

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267028 (CHEMBL4073525) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267031 (CHEMBL4079930) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267009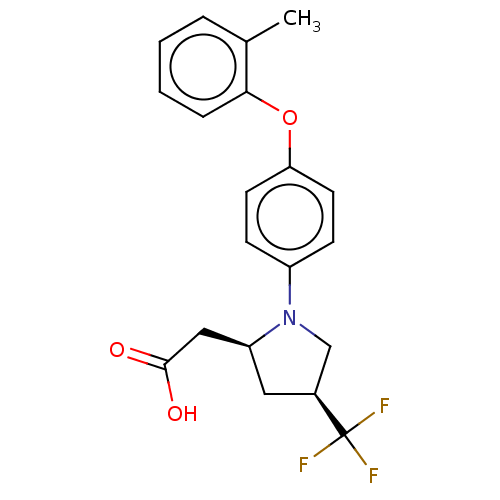 (CHEMBL4089171) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267029 (CHEMBL4069191) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267010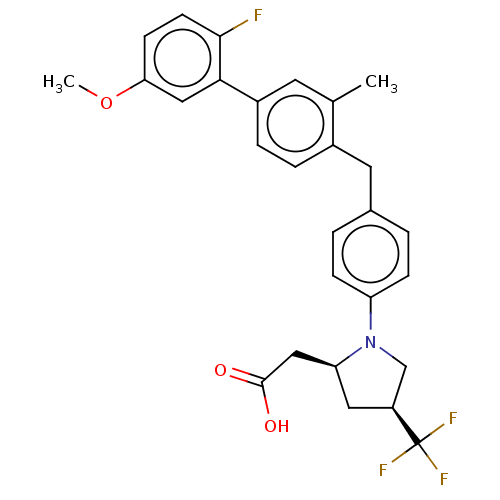 (CHEMBL4082395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267117 (CHEMBL4060499) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267030 (CHEMBL4090240) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267044 (CHEMBL4069764) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267039 (CHEMBL4090766) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267049 (CHEMBL4095599) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267035 (CHEMBL4071899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267038 (CHEMBL4061093) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267046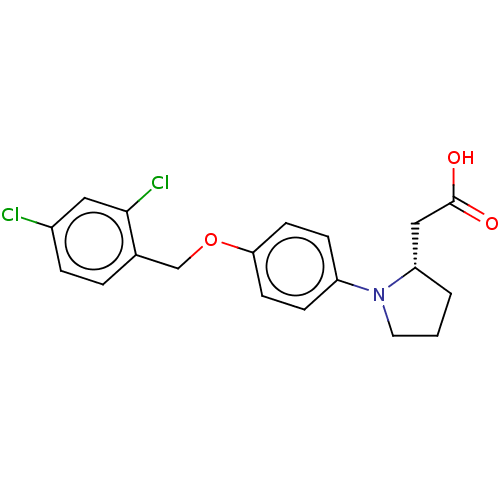 (CHEMBL4103729) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Agonist activity at human GPR40 expressed in CHO-A12 cells assessed as increase in intracellular calcium flux measured for 100 secs by FLIPR assay | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267066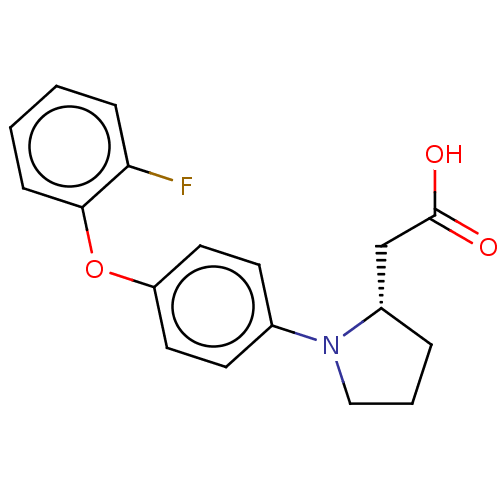 (CHEMBL4067052) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267118 (CHEMBL4096887) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267059 (CHEMBL4091552) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267047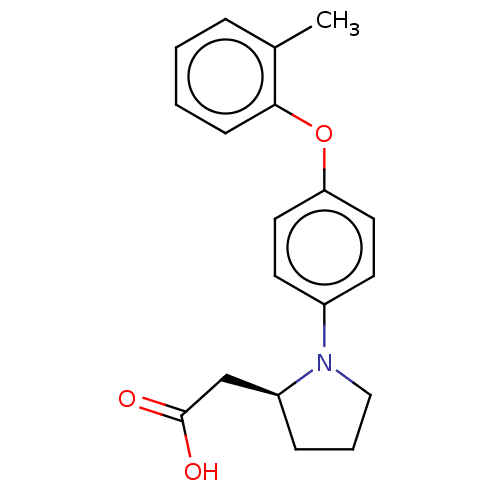 (CHEMBL4078852) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267064 (CHEMBL4070703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267045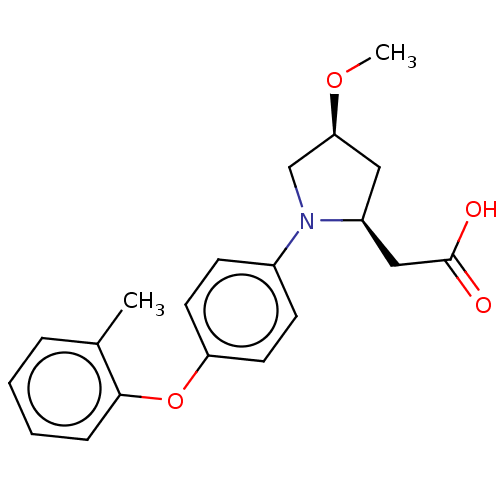 (CHEMBL4063889) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267048 (CHEMBL4075190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267021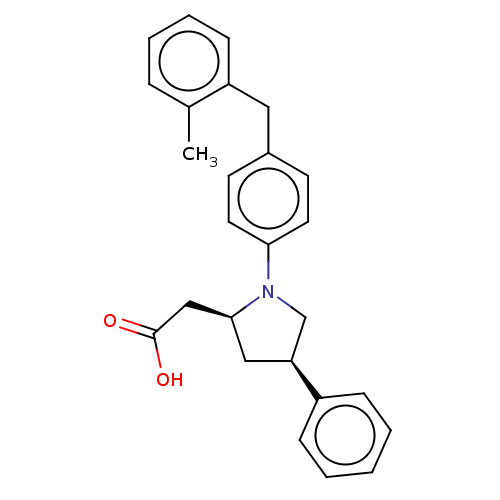 (CHEMBL4091669) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Free fatty acid receptor 1 (Homo sapiens (Human)) | BDBM50267113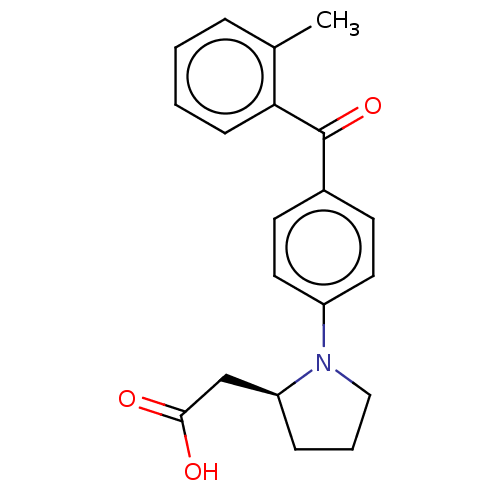 (CHEMBL4105565) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Binding affinity to human GPR40 expressed in HEK293 cell membranes after 1 hr by radioligand displacement based scintillation counting method | J Med Chem 60: 1417-1431 (2017) Article DOI: 10.1021/acs.jmedchem.6b01559 BindingDB Entry DOI: 10.7270/Q25X2CD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322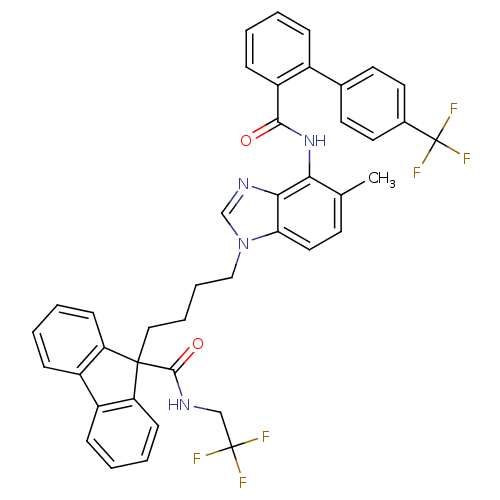 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326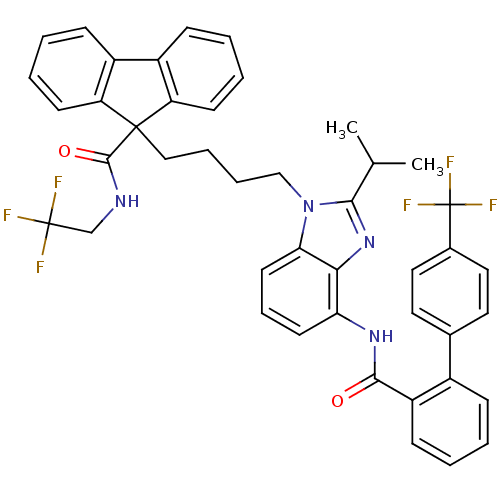 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324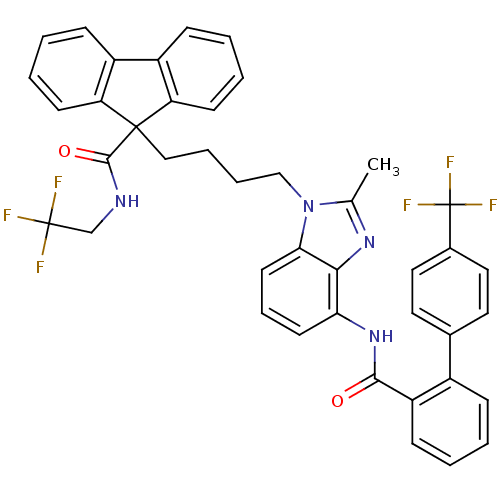 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098325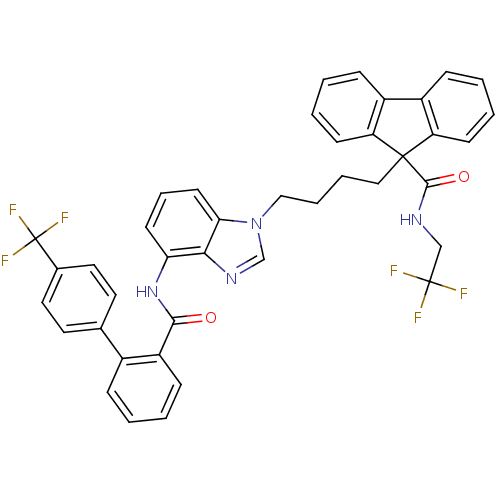 (9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50031845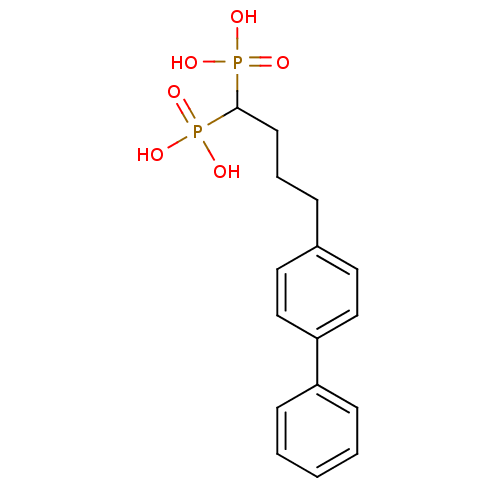 ((4-Biphenyl-4-yl-1-phosphono-butyl)-phosphonic aci...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098320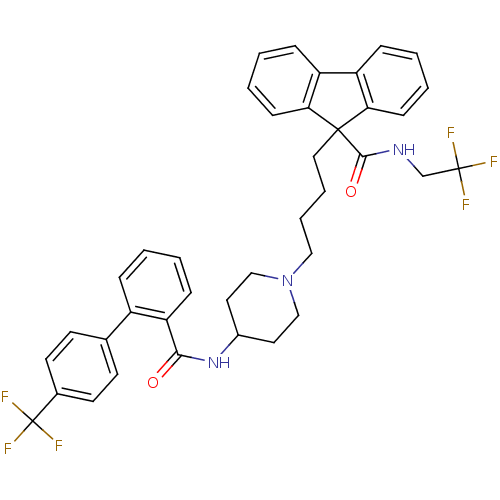 (9-(4-{4-[(4''-Trifluoromethyl-biphenyl-2-carbonyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human microsomal triglyceride transfer protein in HepG2 cells using apoB secretion assay | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098324 (9-(4-{2-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098321 (9-(4-{2,5-Dimethyl-4-[(4'-trifluoromethyl-biphenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049232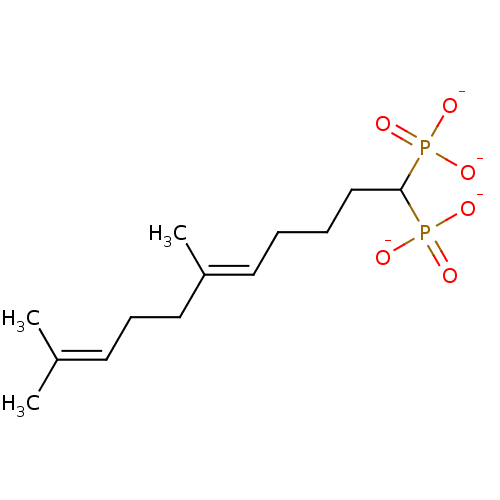 (CHEMBL348349 | Tetrasodium salt of 4-(4'-Methyl-bi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098322 (9-(4-{5-Methyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098323 (9-(4-{2-Propyl-4-[(4'-trifluoromethyl-biphenyl-2-c...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356439 (CHEMBL1911707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50356461 (CHEMBL1911688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356449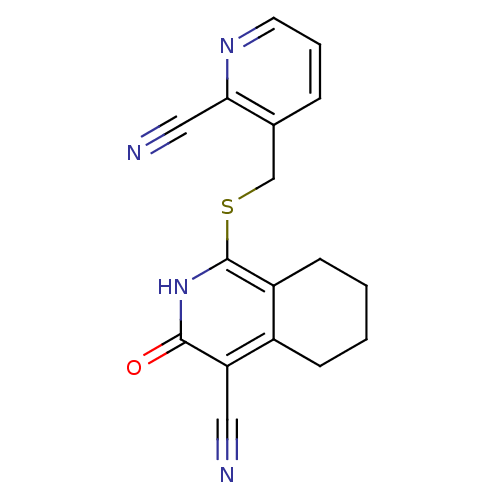 (CHEMBL1911697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356451 (CHEMBL1911695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356461 (CHEMBL1911688) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356444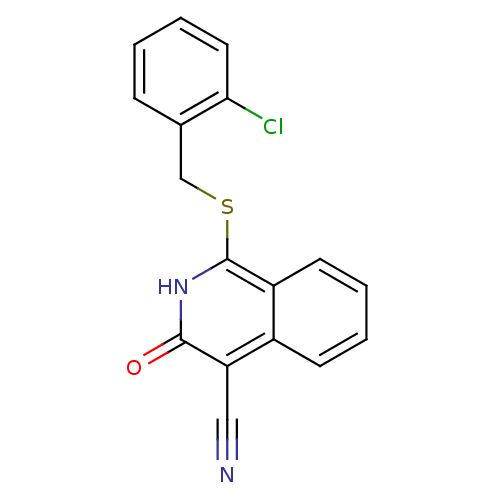 (CHEMBL1911702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098326 (9-(4-{2-Isopropyl-4-[(4'-trifluoromethyl-biphenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein, (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50356435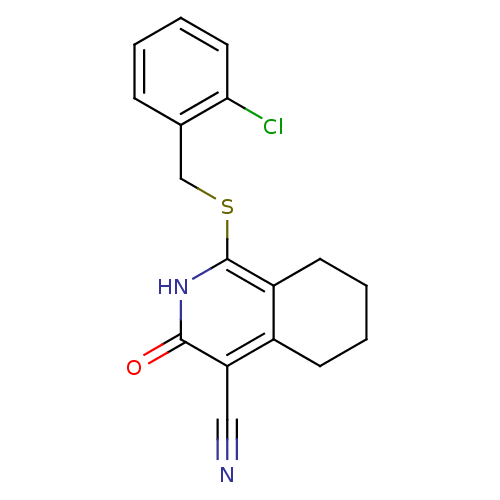 (CHEMBL1911585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microsomal triglyceride transfer protein large subunit (Homo sapiens (Human)) | BDBM50098325 (9-(4-{4-[(4'-Trifluoromethyl-biphenyl-2-carbonyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description In vitro inhibition of human Microsomal Triglyceride Transfer Protein (triglyceride transfer assay) | J Med Chem 44: 851-6 (2001) BindingDB Entry DOI: 10.7270/Q2N015S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356435 (CHEMBL1911585) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356448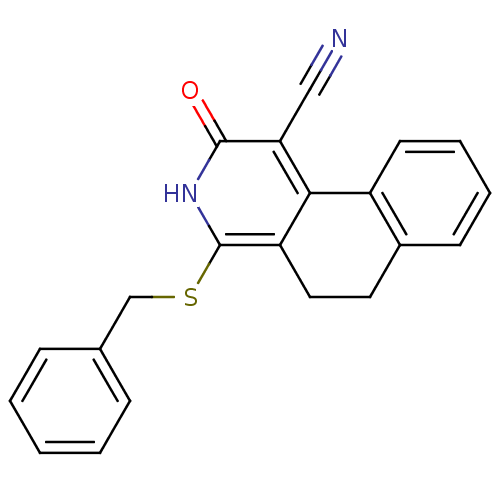 (CHEMBL1911698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Squalene synthase (Rattus norvegicus) | BDBM50049227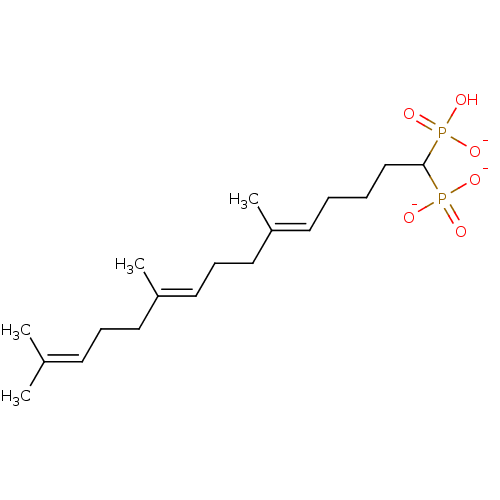 (CHEMBL158707 | Trisodium salt of [1-(Dimethoxy-pho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against rat liver microsomal squalene synthase was determined using [3H]-FPP as radioligand | J Med Chem 39: 657-60 (1996) Article DOI: 10.1021/jm9507340 BindingDB Entry DOI: 10.7270/Q2N29W1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50356452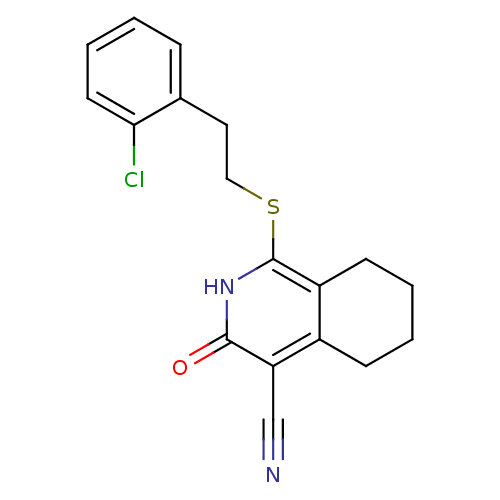 (CHEMBL1911694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50356451 (CHEMBL1911695) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50356462 (CHEMBL1911592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of mouse 11beta-HSD1 | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50356442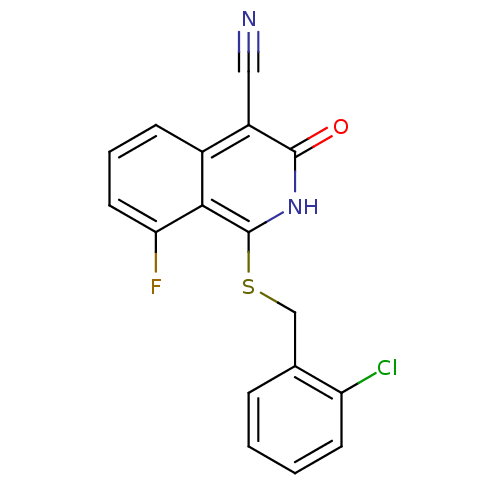 (CHEMBL1911704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Inhibition of human 11beta-HSD1 expressed in HEK293 cells assessed as conversion of [3H]-cortisone to [3H]-cortisol by scintillation plate reader | Bioorg Med Chem Lett 21: 6693-8 (2011) Article DOI: 10.1016/j.bmcl.2011.09.058 BindingDB Entry DOI: 10.7270/Q2FN16M4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 587 total ) | Next | Last >> |