

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303320 ((S)-2-[(R)-3-[((R)-1-Benzyloxycarbonylamino-2-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50018849 (4-Cyclohexyl-1-{2-[hydroxy-(4-phenyl-butyl)-phosph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of ACE | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50393210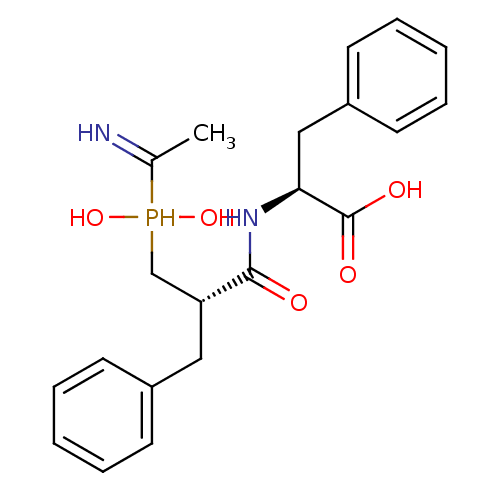 (CHEMBL1235787) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of APN | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50393211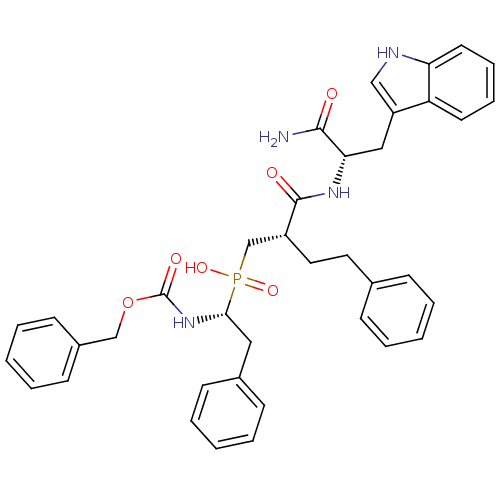 (CHEMBL2153737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of MMP-8 | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303327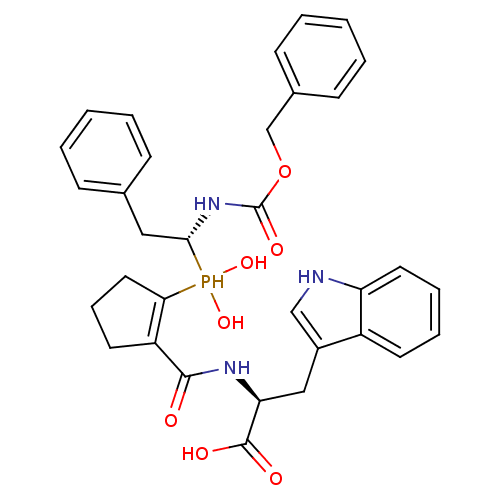 ((2S)-2-((1S,2S)-2-(((R)-1-(benzyloxycarbonylamino)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of human ACE C-terminal domain expressed in CHO cells after 90 mins by fluorescence assay | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Stromelysin-3 (Homo sapiens (Human)) | BDBM50393211 (CHEMBL2153737) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of MMP-11 | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50393213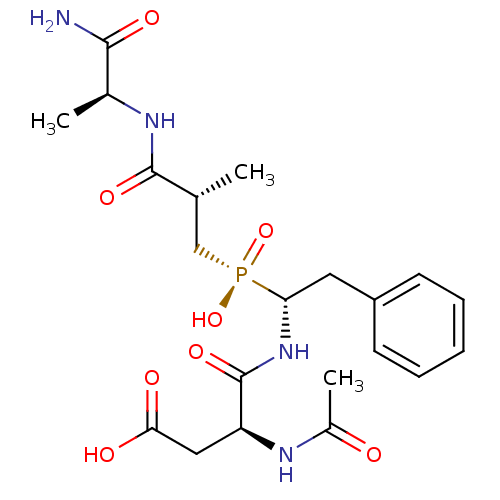 (CHEMBL1235767) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of human ACE N-terminal domain expressed in CHO cells after 90 mins by fluorescence assay | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50393211 (CHEMBL2153737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of MMP-9 | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135836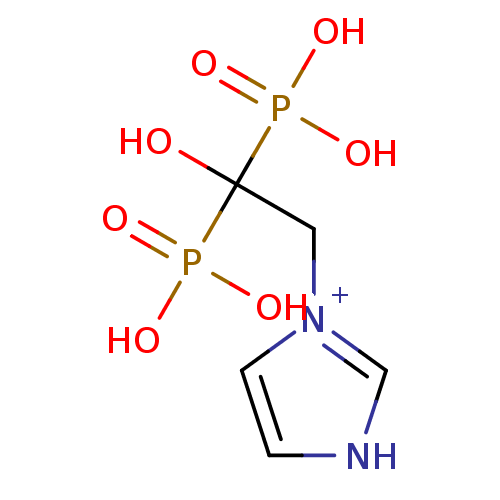 (1-(2-Hydroxy-2,2-bis-phosphono-ethyl)-3H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Leishmania major) | BDBM50098390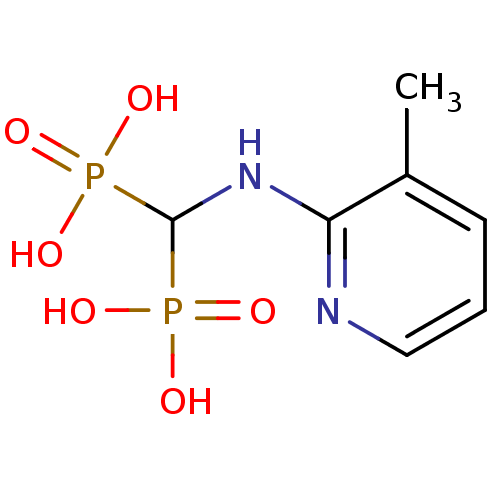 ((3-methylpyridin-2-ylamino)methylenediphosphonic a...) | PDB KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of Leishmania major FPPS | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098390 ((3-methylpyridin-2-ylamino)methylenediphosphonic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098378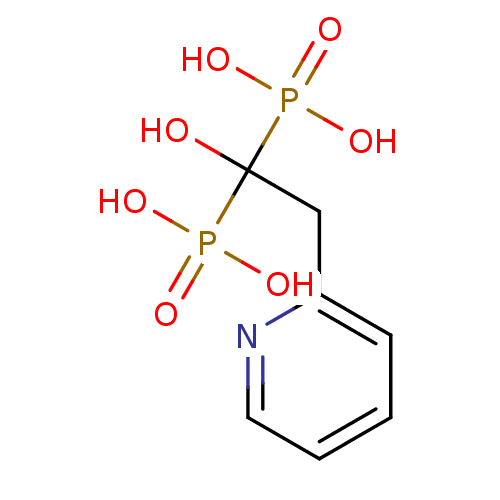 ((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135818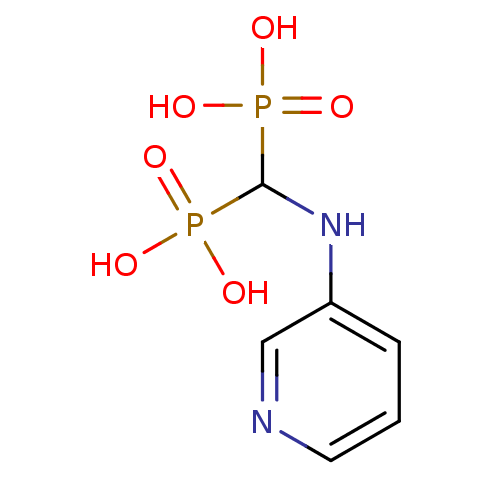 ((pyridin-3-ylamino)methylenediphosphonic acid | 3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115115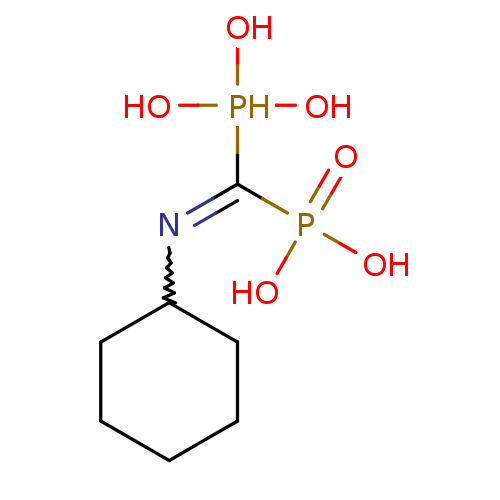 ((Cyclohexylamino-phosphono-methyl)-phosphonic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115106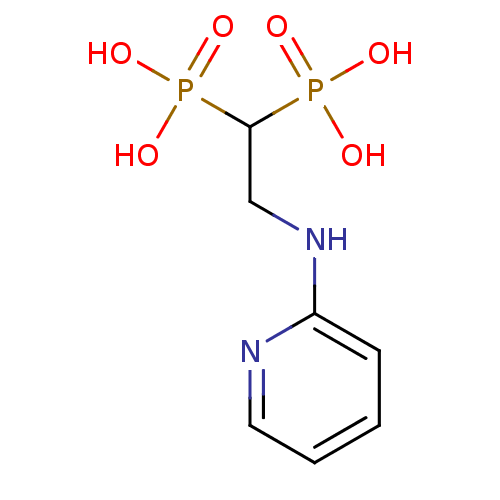 (2-(pyridin-2-ylamino)ethane-1,1-diyldiphosphonic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12576 (Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50113291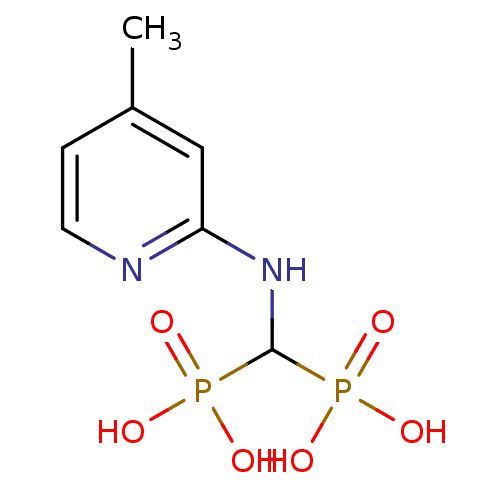 ((4-methylpyridin-2-ylamino)methylenediphosphonic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115104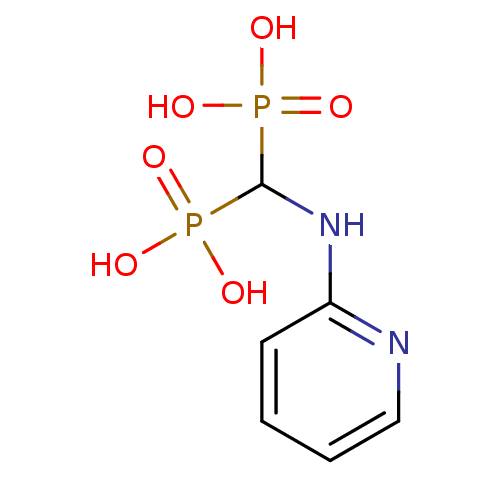 ((pyridin-2-ylamino)methylenediphosphonic acid | 2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50393211 (CHEMBL2153737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of MMP-2 | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135817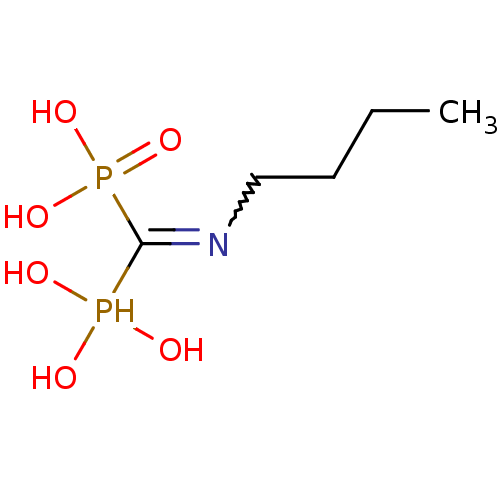 ((Bis-phosphono-methyl)-butyl-ammonium | (Butylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135821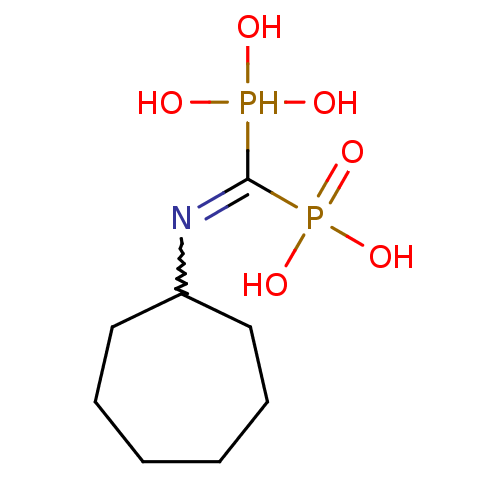 ((Bis-phosphono-methyl)-cycloheptyl-ammonium | (Cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115109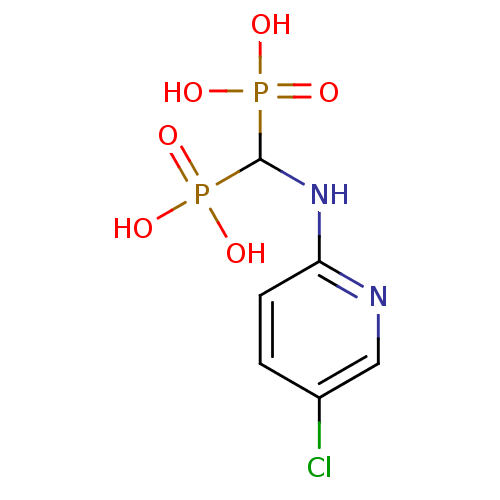 ((5-chloropyridin-2-ylamino)methylenediphosphonic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135839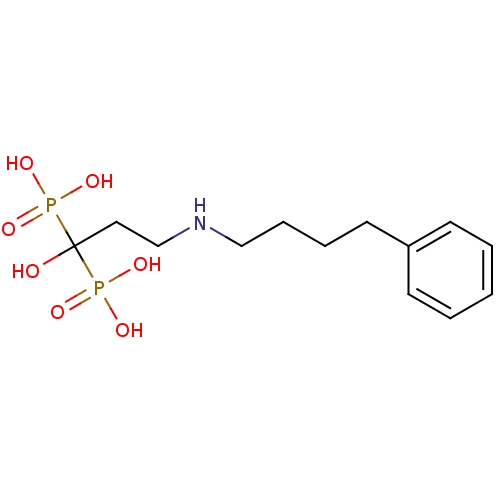 (CHEMBL55464 | [1-Hydroxy-3-(4-phenyl-butylamino)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50393212 (CHEMBL2153739) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of MMP-13 | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50117260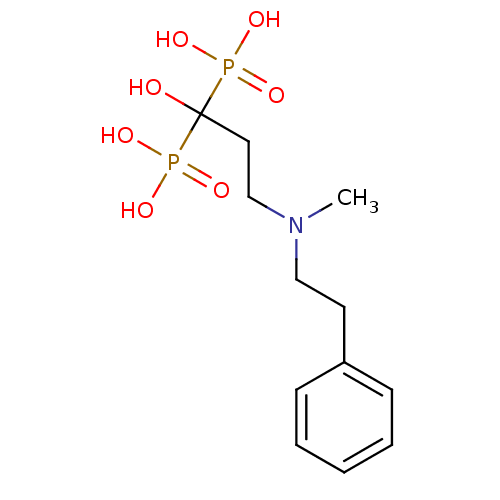 (1-hydroxy-3-(methyl(phenethyl)amino)propane-1,1-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50098389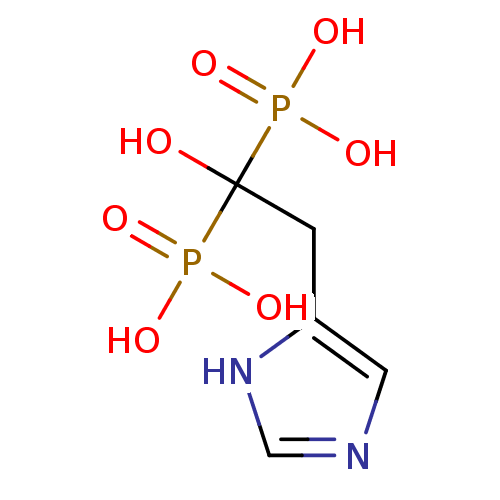 (1-hydroxy-2-(1H-imidazol-5-yl)ethane-1,1-diyldipho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50017478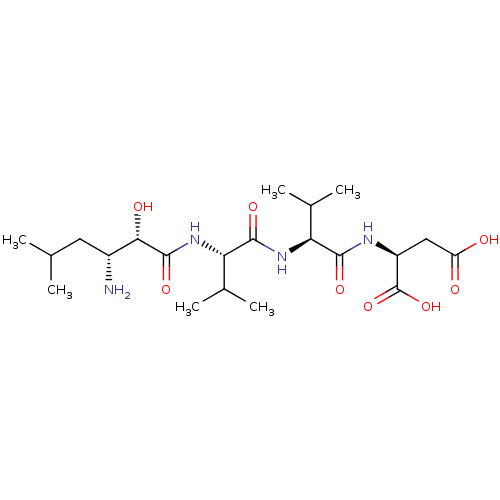 (Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValVal...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | MMDB Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115110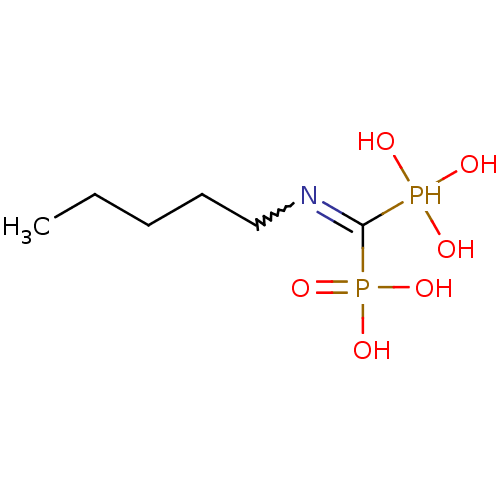 ((Pentylamino-phosphono-methyl)-phosphonic acid | (...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135833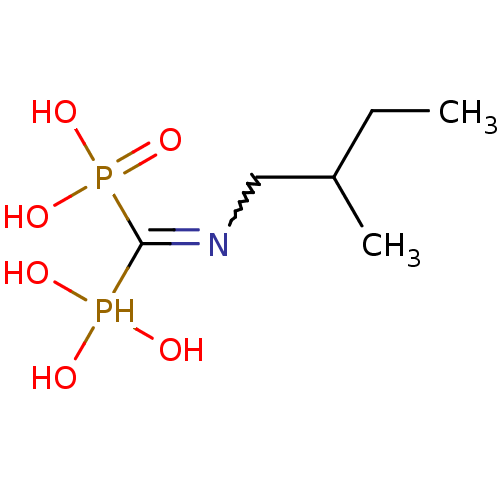 ((2-methylbutyl)aminomethylene-1,1-bisphosphonate |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50129681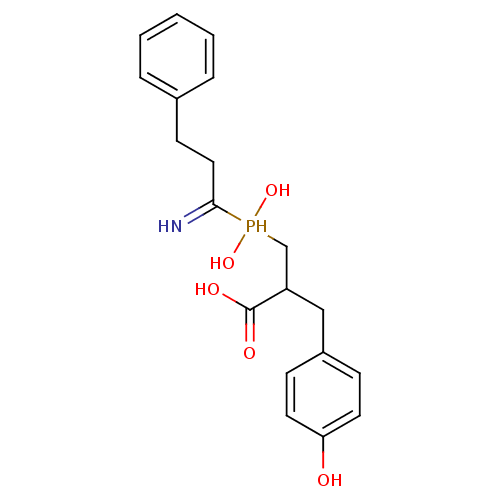 (3-[(1-Amino-3-phenyl-propyl)-hydroxy-phosphinoyl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity towards aminopeptidase N (APN) from pig kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135820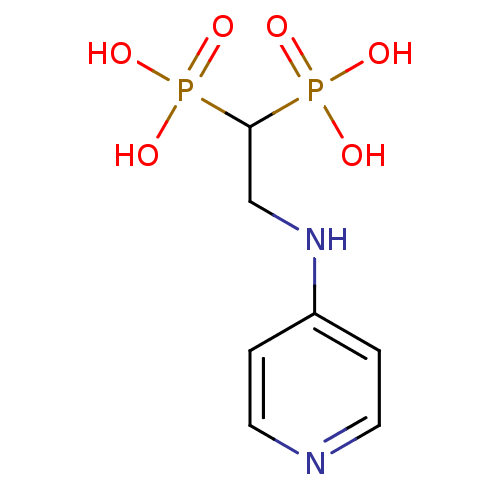 (2-(pyridin-4-ylamino)ethane-1,1-diyldiphosphonic a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135835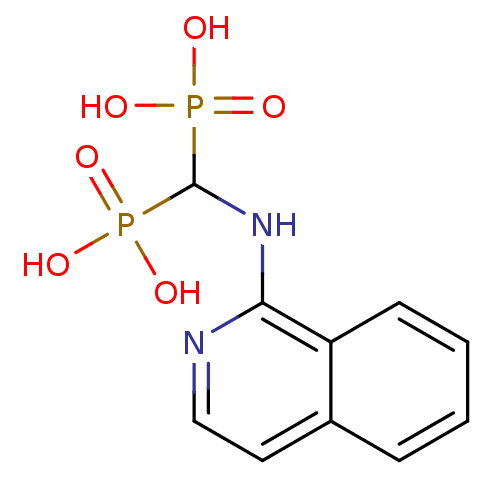 ((isoquinolin-1-ylamino)methylenediphosphonic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135831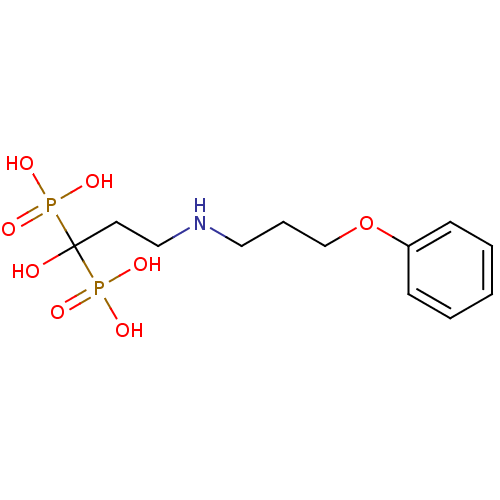 (CHEMBL316844 | [1-Hydroxy-3-(3-phenoxy-propylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50225458 ((2S)-3-[(R)-[(1S)-1-amino-3-phenylpropyl](hydroxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of pig kidney cytosolic leucine aminopeptidase | Bioorg Med Chem Lett 18: 1550-4 (2008) Article DOI: 10.1016/j.bmcl.2008.01.107 BindingDB Entry DOI: 10.7270/Q28K78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM25290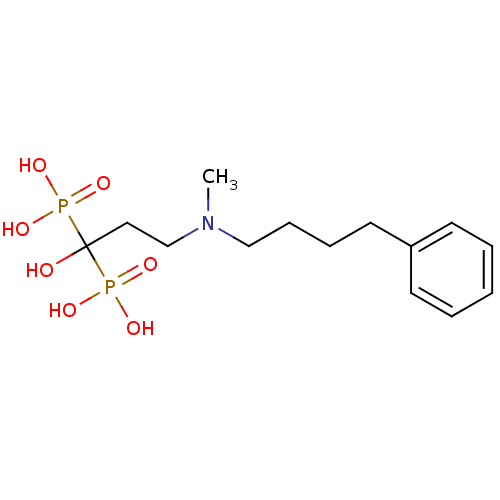 (CHEMBL56073 | bisphosphonate, 39 | {1-hydroxy-3-[m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM12577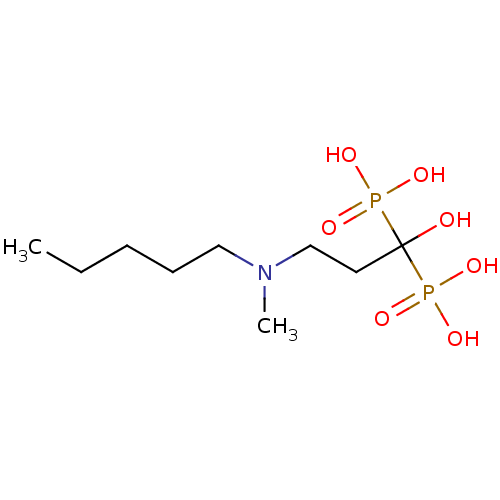 (Bisphosphonate 2 | CHEMBL997 | JMC515594 Compound ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50115112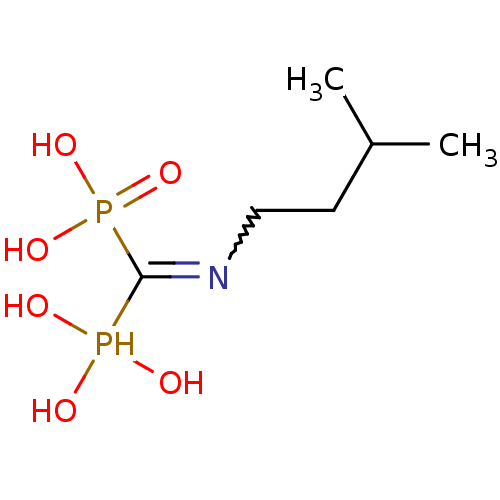 ((Bis-phosphono-methyl)-(3-methyl-butyl)-ammonium |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50117257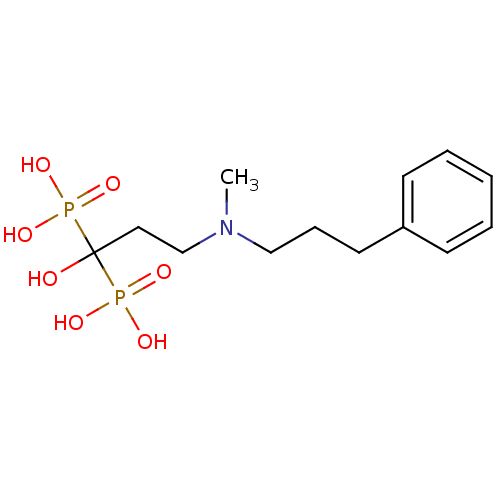 (1-hydroxy-3-(methyl(3-phenylpropyl)amino)propane-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135838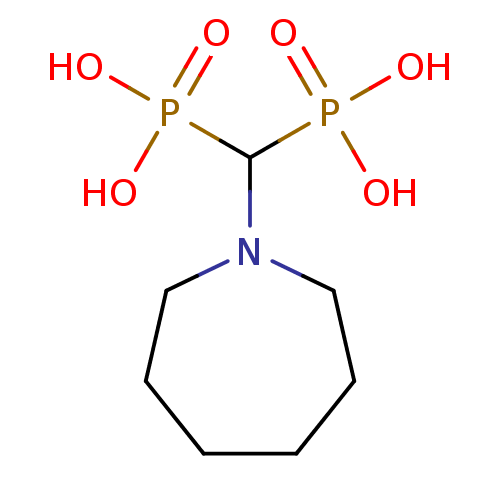 ((Azepan-1-yl-phosphono-methyl)-phosphonic acid | 1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135824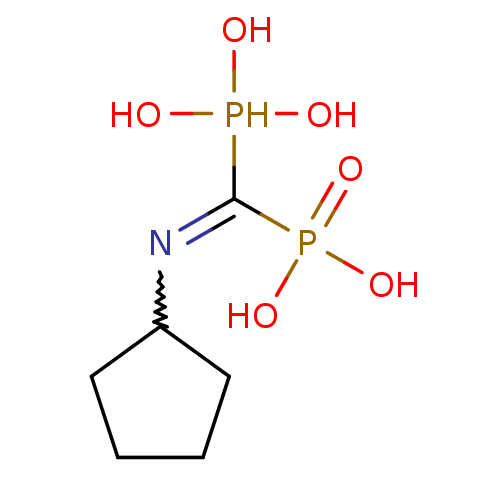 ((Bis-phosphono-methyl)-cyclopentyl-ammonium | (Cyc...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129685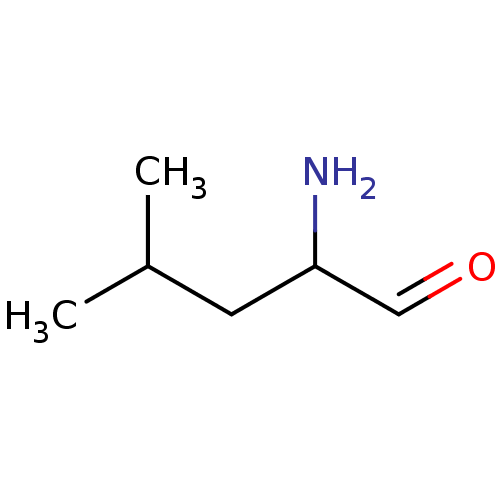 (2-Amino-4-methyl-pentanal | CHEMBL88476) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129683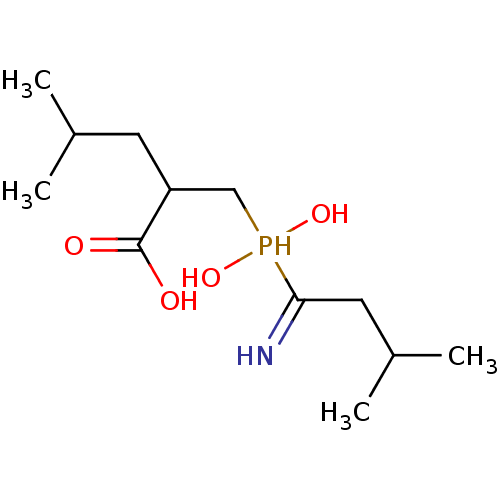 (2-[(1-Amino-3-methyl-butyl)-hydroxy-phosphinoylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129683 (2-[(1-Amino-3-methyl-butyl)-hydroxy-phosphinoylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Inhibition of LAP | J Med Chem 54: 5955-80 (2011) Article DOI: 10.1021/jm200587f BindingDB Entry DOI: 10.7270/Q2PV6MGF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129683 (2-[(1-Amino-3-methyl-butyl)-hydroxy-phosphinoylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129684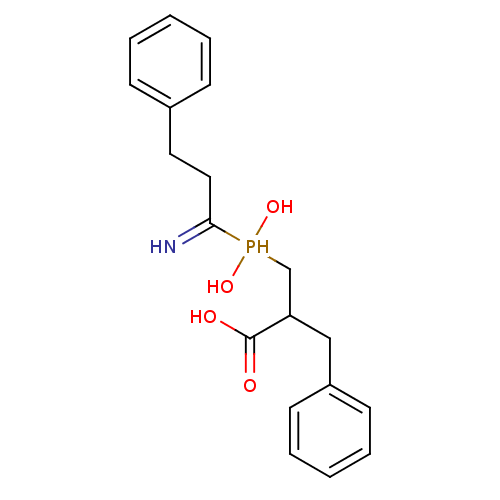 (3-[(1-amino-3-phenyl-propyl)-hydroxy-phosphinoyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129684 (3-[(1-amino-3-phenyl-propyl)-hydroxy-phosphinoyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroc£?aw University of Technology Curated by ChEMBL | Assay Description Inhibition of pig kidney cytosolic leucine aminopeptidase | Bioorg Med Chem Lett 18: 1550-4 (2008) Article DOI: 10.1016/j.bmcl.2008.01.107 BindingDB Entry DOI: 10.7270/Q28K78TP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129681 (3-[(1-Amino-3-phenyl-propyl)-hydroxy-phosphinoyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytosol aminopeptidase (Homo sapiens (Human)) | BDBM50129682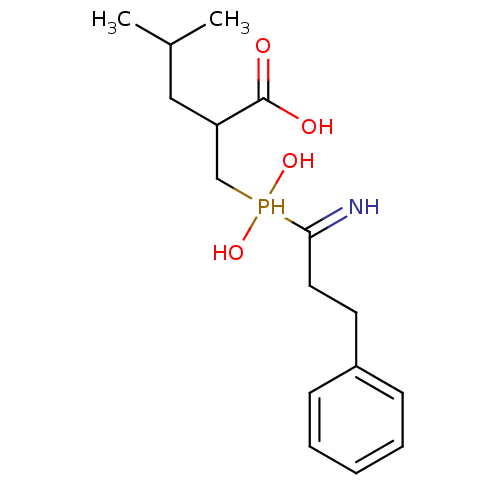 (2-[(1-Amino-3-phenyl-propyl)-hydroxy-phosphinoylme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wroclaw University of Technology Curated by ChEMBL | Assay Description Binding affinity for cytosolic leucine aminopeptidase (LAP) from porcine kidney | J Med Chem 46: 2641-55 (2003) Article DOI: 10.1021/jm030795v BindingDB Entry DOI: 10.7270/Q27P903F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Farnesyl pyrophosphate synthase (Homo sapiens (Human)) | BDBM50135823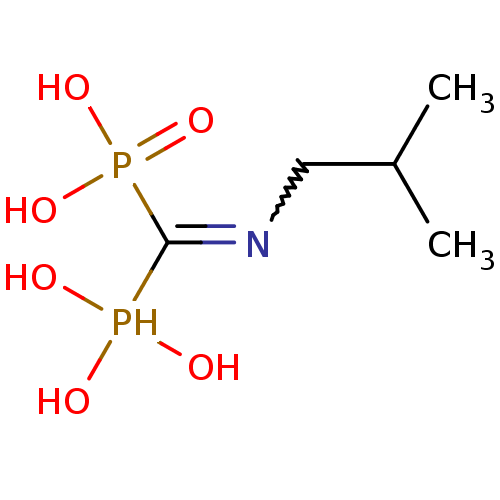 ((Isobutylamino-phosphono-methyl)-phosphonic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Urbana-Champaign Curated by ChEMBL | Assay Description Binding affinity towards farnesyl Pyrophosphate Synthase using [14C]- isopentenyl pyrophosphate as radioligand | J Med Chem 46: 5171-83 (2003) Article DOI: 10.1021/jm0302344 BindingDB Entry DOI: 10.7270/Q24M93Z8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 425 total ) | Next | Last >> |