;40.89,3.63,;40.89,2.09,;42.23,4.39,;38.22,2.1,;36.89,1.34,)| Show InChI InChI=1S/C21H24N4O3/c22-20-19-17(23-12-24-20)9-10-25(21(19)28)16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-18(26)27/h5-8,12-14H,1-4,9-11H2,(H,26,27)(H2,22,23,24)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human microsomal DGAT-1 expressed in baculovirus infected insect Sf9 cells using [14C]decanoylCoA as substrate after 1.5 hr... |
ACS Med Chem Lett 2: 407-412 (2011)
Article DOI: 10.1021/ml200051p
BindingDB Entry DOI: 10.7270/Q2MW2HM6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Rattus norvegicus (rat)) | BDBM50355142

(CHEMBL1835919)Show SMILES Nc1ncnc2OCCN(c3ccc(cc3)[C@H]3CC[C@H](CC(O)=O)CC3)C(=O)c12 |r,wU:16.16,wD:19.20,(-8.41,-1.48,;-8.4,-3.02,;-9.73,-3.79,;-9.73,-5.34,;-8.4,-6.11,;-7.06,-5.34,;-5.73,-6.38,;-4.15,-6.01,;-3.48,-4.52,;-4.27,-3.04,;-3.51,-1.7,;-1.98,-1.68,;-1.22,-.34,;-2.01,.98,;-3.56,.96,;-4.3,-.39,;-1.26,2.32,;-2.05,3.64,;-1.29,4.98,;.25,5,;1.01,6.34,;2.55,6.36,;3.33,5.03,;3.3,7.7,;1.03,3.67,;.28,2.34,;-5.83,-2.78,;-6.22,-1.29,;-7.07,-3.79,)| Show InChI InChI=1S/C21H24N4O4/c22-19-18-20(24-12-23-19)29-10-9-25(21(18)28)16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-17(26)27/h5-8,12-14H,1-4,9-11H2,(H,26,27)(H2,22,23,24)/t13-,14- | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of rat DGAT-1 |
ACS Med Chem Lett 2: 407-412 (2011)
Article DOI: 10.1021/ml200051p
BindingDB Entry DOI: 10.7270/Q2MW2HM6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50363956

(CHEMBL1952025)Show SMILES Nc1ncnc2CCN(C(=O)c12)c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:18.20,wD:21.24,(26.2,1.37,;26.2,-.17,;24.87,-.94,;24.87,-2.48,;26.21,-3.25,;27.53,-2.49,;28.87,-3.26,;30.21,-2.49,;30.22,-.94,;28.87,-.15,;28.87,1.38,;27.54,-.93,;31.55,-.18,;32.88,-.95,;34.21,-.19,;34.22,1.35,;32.88,2.13,;31.55,1.36,;35.56,2.12,;35.56,3.65,;36.9,4.42,;38.23,3.64,;39.57,4.4,;40.89,3.63,;40.89,2.09,;42.23,4.39,;38.22,2.1,;36.89,1.34,)| Show InChI InChI=1S/C21H24N4O3/c22-20-19-17(23-12-24-20)9-10-25(21(19)28)16-7-5-15(6-8-16)14-3-1-13(2-4-14)11-18(26)27/h5-8,12-14H,1-4,9-11H2,(H,26,27)(H2,22,23,24)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1-mediated triglyceride synthesis in human HT-29 cells using [3H]glycerol as substrate after 6 hrs by beta counting |
ACS Med Chem Lett 2: 407-412 (2011)
Article DOI: 10.1021/ml200051p
BindingDB Entry DOI: 10.7270/Q2MW2HM6 |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM27947

(2-[4-(4-{4-amino-7,7-dimethyl-7H-pyrimido[4,5-b][1...)Show SMILES CC1(C)Oc2ncnc(N)c2N=C1c1ccc(cc1)[C@H]1CC[C@H](CC(O)=O)CC1 |r,wU:19.21,wD:22.25,c:12,(1.74,.99,;.97,2.32,;2.46,2.72,;-.37,1.55,;-1.7,2.32,;-3.03,1.55,;-4.37,2.32,;-4.37,3.86,;-3.03,4.63,;-3.03,6.17,;-1.7,3.86,;-.37,4.63,;.97,3.86,;2.3,4.63,;2.3,6.17,;3.63,6.94,;4.97,6.17,;4.97,4.63,;3.63,3.86,;6.19,6.75,;6.19,8.3,;7.53,9.07,;8.86,8.3,;10.19,9.07,;11.53,8.3,;12.86,9.07,;11.53,6.76,;8.86,6.75,;7.53,5.98,)| Show InChI InChI=1S/C22H26N4O3/c1-22(2)19(26-18-20(23)24-12-25-21(18)29-22)16-9-7-15(8-10-16)14-5-3-13(4-6-14)11-17(27)28/h7-10,12-14H,3-6,11H2,1-2H3,(H,27,28)(H2,23,24,25)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of full length human microsomal DGAT-1 expressed in baculovirus infected insect Sf9 cells using [14C]decanoylCoA as substrate after 1.5 hr... |
ACS Med Chem Lett 2: 407-412 (2011)
Article DOI: 10.1021/ml200051p
BindingDB Entry DOI: 10.7270/Q2MW2HM6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065954

((S)-5-chloro-N-(1-(dimethylamino)-1-oxo-3-phenylpr...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H20ClN3O2/c1-24(2)20(26)18(10-13-6-4-3-5-7-13)23-19(25)17-12-14-11-15(21)8-9-16(14)22-17/h3-9,11-12,18,22H,10H2,1-2H3,(H,23,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158310
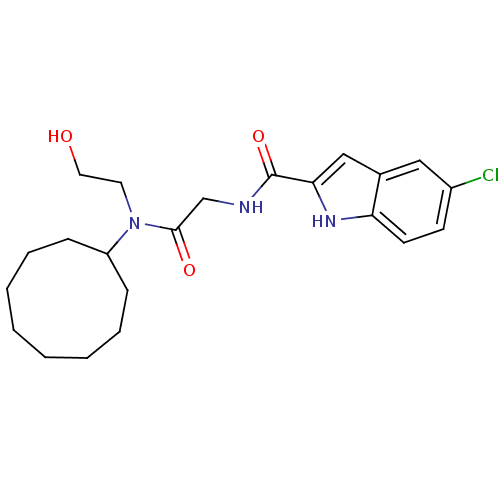
(5-Chloro-1H-indole-2-carboxylic acid {[cyclononyl-...)Show SMILES OCCN(C1CCCCCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C22H30ClN3O3/c23-17-9-10-19-16(13-17)14-20(25-19)22(29)24-15-21(28)26(11-12-27)18-7-5-3-1-2-4-6-8-18/h9-10,13-14,18,25,27H,1-8,11-12,15H2,(H,24,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158315
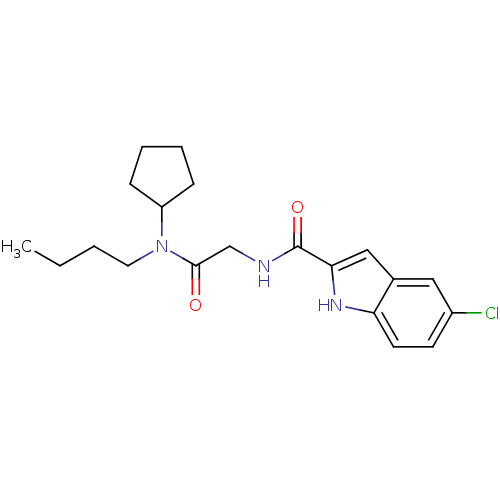
(5-Chloro-1H-indole-2-carboxylic acid [(butyl-cyclo...)Show SMILES CCCCN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O2/c1-2-3-10-24(16-6-4-5-7-16)19(25)13-22-20(26)18-12-14-11-15(21)8-9-17(14)23-18/h8-9,11-12,16,23H,2-7,10,13H2,1H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158261

(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES CC(O)(CO)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O4/c1-20(28,12-25)11-24(15-4-2-3-5-15)18(26)10-22-19(27)17-9-13-8-14(21)6-7-16(13)23-17/h6-9,15,23,25,28H,2-5,10-12H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158264
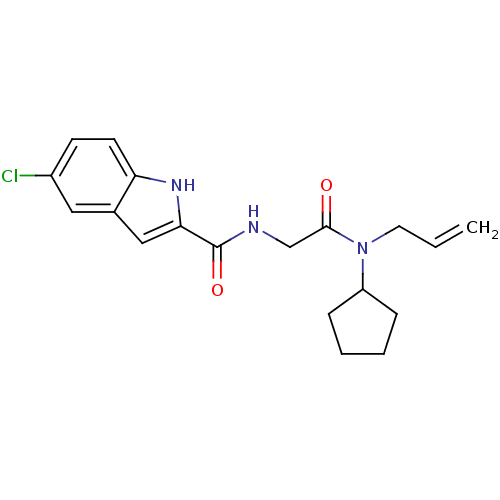
(5-Chloro-1H-indole-2-carboxylic acid [(allyl-cyclo...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NCC(=O)N(CC=C)C1CCCC1 Show InChI InChI=1S/C19H22ClN3O2/c1-2-9-23(15-5-3-4-6-15)18(24)12-21-19(25)17-11-13-10-14(20)7-8-16(13)22-17/h2,7-8,10-11,15,22H,1,3-6,9,12H2,(H,21,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065955

(5-Bromo-1H-indole-2-carboxylic acid ((1S,2R)-1-ben...)Show SMILES CN(C)C(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Br)ccc2[nH]1 Show InChI InChI=1S/C21H22BrN3O3/c1-25(2)21(28)19(26)17(10-13-6-4-3-5-7-13)24-20(27)18-12-14-11-15(22)8-9-16(14)23-18/h3-9,11-12,17,19,23,26H,10H2,1-2H3,(H,24,27)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158274

(5-Chloro-1H-indole-2-carboxylic acid {[cyclohexyl-...)Show SMILES OCCN(C1CCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H24ClN3O3/c20-14-6-7-16-13(10-14)11-17(22-16)19(26)21-12-18(25)23(8-9-24)15-4-2-1-3-5-15/h6-7,10-11,15,22,24H,1-5,8-9,12H2,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158276
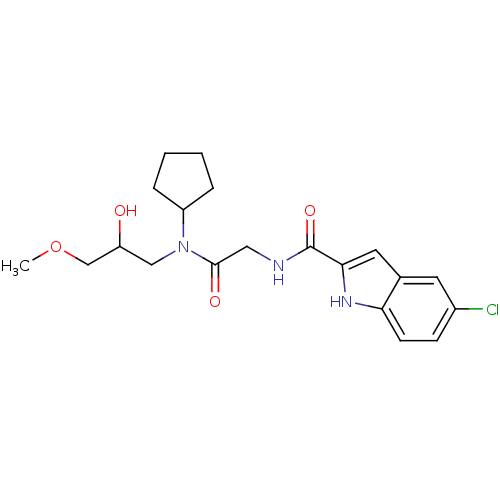
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES COCC(O)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O4/c1-28-12-16(25)11-24(15-4-2-3-5-15)19(26)10-22-20(27)18-9-13-8-14(21)6-7-17(13)23-18/h6-9,15-16,23,25H,2-5,10-12H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065965
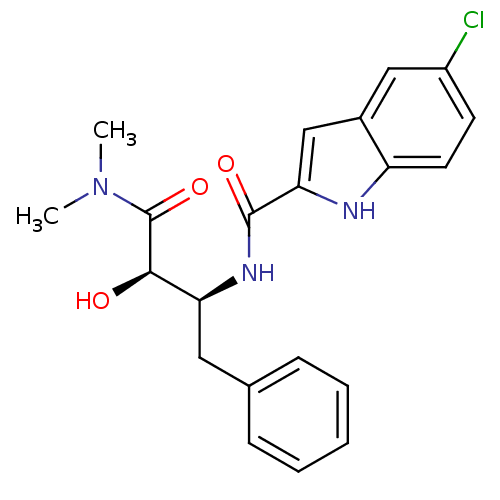
(5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...)Show SMILES CN(C)C(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H22ClN3O3/c1-25(2)21(28)19(26)17(10-13-6-4-3-5-7-13)24-20(27)18-12-14-11-15(22)8-9-16(14)23-18/h3-9,11-12,17,19,23,26H,10H2,1-2H3,(H,24,27)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065965

(5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...)Show SMILES CN(C)C(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C21H22ClN3O3/c1-25(2)21(28)19(26)17(10-13-6-4-3-5-7-13)24-20(27)18-12-14-11-15(22)8-9-16(14)23-18/h3-9,11-12,17,19,23,26H,10H2,1-2H3,(H,24,27)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065944
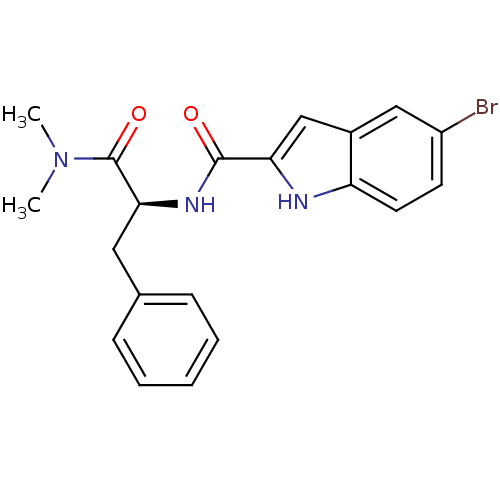
(5-Bromo-1H-indole-2-carboxylic acid ((S)-1-dimethy...)Show SMILES CN(C)C(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Br)ccc2[nH]1 Show InChI InChI=1S/C20H20BrN3O2/c1-24(2)20(26)18(10-13-6-4-3-5-7-13)23-19(25)17-12-14-11-15(21)8-9-16(14)22-17/h3-9,11-12,18,22H,10H2,1-2H3,(H,23,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065951
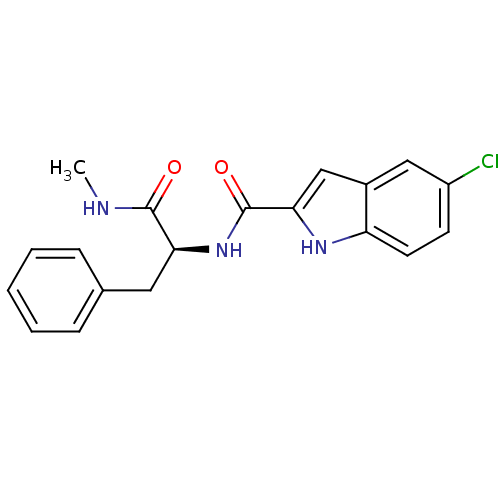
(5-Chloro-1H-indole-2-carboxylic acid ((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H18ClN3O2/c1-21-18(24)16(9-12-5-3-2-4-6-12)23-19(25)17-11-13-10-14(20)7-8-15(13)22-17/h2-8,10-11,16,22H,9H2,1H3,(H,21,24)(H,23,25)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158288
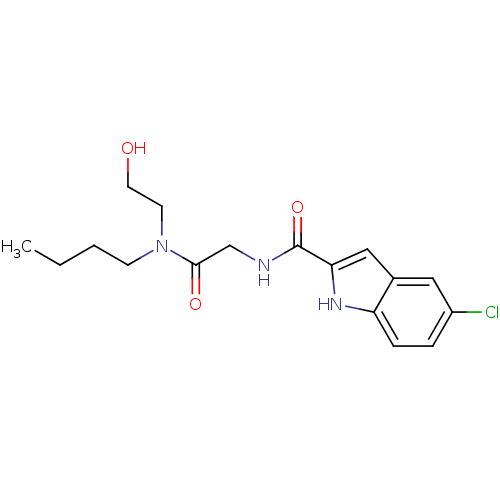
(5-Chloro-1H-indole-2-carboxylic acid {[butyl-(2-hy...)Show InChI InChI=1S/C17H22ClN3O3/c1-2-3-6-21(7-8-22)16(23)11-19-17(24)15-10-12-9-13(18)4-5-14(12)20-15/h4-5,9-10,20,22H,2-3,6-8,11H2,1H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158272
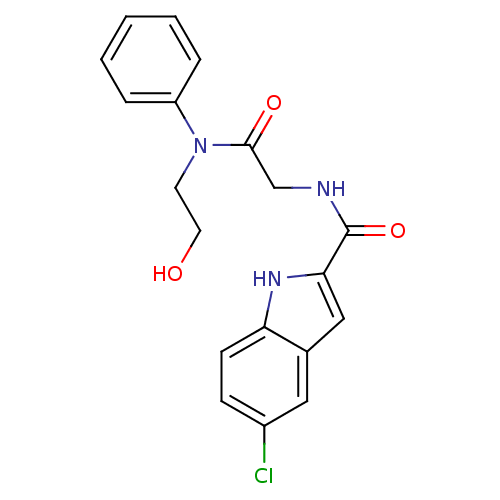
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show SMILES OCCN(C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1)c1ccccc1 Show InChI InChI=1S/C19H18ClN3O3/c20-14-6-7-16-13(10-14)11-17(22-16)19(26)21-12-18(25)23(8-9-24)15-4-2-1-3-5-15/h1-7,10-11,22,24H,8-9,12H2,(H,21,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158248
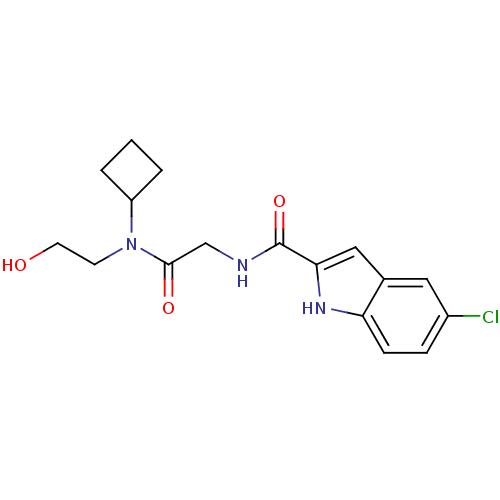
(5-Chloro-1H-indole-2-carboxylic acid {[cyclobutyl-...)Show SMILES OCCN(C1CCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C17H20ClN3O3/c18-12-4-5-14-11(8-12)9-15(20-14)17(24)19-10-16(23)21(6-7-22)13-2-1-3-13/h4-5,8-9,13,20,22H,1-3,6-7,10H2,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065943

((S)-2-[(5-Chloro-1H-indole-2-carbonyl)-amino]-3-ph...)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C19H17ClN2O3/c1-25-19(24)17(9-12-5-3-2-4-6-12)22-18(23)16-11-13-10-14(20)7-8-15(13)21-16/h2-8,10-11,17,21H,9H2,1H3,(H,22,23)/t17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158247
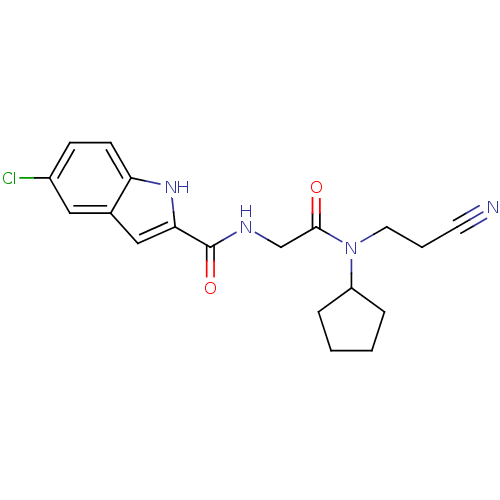
(5-Chloro-1H-indole-2-carboxylic acid {[(2-cyano-et...)Show SMILES Clc1ccc2[nH]c(cc2c1)C(=O)NCC(=O)N(CCC#N)C1CCCC1 Show InChI InChI=1S/C19H21ClN4O2/c20-14-6-7-16-13(10-14)11-17(23-16)19(26)22-12-18(25)24(9-3-8-21)15-4-1-2-5-15/h6-7,10-11,15,23H,1-5,9,12H2,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158301
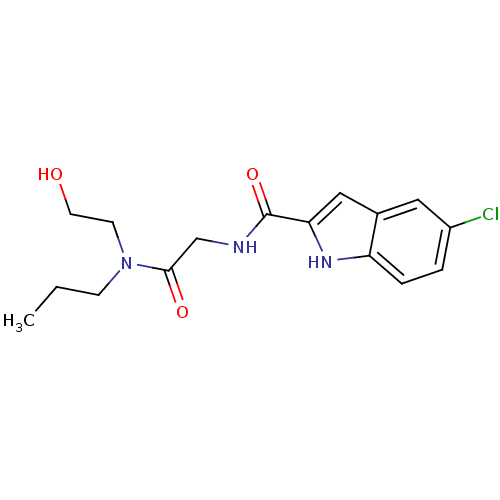
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show InChI InChI=1S/C16H20ClN3O3/c1-2-5-20(6-7-21)15(22)10-18-16(23)14-9-11-8-12(17)3-4-13(11)19-14/h3-4,8-9,19,21H,2,5-7,10H2,1H3,(H,18,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158277
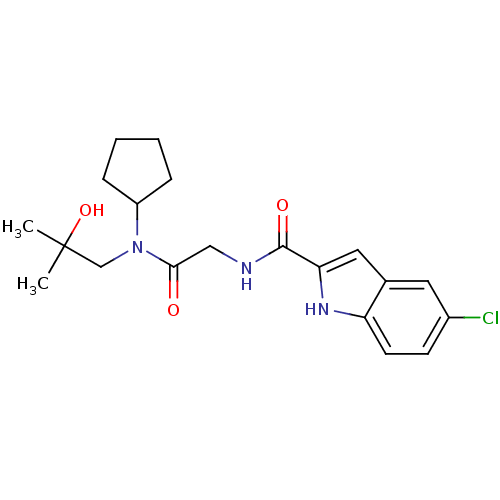
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES CC(C)(O)CN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O3/c1-20(2,27)12-24(15-5-3-4-6-15)18(25)11-22-19(26)17-10-13-9-14(21)7-8-16(13)23-17/h7-10,15,23,27H,3-6,11-12H2,1-2H3,(H,22,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158294
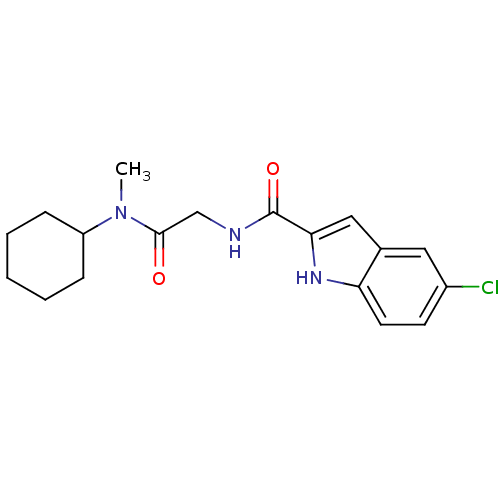
(5-Chloro-1H-indole-2-carboxylic acid [(cyclohexyl-...)Show SMILES CN(C1CCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C18H22ClN3O2/c1-22(14-5-3-2-4-6-14)17(23)11-20-18(24)16-10-12-9-13(19)7-8-15(12)21-16/h7-10,14,21H,2-6,11H2,1H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158290
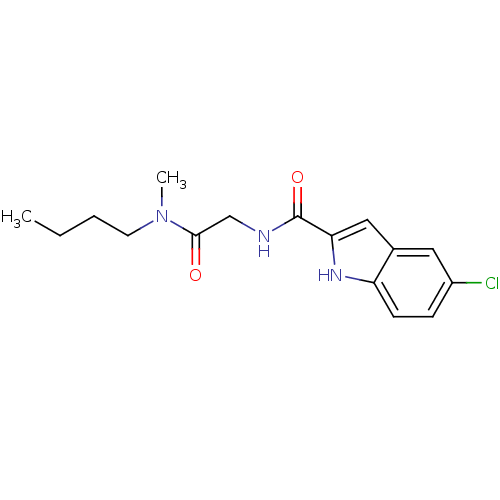
(5-Chloro-1H-indole-2-carboxylic acid [(butyl-methy...)Show InChI InChI=1S/C16H20ClN3O2/c1-3-4-7-20(2)15(21)10-18-16(22)14-9-11-8-12(17)5-6-13(11)19-14/h5-6,8-9,19H,3-4,7,10H2,1-2H3,(H,18,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158252
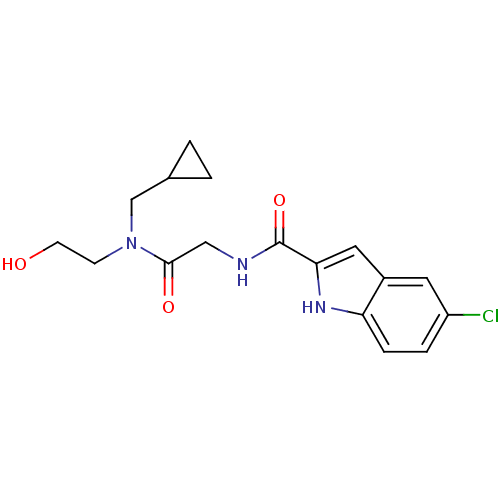
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopropyl...)Show SMILES OCCN(CC1CC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C17H20ClN3O3/c18-13-3-4-14-12(7-13)8-15(20-14)17(24)19-9-16(23)21(5-6-22)10-11-1-2-11/h3-4,7-8,11,20,22H,1-2,5-6,9-10H2,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50065962
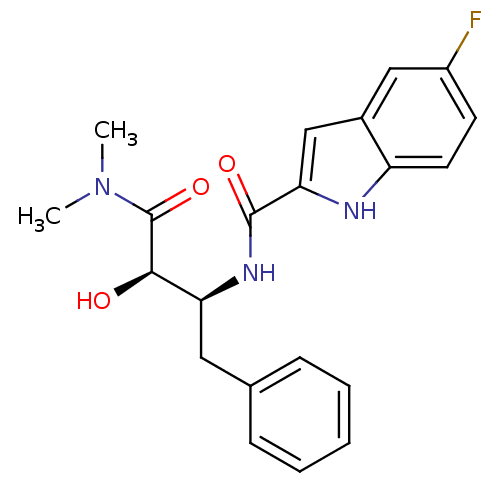
(5-Fluoro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...)Show SMILES CN(C)C(=O)[C@H](O)[C@H](Cc1ccccc1)NC(=O)c1cc2cc(F)ccc2[nH]1 Show InChI InChI=1S/C21H22FN3O3/c1-25(2)21(28)19(26)17(10-13-6-4-3-5-7-13)24-20(27)18-12-14-11-15(22)8-9-16(14)23-18/h3-9,11-12,17,19,23,26H,10H2,1-2H3,(H,24,27)/t17-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158296
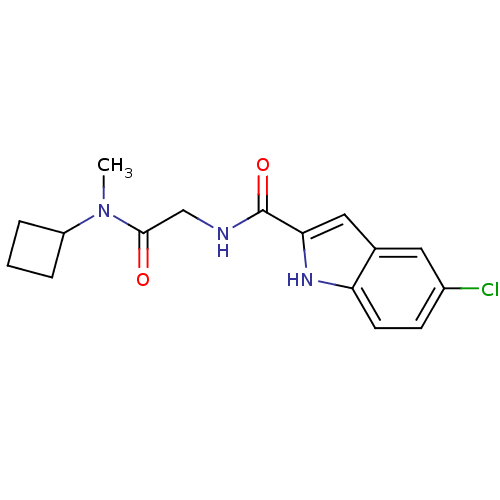
(5-Chloro-1H-indole-2-carboxylic acid [(cyclobutyl-...)Show InChI InChI=1S/C16H18ClN3O2/c1-20(12-3-2-4-12)15(21)9-18-16(22)14-8-10-7-11(17)5-6-13(10)19-14/h5-8,12,19H,2-4,9H2,1H3,(H,18,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158240
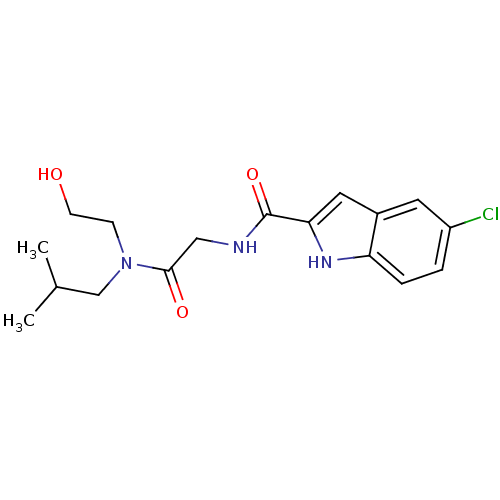
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show SMILES CC(C)CN(CCO)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C17H22ClN3O3/c1-11(2)10-21(5-6-22)16(23)9-19-17(24)15-8-12-7-13(18)3-4-14(12)20-15/h3-4,7-8,11,20,22H,5-6,9-10H2,1-2H3,(H,19,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158287
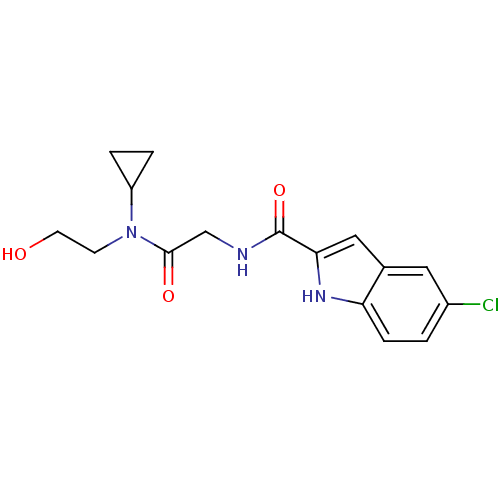
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopropyl...)Show SMILES OCCN(C1CC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C16H18ClN3O3/c17-11-1-4-13-10(7-11)8-14(19-13)16(23)18-9-15(22)20(5-6-21)12-2-3-12/h1,4,7-8,12,19,21H,2-3,5-6,9H2,(H,18,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM35346
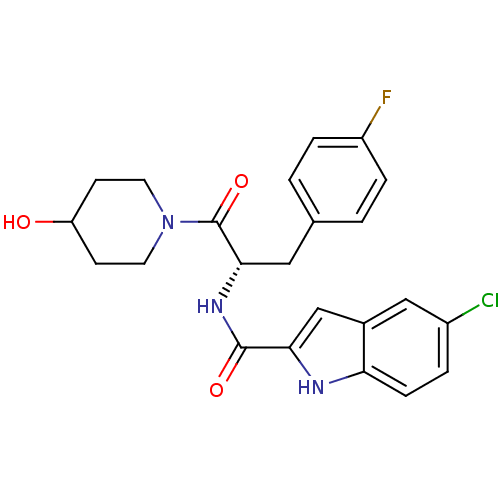
((S)-5-chloro-N-(3-(4-fluorophenyl)-1-(4-hydroxypip...)Show SMILES OC1CCN(CC1)C(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc2cc(Cl)ccc2[nH]1 |r| Show InChI InChI=1S/C23H23ClFN3O3/c24-16-3-6-19-15(12-16)13-20(26-19)22(30)27-21(11-14-1-4-17(25)5-2-14)23(31)28-9-7-18(29)8-10-28/h1-6,12-13,18,21,26,29H,7-11H2,(H,27,30)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 205 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... |
J Med Chem 41: 2934-8 (1998)
Article DOI: 10.1021/jm980264k
BindingDB Entry DOI: 10.7270/Q2NS0T1T |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158286
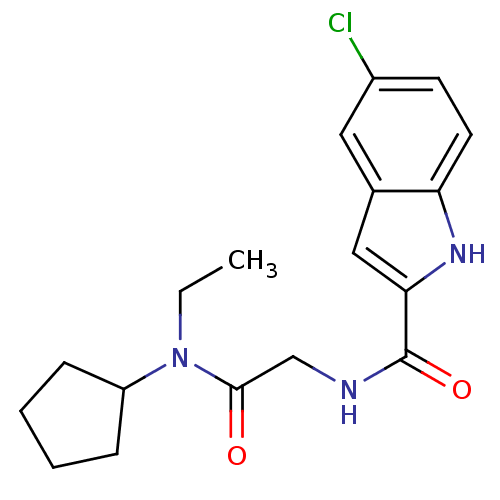
(5-Chloro-1H-indole-2-carboxylic acid [(cyclopentyl...)Show SMILES CCN(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C18H22ClN3O2/c1-2-22(14-5-3-4-6-14)17(23)11-20-18(24)16-10-12-9-13(19)7-8-15(12)21-16/h7-10,14,21H,2-6,11H2,1H3,(H,20,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158307
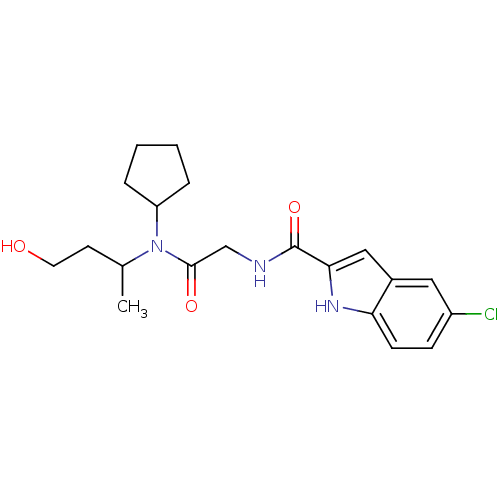
(5-Chloro-1H-indole-2-carboxylic acid {[cyclopentyl...)Show SMILES CC(CCO)N(C1CCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C20H26ClN3O3/c1-13(8-9-25)24(16-4-2-3-5-16)19(26)12-22-20(27)18-11-14-10-15(21)6-7-17(14)23-18/h6-7,10-11,13,16,23,25H,2-5,8-9,12H2,1H3,(H,22,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158271
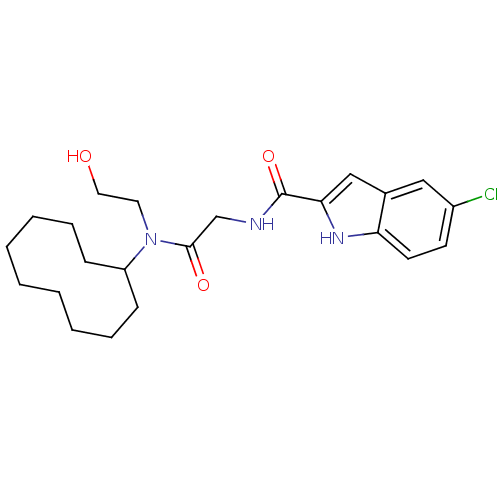
(5-Chloro-1H-indole-2-carboxylic acid {[cyclodecyl-...)Show SMILES OCCN(C1CCCCCCCCC1)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C23H32ClN3O3/c24-18-10-11-20-17(14-18)15-21(26-20)23(30)25-16-22(29)27(12-13-28)19-8-6-4-2-1-3-5-7-9-19/h10-11,14-15,19,26,28H,1-9,12-13,16H2,(H,25,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50158262
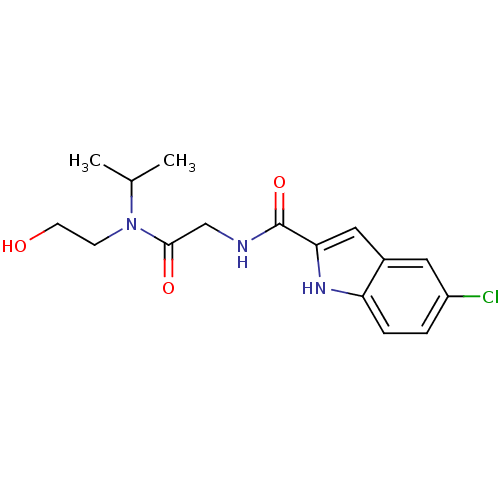
(5-Chloro-1H-indole-2-carboxylic acid {[(2-hydroxy-...)Show SMILES CC(C)N(CCO)C(=O)CNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C16H20ClN3O3/c1-10(2)20(5-6-21)15(22)9-18-16(23)14-8-11-7-12(17)3-4-13(11)19-14/h3-4,7-8,10,19,21H,5-6,9H2,1-2H3,(H,18,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory concentration against glycogen phosphorylase |
Bioorg Med Chem Lett 15: 459-65 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.048
BindingDB Entry DOI: 10.7270/Q26W99K6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data