 Found 249 hits with Last Name = 'hruza' and Initial = 'aw'
Found 249 hits with Last Name = 'hruza' and Initial = 'aw' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM330035
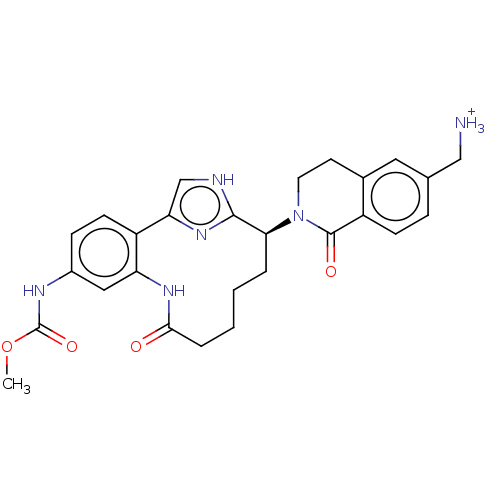
(Methyl [(7S)-7-[6-(aminomethyl)-1-oxo-3,4-dihydroi...)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCCCC(=O)Nc2c1)N1CCc2cc(C[NH3+])ccc2C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US9663527 (2017)
BindingDB Entry DOI: 10.7270/Q2862JJQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM330036
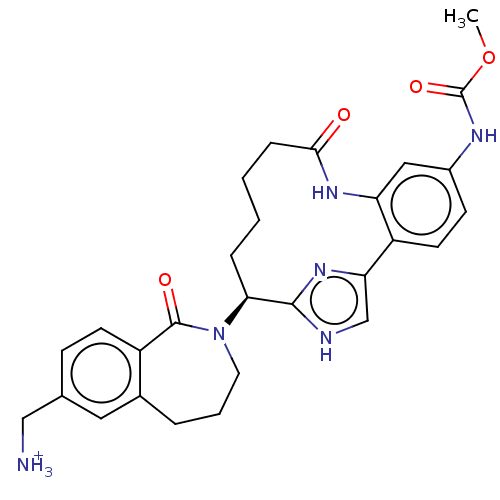
(US9663527, Example 2)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCCCC(=O)Nc2c1)N1CCCc2cc(C[NH3+])ccc2C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US9663527 (2017)
BindingDB Entry DOI: 10.7270/Q2862JJQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388640
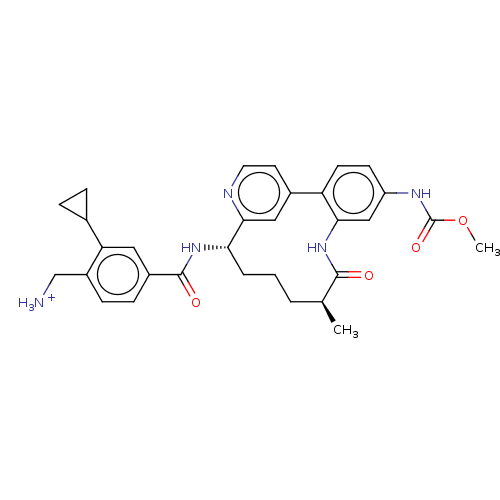
(US9944643, 4)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@@H](C)CCC[C@H](NC(=O)c3ccc(C[NH3+])c(c3)C3CC3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C31H35N5O4/c1-18-4-3-5-26(35-30(38)21-8-9-22(17-32)25(14-21)19-6-7-19)28-15-20(12-13-33-28)24-11-10-23(34-31(39)40-2)16-27(24)36-29(18)37/h8-16,18-19,26H,3-7,17,32H2,1-2H3,(H,34,39)(H,35,38)(H,36,37)/p+1/t18-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388641
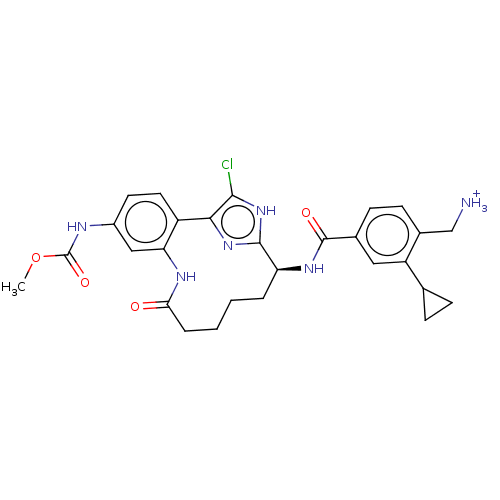
(US9944643, 5)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](CCCCC(=O)Nc2c1)NC(=O)c1ccc(C[NH3+])c(c1)C1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM330037
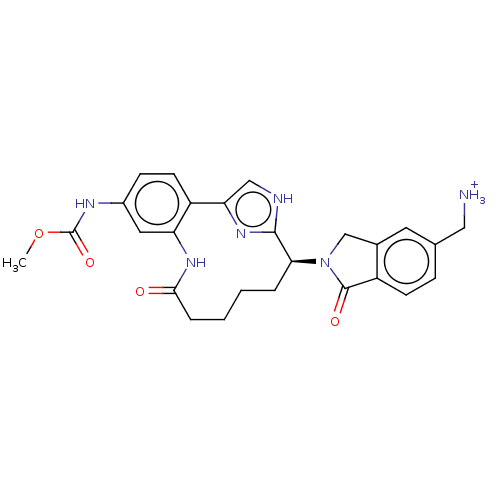
(US9663527, Example 3)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCCCC(=O)Nc2c1)N1Cc2cc(C[NH3+])ccc2C1=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US9663527 (2017)
BindingDB Entry DOI: 10.7270/Q2862JJQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444932
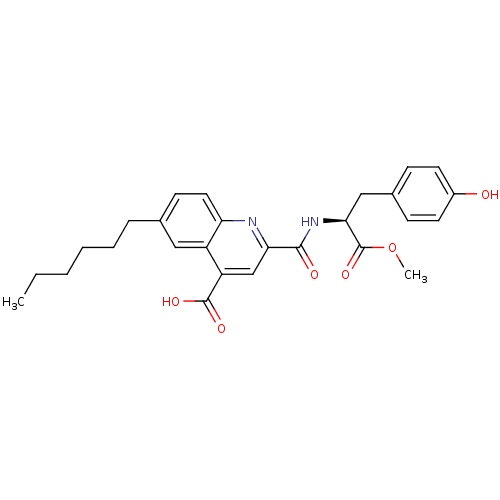
(CHEMBL3099764)Show SMILES CCCCCCc1ccc2nc(cc(C(O)=O)c2c1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)OC |r| Show InChI InChI=1S/C27H30N2O6/c1-3-4-5-6-7-17-10-13-22-20(14-17)21(26(32)33)16-23(28-22)25(31)29-24(27(34)35-2)15-18-8-11-19(30)12-9-18/h8-14,16,24,30H,3-7,15H2,1-2H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388554
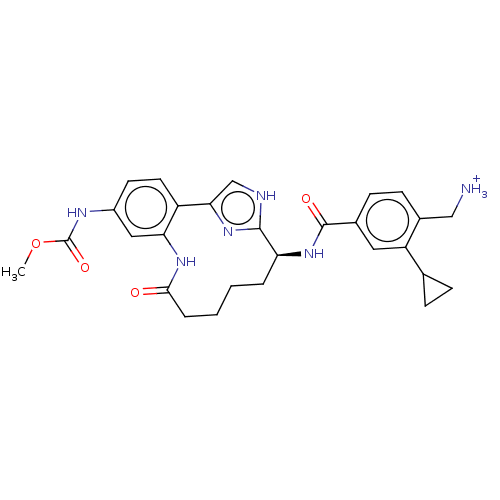
(Methyl [(7S)-7-({[4-(aminomethyl)-3-cyclopropylphe...)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCCCC(=O)Nc2c1)NC(=O)c1ccc(C[NH3+])c(c1)C1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444933
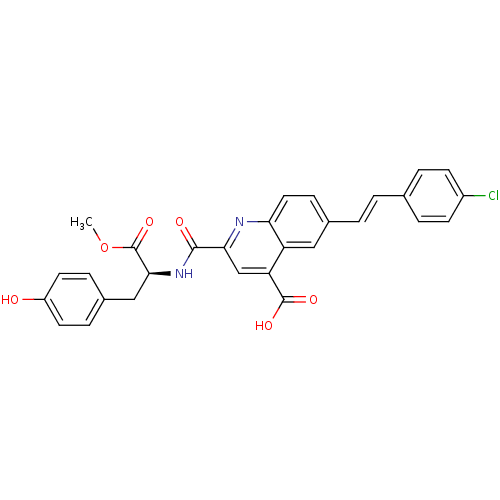
(CHEMBL3099763)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H23ClN2O6/c1-38-29(37)26(15-19-6-11-21(33)12-7-19)32-27(34)25-16-23(28(35)36)22-14-18(8-13-24(22)31-25)3-2-17-4-9-20(30)10-5-17/h2-14,16,26,33H,15H2,1H3,(H,32,34)(H,35,36)/b3-2+/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388639
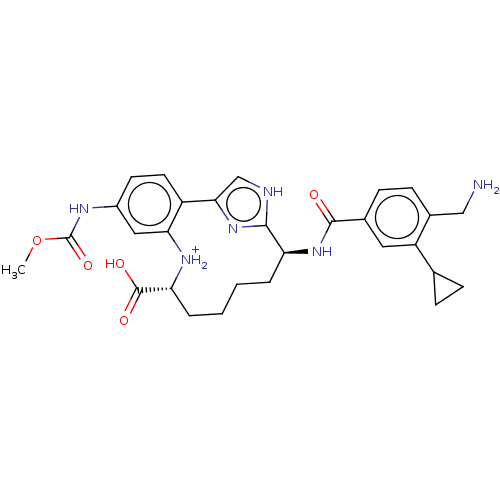
(US9944643, 3)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCCC[C@@H]([NH2+]c2c1)C(O)=O)NC(=O)c1ccc(CN)c(c1)C1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388646
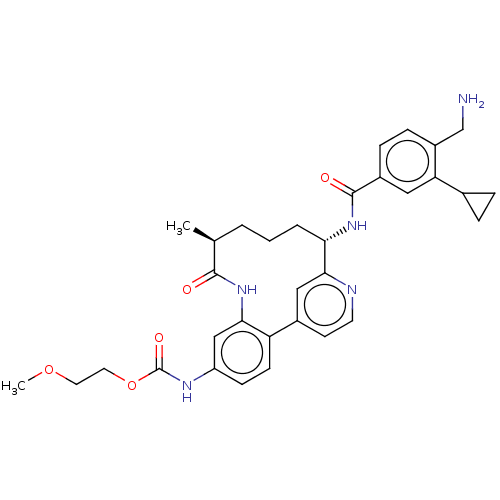
(US9944643, 8)Show SMILES COCCOC(=O)Nc1ccc-2c(NC(=O)[C@@H](C)CCC[C@H](NC(=O)c3ccc(CN)c(c3)C3CC3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C33H39N5O5/c1-20-4-3-5-28(37-32(40)23-8-9-24(19-34)27(16-23)21-6-7-21)30-17-22(12-13-35-30)26-11-10-25(18-29(26)38-31(20)39)36-33(41)43-15-14-42-2/h8-13,16-18,20-21,28H,3-7,14-15,19,34H2,1-2H3,(H,36,41)(H,37,40)(H,38,39)/t20-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444918
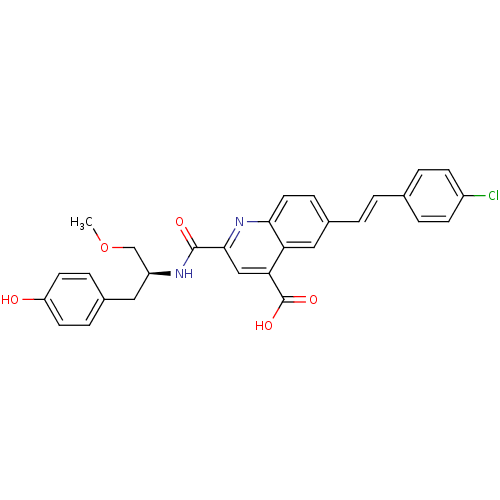
(CHEMBL3099753)Show SMILES COC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H25ClN2O5/c1-37-17-22(14-19-6-11-23(33)12-7-19)31-28(34)27-16-25(29(35)36)24-15-20(8-13-26(24)32-27)3-2-18-4-9-21(30)10-5-18/h2-13,15-16,22,33H,14,17H2,1H3,(H,31,34)(H,35,36)/b3-2+/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444921

(CHEMBL3099750)Show SMILES CNC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H24ClN3O5/c1-31-27(35)25(15-19-6-11-21(34)12-7-19)33-28(36)26-16-23(29(37)38)22-14-18(8-13-24(22)32-26)3-2-17-4-9-20(30)10-5-17/h2-14,16,25,34H,15H2,1H3,(H,31,35)(H,33,36)(H,37,38)/b3-2+/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444919
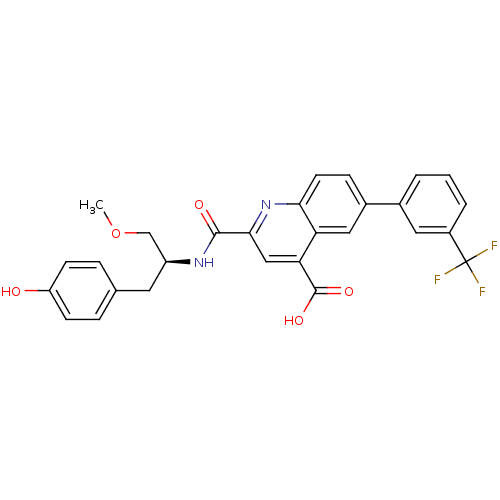
(CHEMBL3099752)Show SMILES COC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H23F3N2O5/c1-38-15-20(11-16-5-8-21(34)9-6-16)32-26(35)25-14-23(27(36)37)22-13-18(7-10-24(22)33-25)17-3-2-4-19(12-17)28(29,30)31/h2-10,12-14,20,34H,11,15H2,1H3,(H,32,35)(H,36,37)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444935
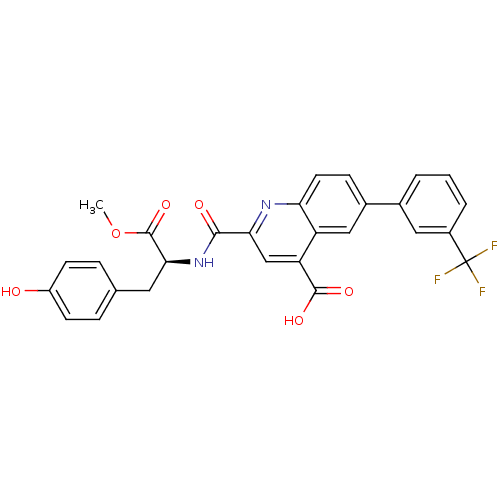
(CHEMBL3099761)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H21F3N2O6/c1-39-27(38)24(11-15-5-8-19(34)9-6-15)33-25(35)23-14-21(26(36)37)20-13-17(7-10-22(20)32-23)16-3-2-4-18(12-16)28(29,30)31/h2-10,12-14,24,34H,11H2,1H3,(H,33,35)(H,36,37)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444934
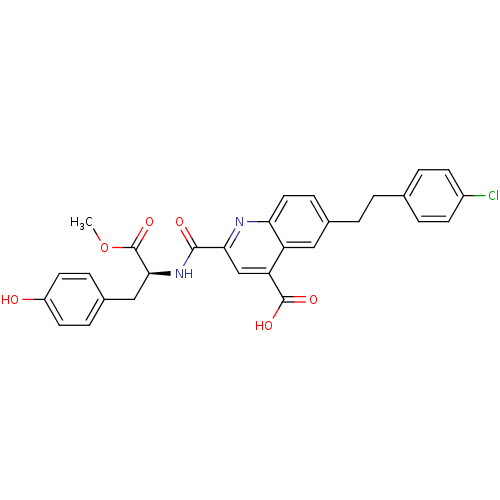
(CHEMBL3099762)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(CCc3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C29H25ClN2O6/c1-38-29(37)26(15-19-6-11-21(33)12-7-19)32-27(34)25-16-23(28(35)36)22-14-18(8-13-24(22)31-25)3-2-17-4-9-20(30)10-5-17/h4-14,16,26,33H,2-3,15H2,1H3,(H,32,34)(H,35,36)/t26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444941
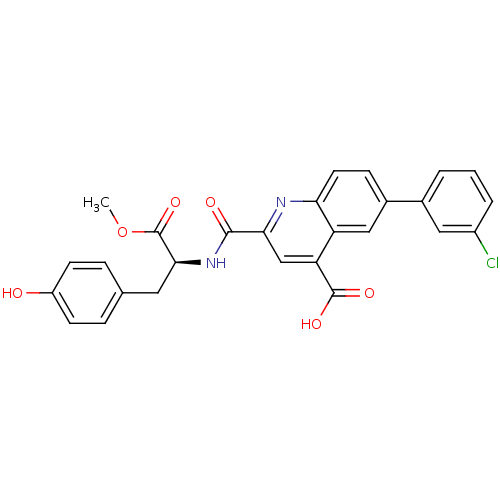
(CHEMBL3099755)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(Cl)c1 |r| Show InChI InChI=1S/C27H21ClN2O6/c1-36-27(35)24(11-15-5-8-19(31)9-6-15)30-25(32)23-14-21(26(33)34)20-13-17(7-10-22(20)29-23)16-3-2-4-18(28)12-16/h2-10,12-14,24,31H,11H2,1H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444920
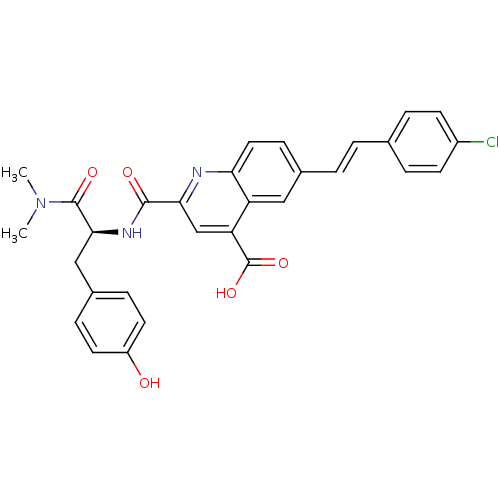
(CHEMBL3099751)Show SMILES CN(C)C(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(\C=C\c3ccc(Cl)cc3)ccc2n1 |r| Show InChI InChI=1S/C30H26ClN3O5/c1-34(2)29(37)27(16-20-7-12-22(35)13-8-20)33-28(36)26-17-24(30(38)39)23-15-19(9-14-25(23)32-26)4-3-18-5-10-21(31)11-6-18/h3-15,17,27,35H,16H2,1-2H3,(H,33,36)(H,38,39)/b4-3+/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM330038
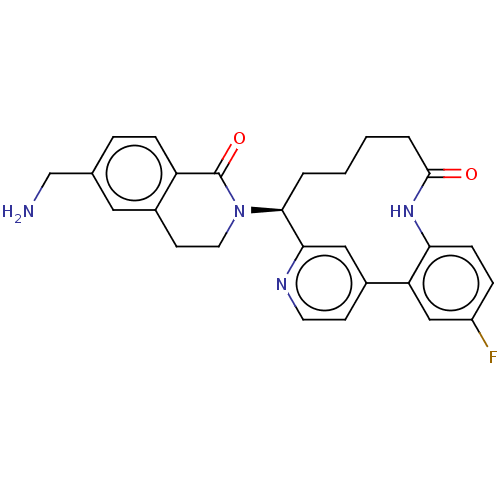
(US9663527, Example 4)Show SMILES NCc1ccc2C(=O)N(CCc2c1)[C@H]1CCCCC(=O)Nc2ccc(F)cc2-c2ccnc1c2 |r| Show InChI InChI=1S/C27H27FN4O2/c28-20-6-8-23-22(15-20)18-9-11-30-24(14-18)25(3-1-2-4-26(33)31-23)32-12-10-19-13-17(16-29)5-7-21(19)27(32)34/h5-9,11,13-15,25H,1-4,10,12,16,29H2,(H,31,33)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... |
US Patent US9663527 (2017)
BindingDB Entry DOI: 10.7270/Q2862JJQ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444938
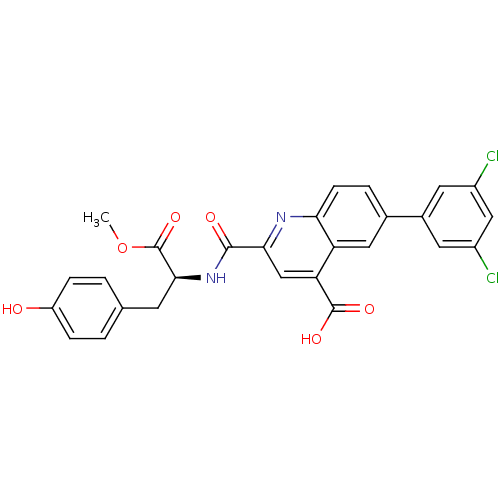
(CHEMBL3099758)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C27H20Cl2N2O6/c1-37-27(36)24(8-14-2-5-19(32)6-3-14)31-25(33)23-13-21(26(34)35)20-11-15(4-7-22(20)30-23)16-9-17(28)12-18(29)10-16/h2-7,9-13,24,32H,8H2,1H3,(H,31,33)(H,34,35)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388643
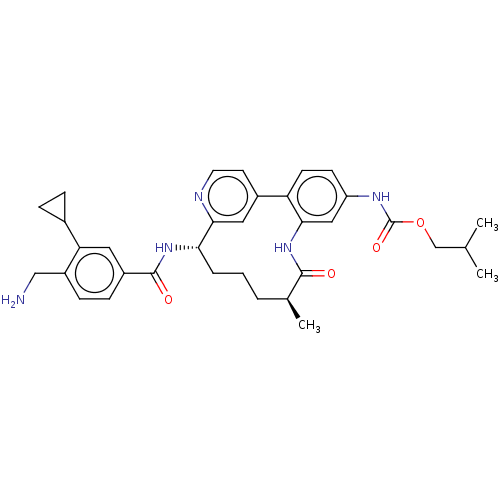
(US9944643, 6)Show SMILES CC(C)COC(=O)Nc1ccc-2c(NC(=O)[C@@H](C)CCC[C@H](NC(=O)c3ccc(CN)c(c3)C3CC3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C34H41N5O4/c1-20(2)19-43-34(42)37-26-11-12-27-23-13-14-36-31(16-23)29(6-4-5-21(3)32(40)39-30(27)17-26)38-33(41)24-9-10-25(18-35)28(15-24)22-7-8-22/h9-17,20-22,29H,4-8,18-19,35H2,1-3H3,(H,37,42)(H,38,41)(H,39,40)/t21-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444922
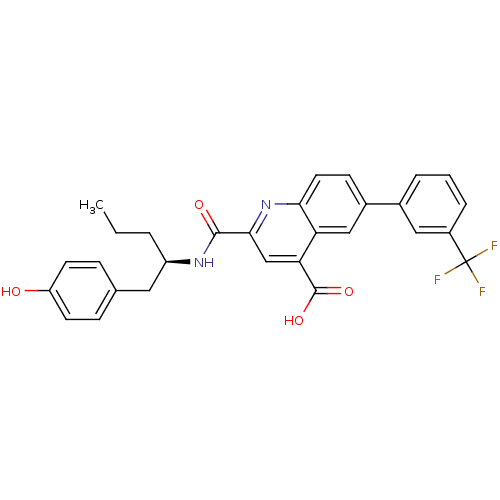
(CHEMBL3099749)Show SMILES CCC[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C29H25F3N2O4/c1-2-4-21(13-17-7-10-22(35)11-8-17)33-27(36)26-16-24(28(37)38)23-15-19(9-12-25(23)34-26)18-5-3-6-20(14-18)29(30,31)32/h3,5-12,14-16,21,35H,2,4,13H2,1H3,(H,33,36)(H,37,38)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388645
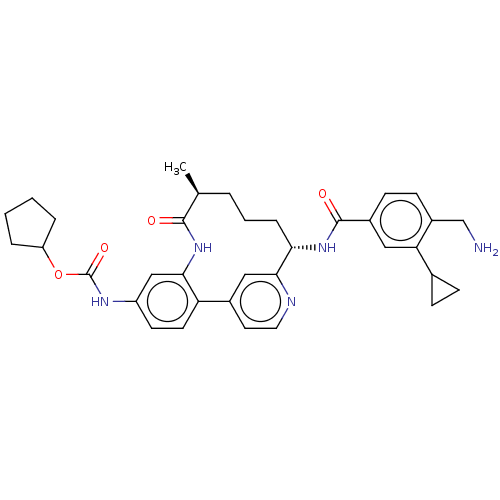
(US9944643, 7)Show SMILES C[C@H]1CCC[C@H](NC(=O)c2ccc(CN)c(c2)C2CC2)c2cc(ccn2)-c2ccc(NC(=O)OC3CCCC3)cc2NC1=O |r| Show InChI InChI=1S/C35H41N5O4/c1-21-5-4-8-30(39-34(42)24-11-12-25(20-36)29(17-24)22-9-10-22)32-18-23(15-16-37-32)28-14-13-26(19-31(28)40-33(21)41)38-35(43)44-27-6-2-3-7-27/h11-19,21-22,27,30H,2-10,20,36H2,1H3,(H,38,43)(H,39,42)(H,40,41)/t21-,30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444940
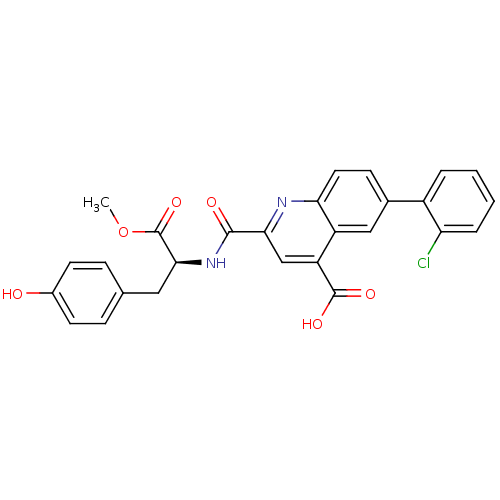
(CHEMBL3099756)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1ccccc1Cl |r| Show InChI InChI=1S/C27H21ClN2O6/c1-36-27(35)24(12-15-6-9-17(31)10-7-15)30-25(32)23-14-20(26(33)34)19-13-16(8-11-22(19)29-23)18-4-2-3-5-21(18)28/h2-11,13-14,24,31H,12H2,1H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444942
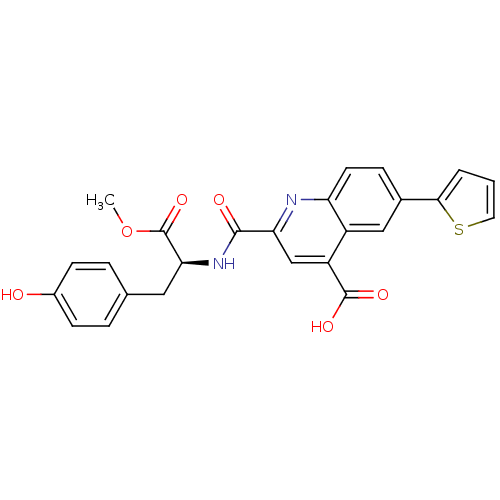
(CHEMBL3099754)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccs1 |r| Show InChI InChI=1S/C25H20N2O6S/c1-33-25(32)21(11-14-4-7-16(28)8-5-14)27-23(29)20-13-18(24(30)31)17-12-15(6-9-19(17)26-20)22-3-2-10-34-22/h2-10,12-13,21,28H,11H2,1H3,(H,27,29)(H,30,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444939
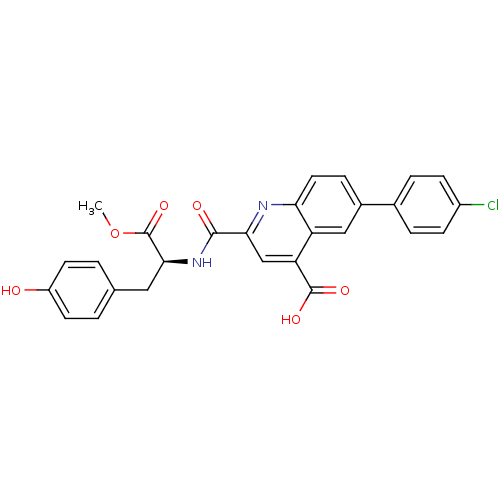
(CHEMBL3099757)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H21ClN2O6/c1-36-27(35)24(12-15-2-9-19(31)10-3-15)30-25(32)23-14-21(26(33)34)20-13-17(6-11-22(20)29-23)16-4-7-18(28)8-5-16/h2-11,13-14,24,31H,12H2,1H3,(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM388638

(US9944643, 2)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c([nH+]3)[C@H](CCCCC(=O)Nc2c1)NC(=O)c1cnc(C[NH3+])c(c1)C1CC1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nippon Shinyaku Co.
| Assay Description
Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; J T B... |
Bioorg Med Chem 17: 119-32 (2009)
BindingDB Entry DOI: 10.7270/Q2XW4N5K |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444923
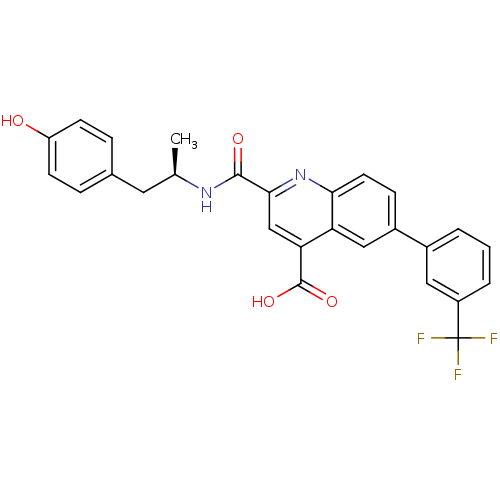
(CHEMBL3099748)Show SMILES C[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H21F3N2O4/c1-15(11-16-5-8-20(33)9-6-16)31-25(34)24-14-22(26(35)36)21-13-18(7-10-23(21)32-24)17-3-2-4-19(12-17)27(28,29)30/h2-10,12-15,33H,11H2,1H3,(H,31,34)(H,35,36)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444928
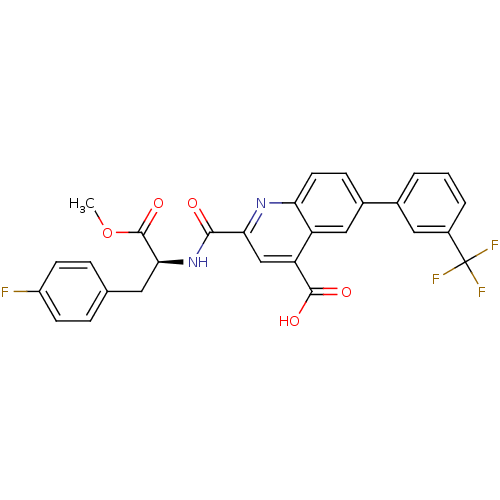
(CHEMBL3099743)Show SMILES COC(=O)[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H20F4N2O5/c1-39-27(38)24(11-15-5-8-19(29)9-6-15)34-25(35)23-14-21(26(36)37)20-13-17(7-10-22(20)33-23)16-3-2-4-18(12-16)28(30,31)32/h2-10,12-14,24H,11H2,1H3,(H,34,35)(H,36,37)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
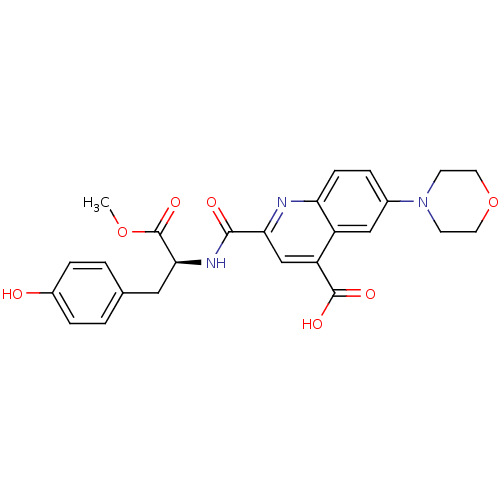
(Homo sapiens (Human)) | BDBM50444936
(CHEMBL3099760)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)N1CCOCC1 |r| Show InChI InChI=1S/C25H25N3O7/c1-34-25(33)22(12-15-2-5-17(29)6-3-15)27-23(30)21-14-19(24(31)32)18-13-16(4-7-20(18)26-21)28-8-10-35-11-9-28/h2-7,13-14,22,29H,8-12H2,1H3,(H,27,30)(H,31,32)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
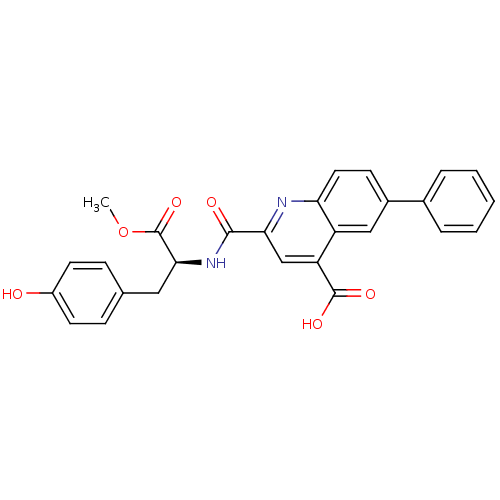
(Homo sapiens (Human)) | BDBM50444937
(CHEMBL3099759)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1ccccc1 |r| Show InChI InChI=1S/C27H22N2O6/c1-35-27(34)24(13-16-7-10-19(30)11-8-16)29-25(31)23-15-21(26(32)33)20-14-18(9-12-22(20)28-23)17-5-3-2-4-6-17/h2-12,14-15,24,30H,13H2,1H3,(H,29,31)(H,32,33)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444924
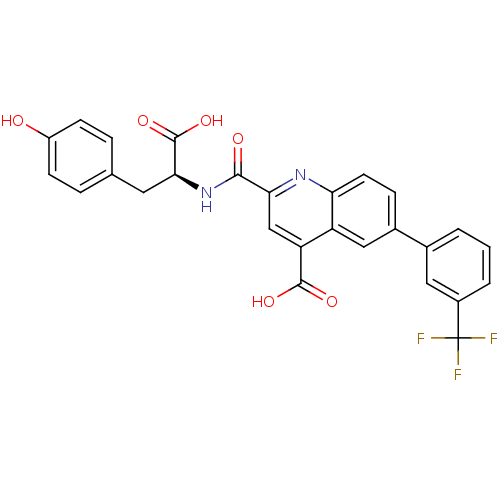
(CHEMBL3099747)Show SMILES OC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C27H19F3N2O6/c28-27(29,30)17-3-1-2-15(11-17)16-6-9-21-19(12-16)20(25(35)36)13-22(31-21)24(34)32-23(26(37)38)10-14-4-7-18(33)8-5-14/h1-9,11-13,23,33H,10H2,(H,32,34)(H,35,36)(H,37,38)/t23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444927
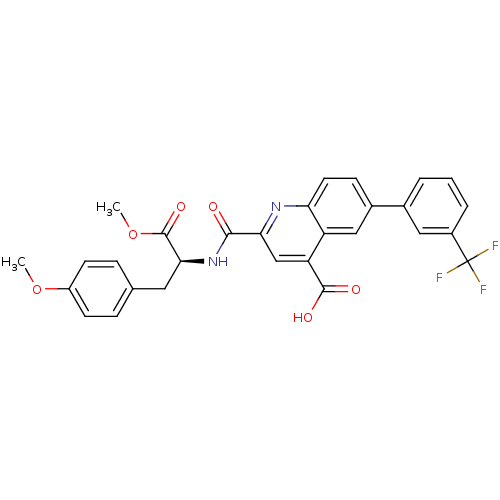
(CHEMBL3099744)Show SMILES COC(=O)[C@H](Cc1ccc(OC)cc1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C29H23F3N2O6/c1-39-20-9-6-16(7-10-20)12-25(28(38)40-2)34-26(35)24-15-22(27(36)37)21-14-18(8-11-23(21)33-24)17-4-3-5-19(13-17)29(30,31)32/h3-11,13-15,25H,12H2,1-2H3,(H,34,35)(H,36,37)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444929
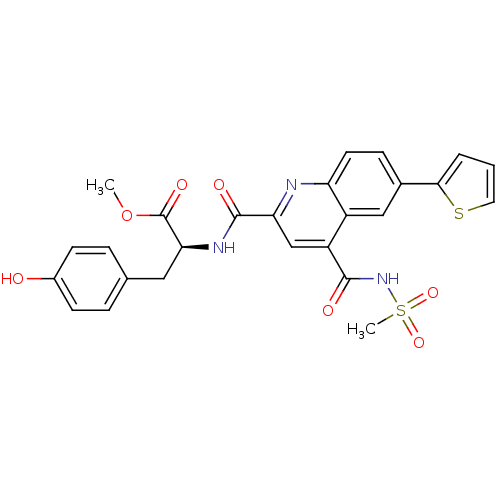
(CHEMBL3099742)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(=O)NS(C)(=O)=O)c2cc(ccc2n1)-c1cccs1 |r| Show InChI InChI=1S/C26H23N3O7S2/c1-36-26(33)22(12-15-5-8-17(30)9-6-15)28-25(32)21-14-19(24(31)29-38(2,34)35)18-13-16(7-10-20(18)27-21)23-4-3-11-37-23/h3-11,13-14,22,30H,12H2,1-2H3,(H,28,32)(H,29,31)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50444926
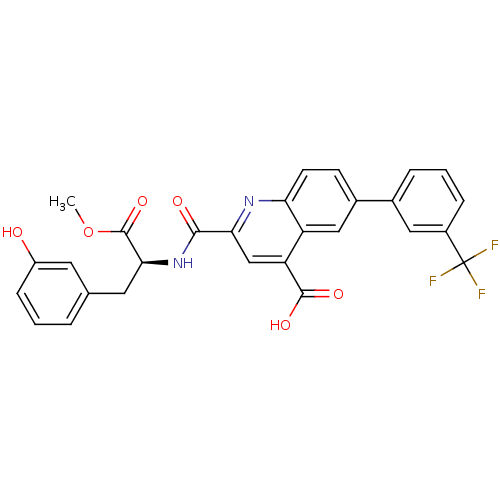
(CHEMBL3099745)Show SMILES COC(=O)[C@H](Cc1cccc(O)c1)NC(=O)c1cc(C(O)=O)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H21F3N2O6/c1-39-27(38)24(11-15-4-2-7-19(34)10-15)33-25(35)23-14-21(26(36)37)20-13-17(8-9-22(20)32-23)16-5-3-6-18(12-16)28(29,30)31/h2-10,12-14,24,34H,11H2,1H3,(H,33,35)(H,36,37)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
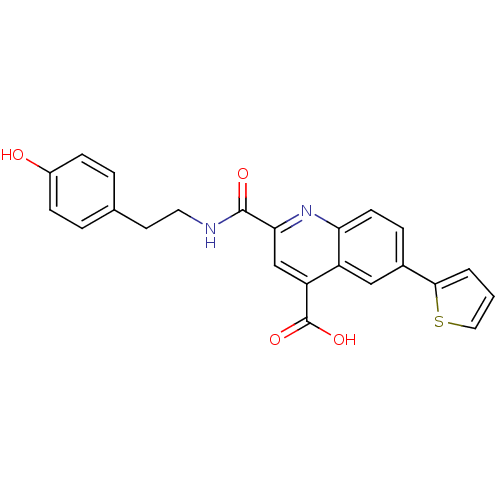
(Homo sapiens (Human)) | BDBM50444925
(CHEMBL3099746)Show SMILES OC(=O)c1cc(nc2ccc(cc12)-c1cccs1)C(=O)NCCc1ccc(O)cc1 Show InChI InChI=1S/C23H18N2O4S/c26-16-6-3-14(4-7-16)9-10-24-22(27)20-13-18(23(28)29)17-12-15(5-8-19(17)25-20)21-2-1-11-30-21/h1-8,11-13,26H,9-10H2,(H,24,27)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
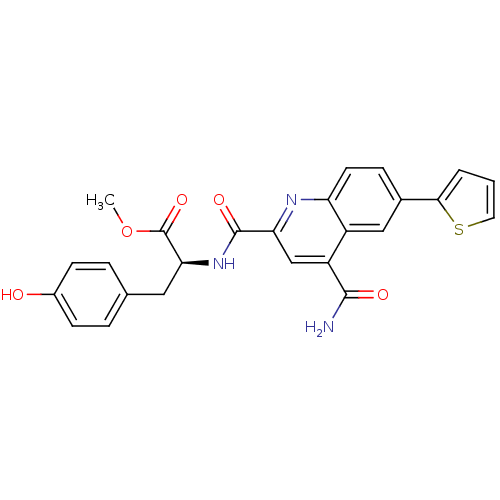
(Homo sapiens (Human)) | BDBM50444930
(CHEMBL3099766)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(C(N)=O)c2cc(ccc2n1)-c1cccs1 |r| Show InChI InChI=1S/C25H21N3O5S/c1-33-25(32)21(11-14-4-7-16(29)8-5-14)28-24(31)20-13-18(23(26)30)17-12-15(6-9-19(17)27-20)22-3-2-10-34-22/h2-10,12-13,21,29H,11H2,1H3,(H2,26,30)(H,28,31)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
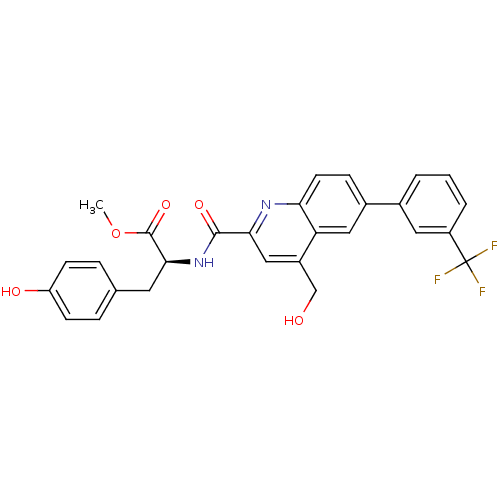
(Homo sapiens (Human)) | BDBM50444931
(CHEMBL3099765)Show SMILES COC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)c1cc(CO)c2cc(ccc2n1)-c1cccc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H23F3N2O5/c1-38-27(37)25(11-16-5-8-21(35)9-6-16)33-26(36)24-14-19(15-34)22-13-18(7-10-23(22)32-24)17-3-2-4-20(12-17)28(29,30)31/h2-10,12-14,25,34-35H,11,15H2,1H3,(H,33,36)/t25-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay |
Bioorg Med Chem Lett 24: 199-203 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.041
BindingDB Entry DOI: 10.7270/Q2SN0BF8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
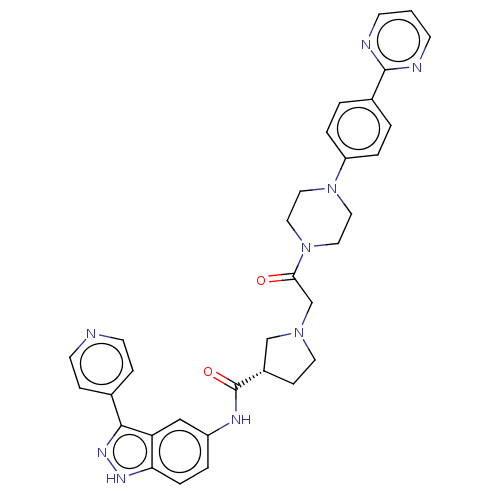
(Mus musculus (Mouse)) | BDBM103302
(SCH772984 | US8546404, 6)Show SMILES O=C(CN1CC[C@@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C33H33N9O2/c43-30(42-18-16-41(17-19-42)27-5-2-24(3-6-27)32-35-11-1-12-36-32)22-40-15-10-25(21-40)33(44)37-26-4-7-29-28(20-26)31(39-38-29)23-8-13-34-14-9-23/h1-9,11-14,20,25H,10,15-19,21-22H2,(H,37,44)(H,38,39)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
Activated ERK2 activity was determined in the IMAP assay format. |
US Patent US8546404 (2013)
BindingDB Entry DOI: 10.7270/Q2959G61 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094464

(CHEMBL3590107 | US10525036, Example SCH772984 | US...)Show SMILES O=C(CN1CC[C@H](C1)C(=O)Nc1ccc2[nH]nc(-c3ccncc3)c2c1)N1CCN(CC1)c1ccc(cc1)-c1ncccn1 |r| Show InChI InChI=1S/C22H27NO3/c1-2-15-23-16-13-20(14-17-23)26-21(24)22(25,18-9-5-3-6-10-18)19-11-7-4-8-12-19/h3-12,20,25H,2,13-17H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50458811
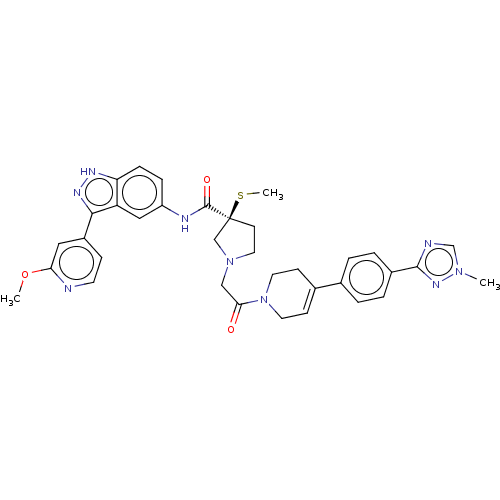
(CHEMBL4209570)Show SMILES COc1cc(ccn1)-c1n[nH]c2ccc(NC(=O)[C@@]3(CCN(CC(=O)N4CCC(=CC4)c4ccc(cc4)-c4ncn(C)n4)C3)SC)cc12 |r,c:29| Show InChI InChI=1S/C35H37N9O3S/c1-42-22-37-33(41-42)25-6-4-23(5-7-25)24-11-15-44(16-12-24)31(45)20-43-17-13-35(21-43,48-3)34(46)38-27-8-9-29-28(19-27)32(40-39-29)26-10-14-36-30(18-26)47-2/h4-11,14,18-19,22H,12-13,15-17,20-21H2,1-3H3,(H,38,46)(H,39,40)/t35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Mus musculus (Mouse)) | BDBM103295
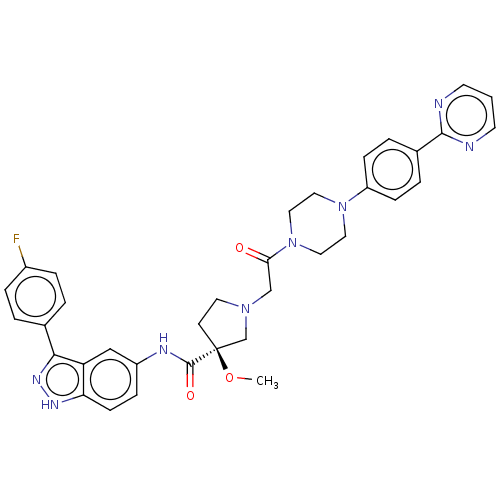
(US8546404, 462)Show SMILES CO[C@]1(CCN(CC(=O)N2CCN(CC2)c2ccc(cc2)-c2ncccn2)C1)C(=O)Nc1ccc2[nH]nc(-c3ccc(F)cc3)c2c1 |r| Show InChI InChI=1S/C35H35FN8O3/c1-47-35(34(46)39-27-9-12-30-29(21-27)32(41-40-30)24-3-7-26(36)8-4-24)13-16-42(23-35)22-31(45)44-19-17-43(18-20-44)28-10-5-25(6-11-28)33-37-14-2-15-38-33/h2-12,14-15,21H,13,16-20,22-23H2,1H3,(H,39,46)(H,40,41)/t35-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
Activated ERK2 activity was determined in the IMAP assay format. |
US Patent US8546404 (2013)
BindingDB Entry DOI: 10.7270/Q2959G61 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Mus musculus (Mouse)) | BDBM34531
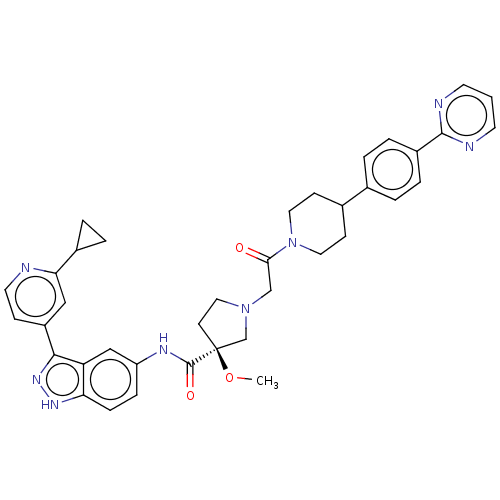
(US8546404, 459)Show SMILES CO[C@]1(CCN(CC(=O)N2CCC(CC2)c2ccc(cc2)-c2ncccn2)C1)C(=O)Nc1ccc2[nH]nc(-c3ccnc(c3)C3CC3)c2c1 |r| Show InChI InChI=1S/C17H13N3O3S/c21-16(15-9-22-13-3-1-2-4-14(13)23-15)20-17-19-12(10-24-17)11-5-7-18-8-6-11/h1-8,10,15H,9H2,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
Activated ERK2 activity was determined in the IMAP assay format. |
US Patent US8546404 (2013)
BindingDB Entry DOI: 10.7270/Q2959G61 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Mus musculus (Mouse)) | BDBM103292
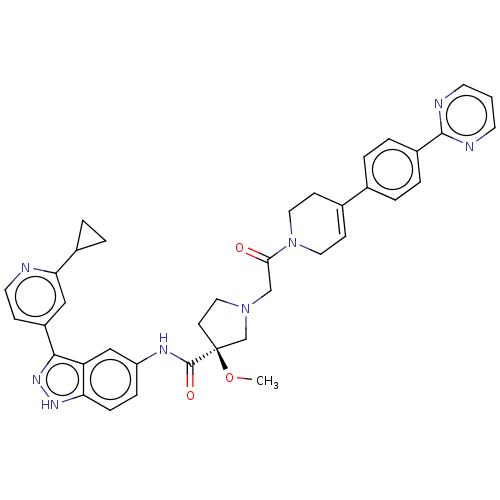
(US8546404, 1729 | US8546404, 469)Show SMILES CO[C@]1(CCN(CC(=O)N2CCC(=CC2)c2ccc(cc2)-c2ncccn2)C1)C(=O)Nc1ccc2[nH]nc(-c3ccnc(c3)C3CC3)c2c1 |r,c:12| Show InChI InChI=1S/C38H38N8O3/c1-49-38(37(48)42-30-9-10-32-31(22-30)35(44-43-32)29-11-17-39-33(21-29)27-5-6-27)14-20-45(24-38)23-34(47)46-18-12-26(13-19-46)25-3-7-28(8-4-25)36-40-15-2-16-41-36/h2-4,7-12,15-17,21-22,27H,5-6,13-14,18-20,23-24H2,1H3,(H,42,48)(H,43,44)/t38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
Activated ERK2 activity was determined in the IMAP assay format. |
US Patent US8546404 (2013)
BindingDB Entry DOI: 10.7270/Q2959G61 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Mus musculus (Mouse)) | BDBM103292

(US8546404, 1729 | US8546404, 469)Show SMILES CO[C@]1(CCN(CC(=O)N2CCC(=CC2)c2ccc(cc2)-c2ncccn2)C1)C(=O)Nc1ccc2[nH]nc(-c3ccnc(c3)C3CC3)c2c1 |r,c:12| Show InChI InChI=1S/C38H38N8O3/c1-49-38(37(48)42-30-9-10-32-31(22-30)35(44-43-32)29-11-17-39-33(21-29)27-5-6-27)14-20-45(24-38)23-34(47)46-18-12-26(13-19-46)25-3-7-28(8-4-25)36-40-15-2-16-41-36/h2-4,7-12,15-17,21-22,27H,5-6,13-14,18-20,23-24H2,1H3,(H,42,48)(H,43,44)/t38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
Activated ERK2 activity was determined in the IMAP assay format. |
US Patent US8546404 (2013)
BindingDB Entry DOI: 10.7270/Q2959G61 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Mus musculus (Mouse)) | BDBM103293

(US8546404, 480)Show SMILES CO[C@]1(CCN(CC(=O)N2CCC(=CC2)c2ccc(cc2)-c2ncccn2)C1)C(=O)Nc1ccc2[nH]nc(-c3ccc(F)cc3)c2c1 |r,c:12| Show InChI InChI=1S/C36H34FN7O3/c1-47-36(35(46)40-29-11-12-31-30(21-29)33(42-41-31)26-7-9-28(37)10-8-26)15-20-43(23-36)22-32(45)44-18-13-25(14-19-44)24-3-5-27(6-4-24)34-38-16-2-17-39-34/h2-13,16-17,21H,14-15,18-20,22-23H2,1H3,(H,40,46)(H,41,42)/t36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
Activated ERK2 activity was determined in the IMAP assay format. |
US Patent US8546404 (2013)
BindingDB Entry DOI: 10.7270/Q2959G61 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Mus musculus (Mouse)) | BDBM103296
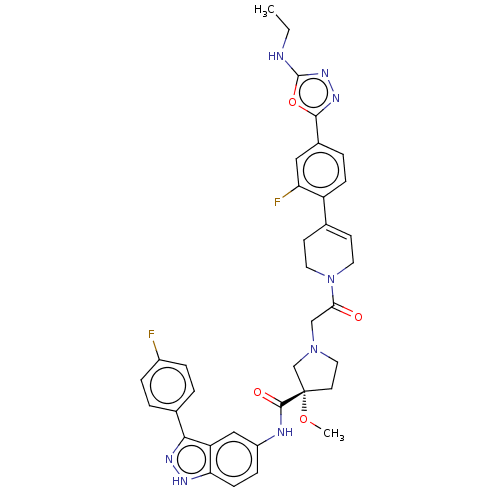
(US8546404, 622)Show SMILES CCNc1nnc(o1)-c1ccc(C2=CCN(CC2)C(=O)CN2CC[C@@](C2)(OC)C(=O)Nc2ccc3[nH]nc(-c4ccc(F)cc4)c3c2)c(F)c1 |r,t:13| Show InChI InChI=1S/C36H36F2N8O4/c1-3-39-35-44-43-33(50-35)24-6-10-27(29(38)18-24)22-12-15-46(16-13-22)31(47)20-45-17-14-36(21-45,49-2)34(48)40-26-9-11-30-28(19-26)32(42-41-30)23-4-7-25(37)8-5-23/h4-12,18-19H,3,13-17,20-21H2,1-2H3,(H,39,44)(H,40,48)(H,41,42)/t36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme
US Patent
| Assay Description
Activated ERK2 activity was determined in the IMAP assay format. |
US Patent US8546404 (2013)
BindingDB Entry DOI: 10.7270/Q2959G61 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50458806

(CHEMBL4205192)Show SMILES CCNc1nnc(o1)-c1ccc(cc1)C1=CCN(CC1)C(=O)CN1CC[C@@](C1)(SC)C(=O)Nc1ccc2[nH]nc(-c3ccc(F)cc3)c2c1 |r,t:16| Show InChI InChI=1S/C36H37FN8O3S/c1-3-38-35-43-42-33(48-35)26-6-4-23(5-7-26)24-14-17-45(18-15-24)31(46)21-44-19-16-36(22-44,49-2)34(47)39-28-12-13-30-29(20-28)32(41-40-30)25-8-10-27(37)11-9-25/h4-14,20H,3,15-19,21-22H2,1-2H3,(H,38,43)(H,39,47)(H,40,41)/t36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50458805
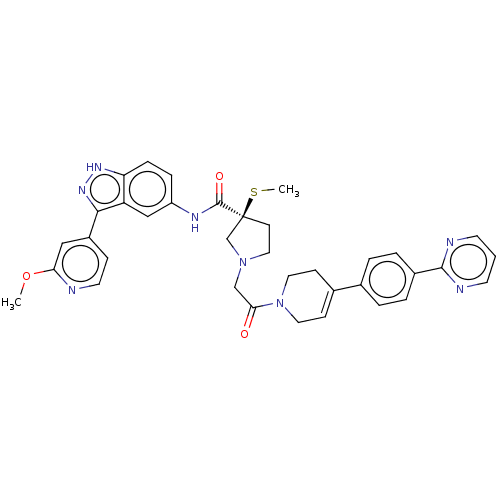
(CHEMBL4206960)Show SMILES COc1cc(ccn1)-c1n[nH]c2ccc(NC(=O)[C@@]3(CCN(CC(=O)N4CCC(=CC4)c4ccc(cc4)-c4ncccn4)C3)SC)cc12 |r,c:29| Show InChI InChI=1S/C36H36N8O3S/c1-47-31-20-27(10-16-37-31)33-29-21-28(8-9-30(29)41-42-33)40-35(46)36(48-2)13-19-43(23-36)22-32(45)44-17-11-25(12-18-44)24-4-6-26(7-5-24)34-38-14-3-15-39-34/h3-11,14-16,20-21H,12-13,17-19,22-23H2,1-2H3,(H,40,46)(H,41,42)/t36-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50458802
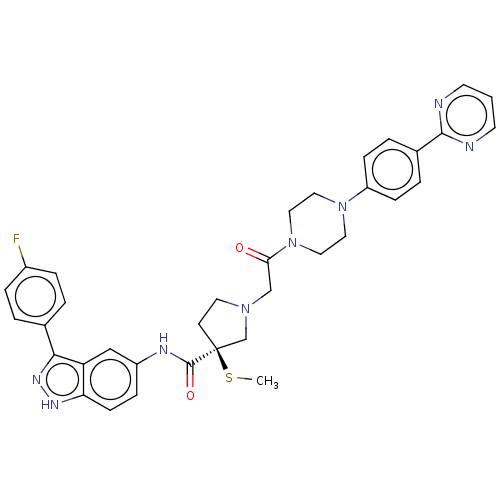
(CHEMBL4215228)Show SMILES CS[C@]1(CCN(CC(=O)N2CCN(CC2)c2ccc(cc2)-c2ncccn2)C1)C(=O)Nc1ccc2[nH]nc(-c3ccc(F)cc3)c2c1 |r| Show InChI InChI=1S/C35H35FN8O2S/c1-47-35(34(46)39-27-9-12-30-29(21-27)32(41-40-30)24-3-7-26(36)8-4-24)13-16-42(23-35)22-31(45)44-19-17-43(18-20-44)28-10-5-25(6-11-28)33-37-14-2-15-38-33/h2-12,14-15,21H,13,16-20,22-23H2,1H3,(H,39,46)(H,40,41)/t35-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50458807

(CHEMBL4216900)Show SMILES CCNc1ncc(s1)-c1ccc(cc1)C1=CCN(CC1)C(=O)CN1CC[C@@](C1)(SC)C(=O)Nc1ccc2[nH]nc(-c3ccnc(C)c3)c2c1 |r,t:16| Show InChI InChI=1S/C37H40N8O2S2/c1-4-38-36-40-21-32(49-36)27-7-5-25(6-8-27)26-12-16-45(17-13-26)33(46)22-44-18-14-37(23-44,48-3)35(47)41-29-9-10-31-30(20-29)34(43-42-31)28-11-15-39-24(2)19-28/h5-12,15,19-21H,4,13-14,16-18,22-23H2,1-3H3,(H,38,40)(H,41,47)(H,42,43)/t37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) using peptide substrate measured after 45 mins by IMAP assay |
ACS Med Chem Lett 9: 761-767 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00220
BindingDB Entry DOI: 10.7270/Q2HX1G9S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data