

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50235402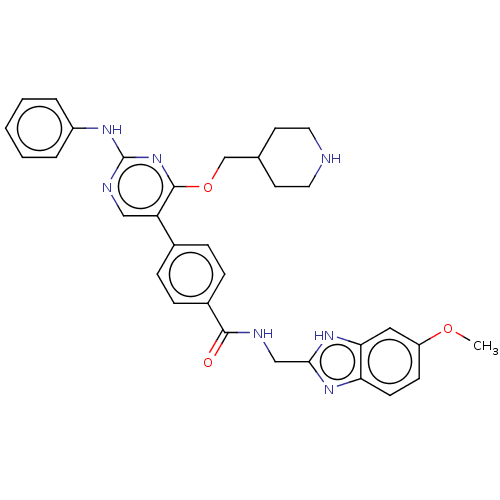 (CHEMBL4077934) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay | J Med Chem 60: 2155-2161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00033 BindingDB Entry DOI: 10.7270/Q2MC928T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185443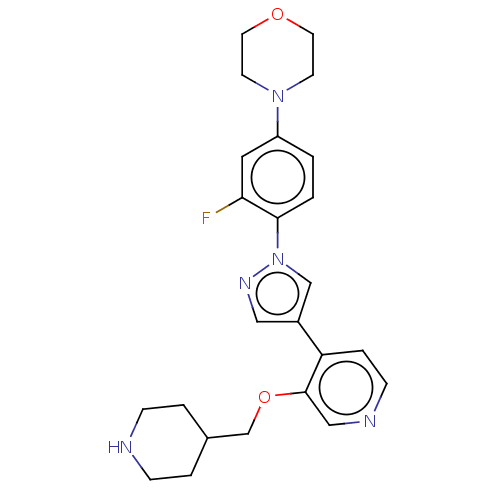 (CHEMBL3824328) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1/2 (Homo sapiens (Human)) | BDBM50531540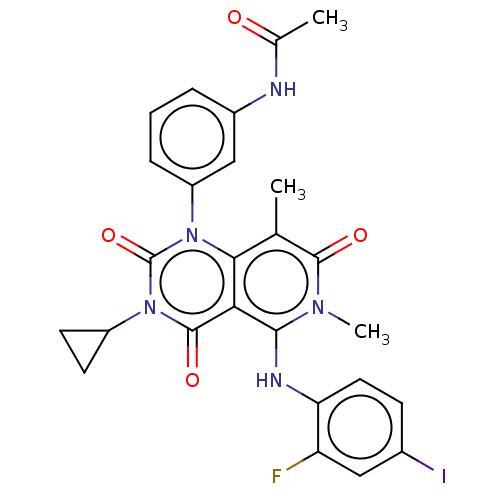 (CHEBI:75998 | GSK-1120212 | GSK1120212 | JTP 74057...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of MEK in human KYSE-520 cells assessed as reduction in p-ERK levels | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01170 BindingDB Entry DOI: 10.7270/Q2CC14BB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185439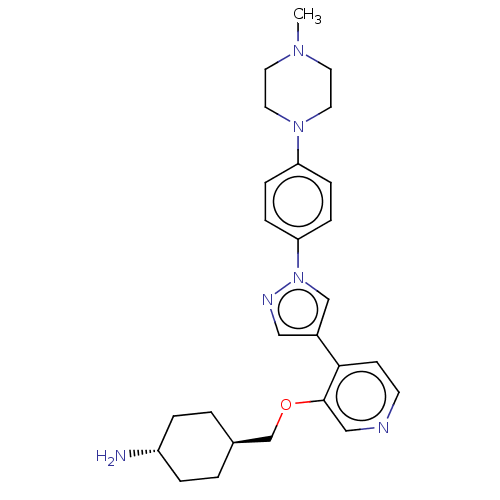 (CHEMBL3823975) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146964 (US8957074, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50235407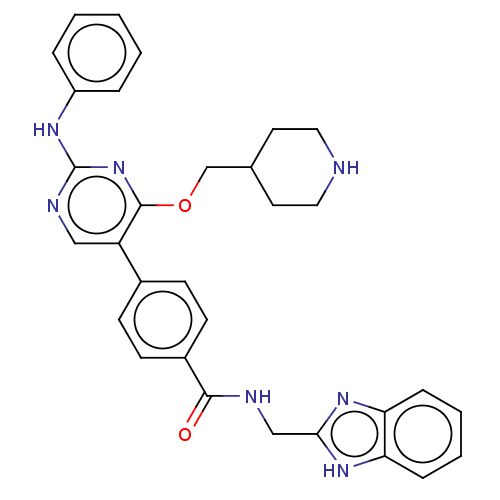 (CHEMBL4088246) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay | J Med Chem 60: 2155-2161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00033 BindingDB Entry DOI: 10.7270/Q2MC928T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50235401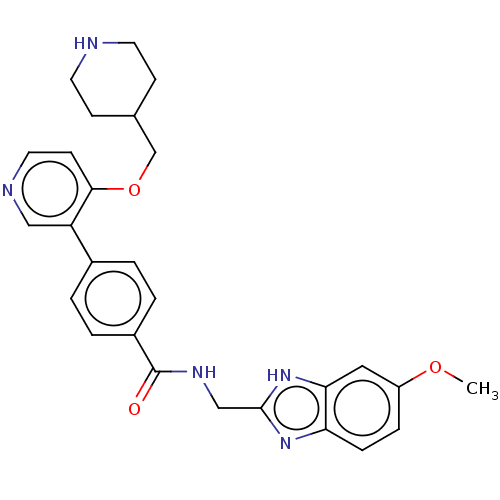 (CHEMBL4071823) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay | J Med Chem 60: 2155-2161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00033 BindingDB Entry DOI: 10.7270/Q2MC928T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50235399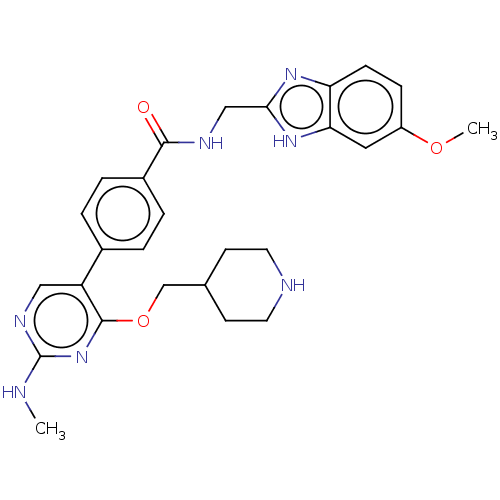 (CHEMBL4092605) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of MELK (unknown origin) containing kinase domain and UBA domain using STK S1 as substrate after 20 mins by HTRF assay | J Med Chem 60: 2155-2161 (2017) Article DOI: 10.1021/acs.jmedchem.7b00033 BindingDB Entry DOI: 10.7270/Q2MC928T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185433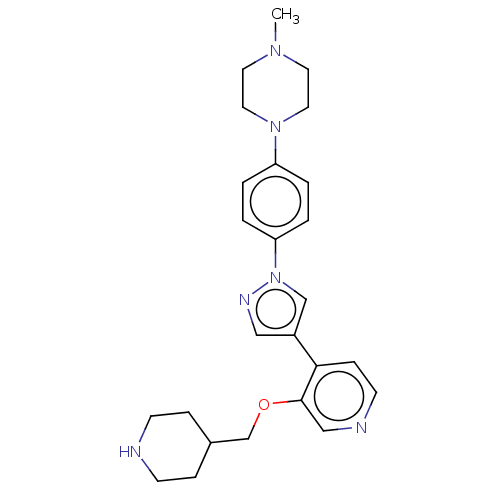 (CHEMBL3824068) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185433 (CHEMBL3824068) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Binding affinity to MELK (unknown origin) expressed in Escherichia coli by SPR analysis | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408072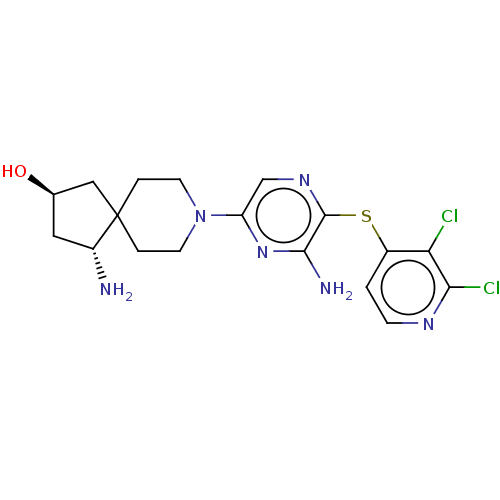 (US10336774, Example 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... | US Patent US10336774 (2019) BindingDB Entry DOI: 10.7270/Q2BR8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147014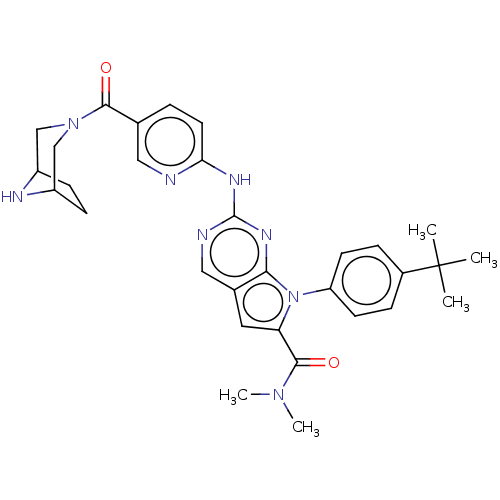 (US8957074, 53) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147015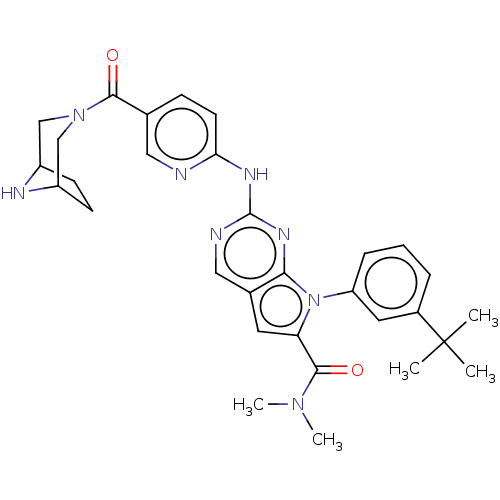 (US8957074, 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147027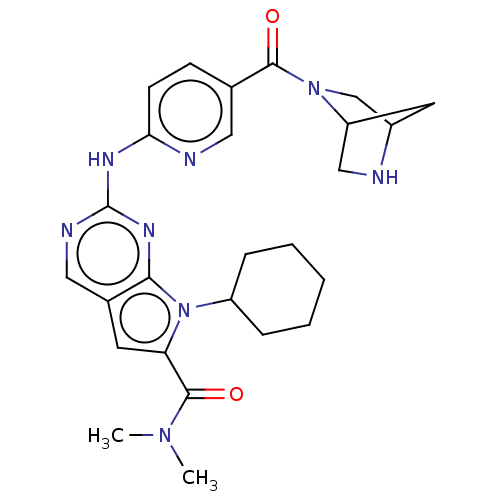 (US8957074, 67) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146997 (US8957074, 36) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147049 (US8957074, 89) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147083 (US8957074, 123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM50420304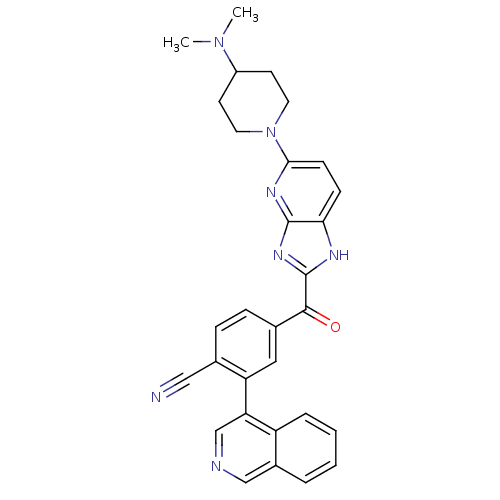 (CHEMBL2089065 | US8598217, 165) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147041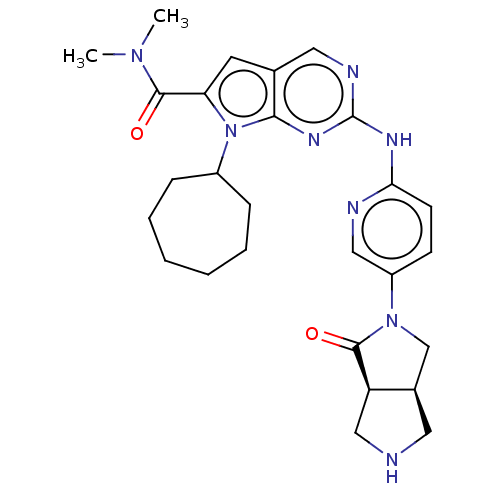 (US8957074, 83) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408094 (US10336774, Example 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... | US Patent US10336774 (2019) BindingDB Entry DOI: 10.7270/Q2BR8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408096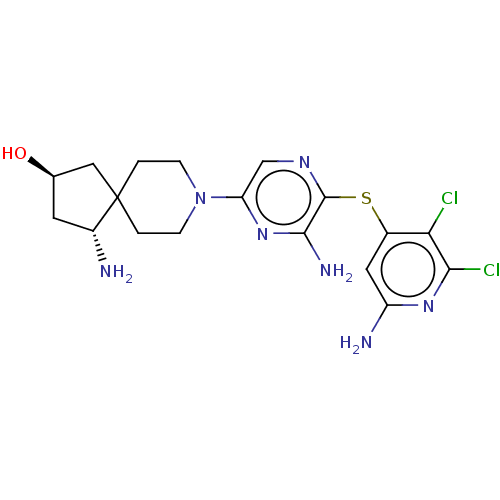 (US10336774, Example 90) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... | US Patent US10336774 (2019) BindingDB Entry DOI: 10.7270/Q2BR8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147091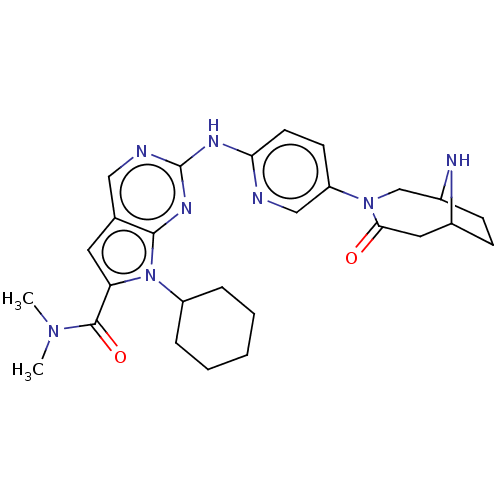 (US8957074, 131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185436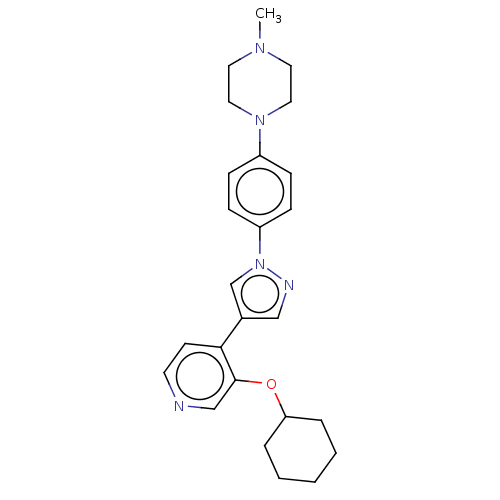 (CHEMBL3823874) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408073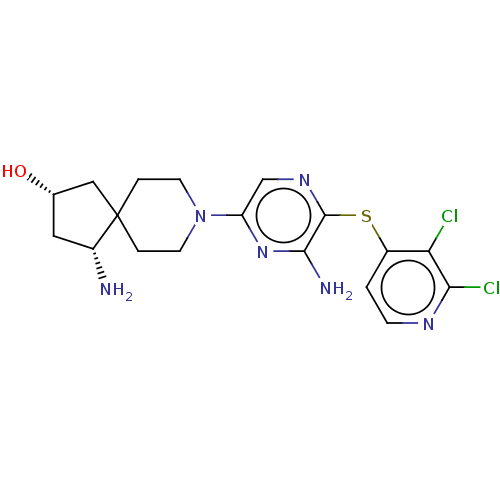 (US10336774, Example 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... | US Patent US10336774 (2019) BindingDB Entry DOI: 10.7270/Q2BR8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146970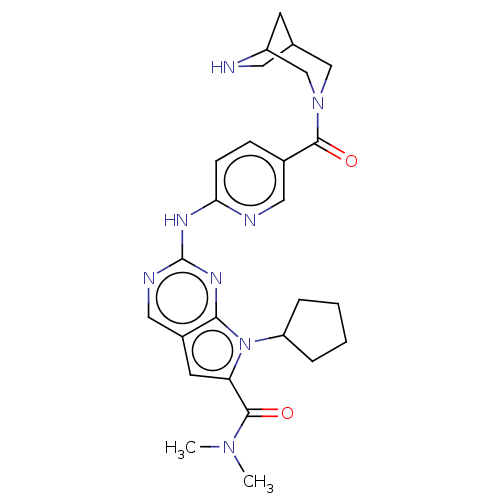 (US8957074, 9) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147042 (US8957074, 82) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107751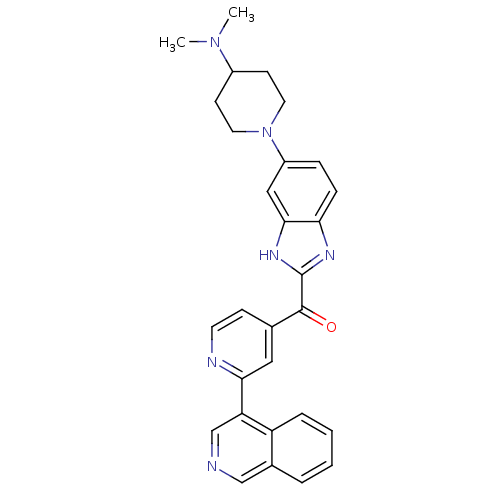 (CHEMBL2089063 | US8598217, 89) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107755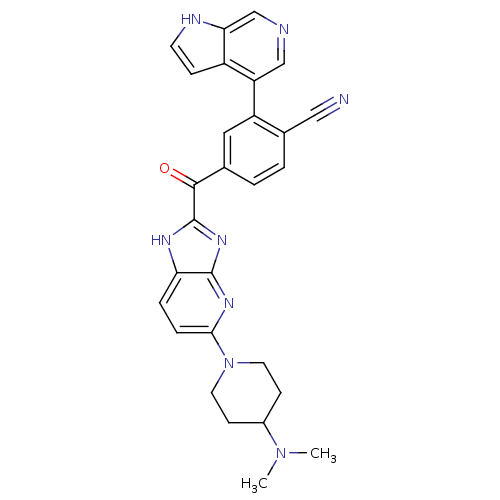 (US8598217, 140) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146965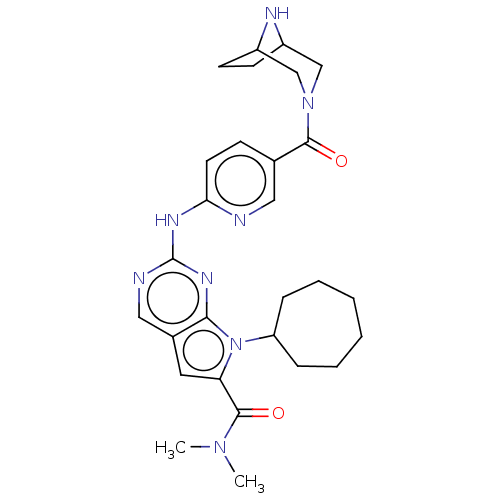 (US8957074, 4) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408075 (US10336774, Example 60) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... | US Patent US10336774 (2019) BindingDB Entry DOI: 10.7270/Q2BR8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM408086 (US10336774, Example 77) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description SHP2 is allosterically activated through binding of bis-tyrosyl-phorphorylated peptides to its Src Homology 2 (SH2) domains. The latter activation st... | US Patent US10336774 (2019) BindingDB Entry DOI: 10.7270/Q2BR8VJV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147087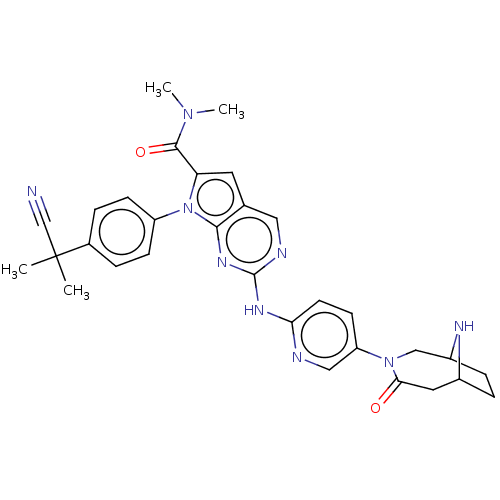 (US8957074, 127) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146971 (US8957074, 10) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146963 (US8957074, 2) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107761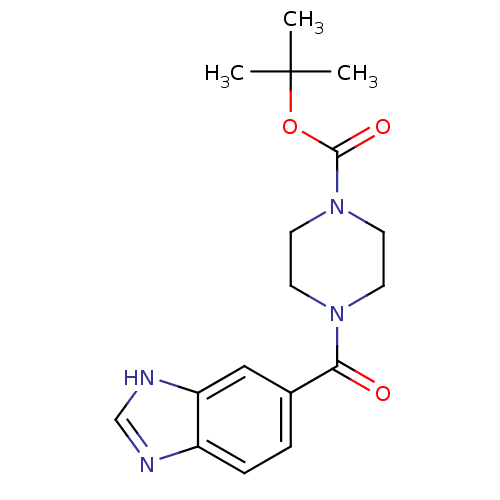 (US8598217, 174) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147053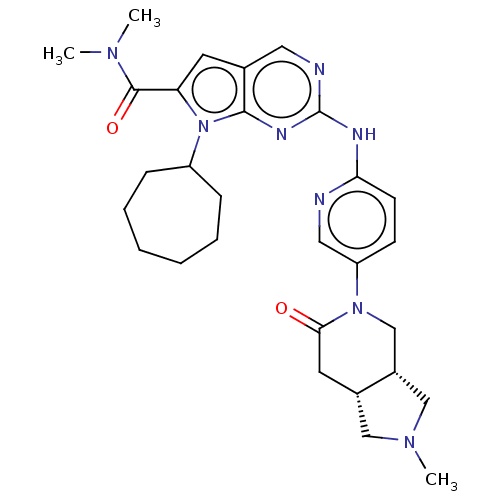 (US8957074, 93) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147093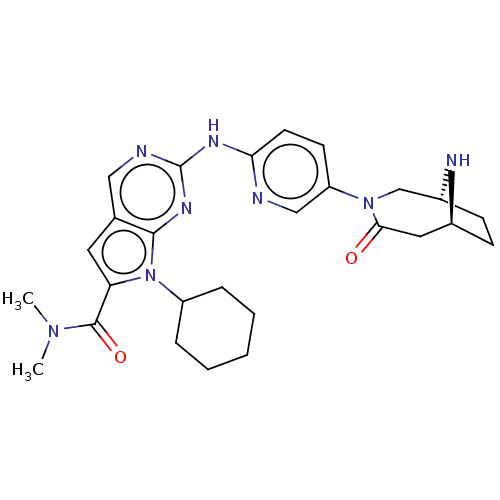 (US8957074, 133) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185433 (CHEMBL3824068) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of full-length MELK (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence of 2... | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM47154 (US8957074, 81) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147044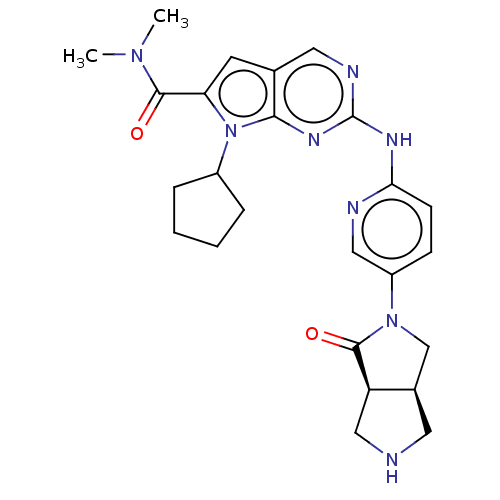 (US8957074, 84 | US8957074, 86) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147047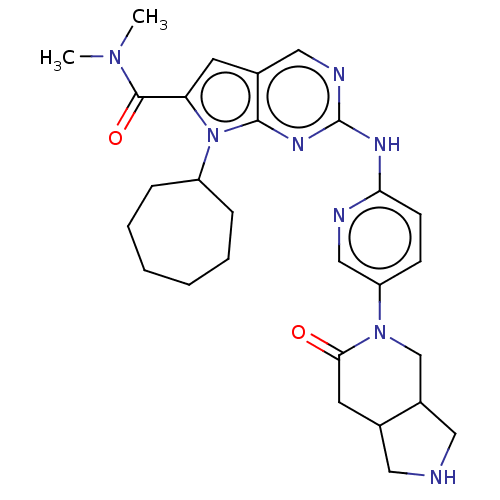 (US8957074, 87) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | <5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147048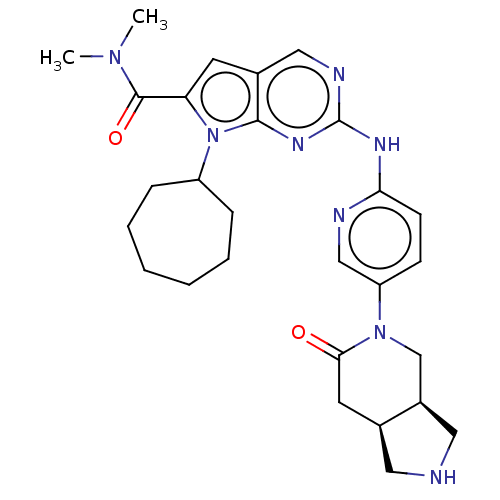 (US8957074, 88) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147050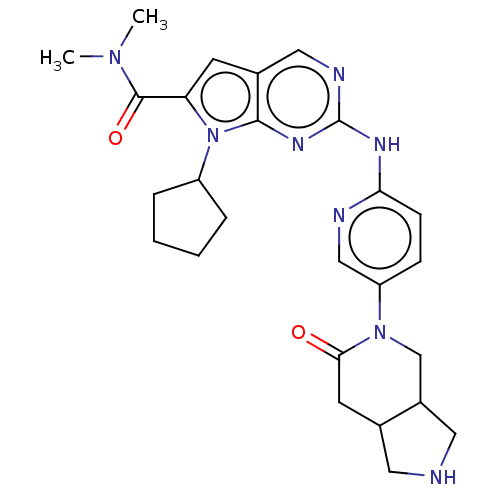 (US8957074, 90) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147026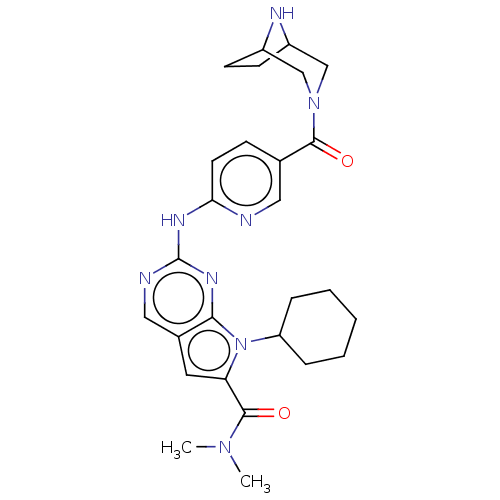 (US8957074, 66) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147044 (US8957074, 84 | US8957074, 86) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147100 (US8957074, 140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM147031 (US8957074, 71) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM146998 (US8957074, 37) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis AG US Patent | Assay Description An assay for monitoring CDK4/cyclin D1-catalyzed phosphorylation of pRb at the Ser780 site was performed using TR-FRET in a 384-well format, and was ... | US Patent US8957074 (2015) BindingDB Entry DOI: 10.7270/Q2K0730S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185434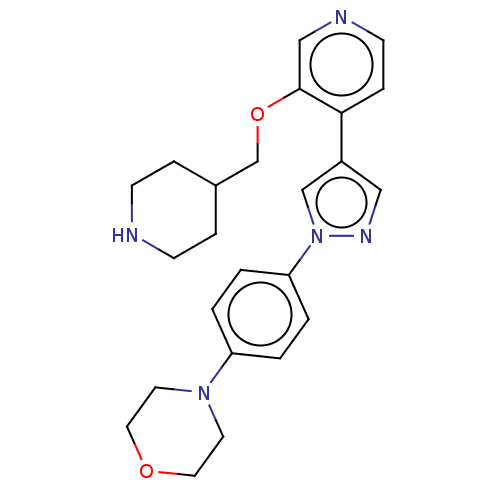 (CHEMBL3823302) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Maternal embryonic leucine zipper kinase (Homo sapiens (Human)) | BDBM50185437 (CHEMBL3822465) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of MELK catalytic domain (unknown origin) preincubated for 20 mins followed by addition of KinEASE STK S1 peptide as substrate in presence... | J Med Chem 59: 4711-23 (2016) Article DOI: 10.1021/acs.jmedchem.6b00052 BindingDB Entry DOI: 10.7270/Q2MK6FTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 565 total ) | Next | Last >> |