 Found 1300 hits with Last Name = 'lem' and Initial = 'g'
Found 1300 hits with Last Name = 'lem' and Initial = 'g' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50118719
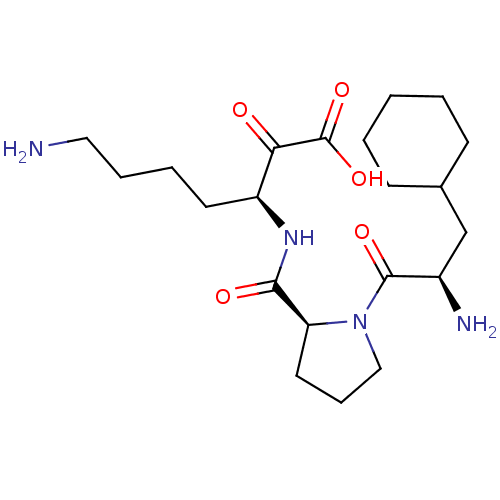
(7-Amino-3-{[1-(2-amino-3-cyclohexyl-propionyl)-pyr...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)C(O)=O Show InChI InChI=1S/C21H36N4O5/c22-11-5-4-9-16(18(26)21(29)30)24-19(27)17-10-6-12-25(17)20(28)15(23)13-14-7-2-1-3-8-14/h14-17H,1-13,22-23H2,(H,24,27)(H,29,30)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118730
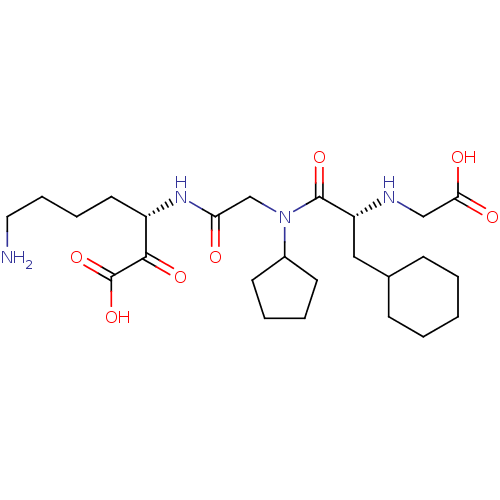
(7-Amino-3-(2-{[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CCCC1)C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C25H42N4O7/c26-13-7-6-12-19(23(33)25(35)36)28-21(30)16-29(18-10-4-5-11-18)24(34)20(27-15-22(31)32)14-17-8-2-1-3-9-17/h17-20,27H,1-16,26H2,(H,28,30)(H,31,32)(H,35,36)/t19-,20+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118739
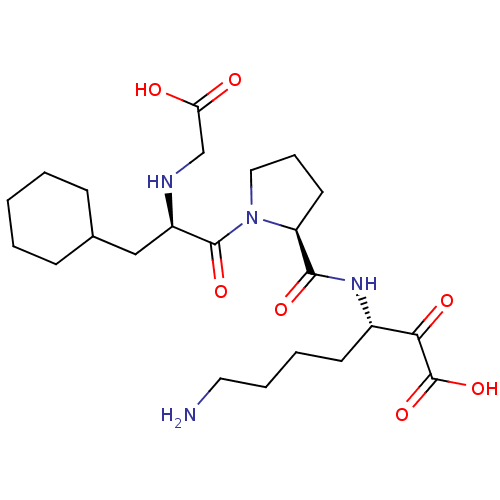
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C23H38N4O7/c24-11-5-4-9-16(20(30)23(33)34)26-21(31)18-10-6-12-27(18)22(32)17(25-14-19(28)29)13-15-7-2-1-3-8-15/h15-18,25H,1-14,24H2,(H,26,31)(H,28,29)(H,33,34)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118728
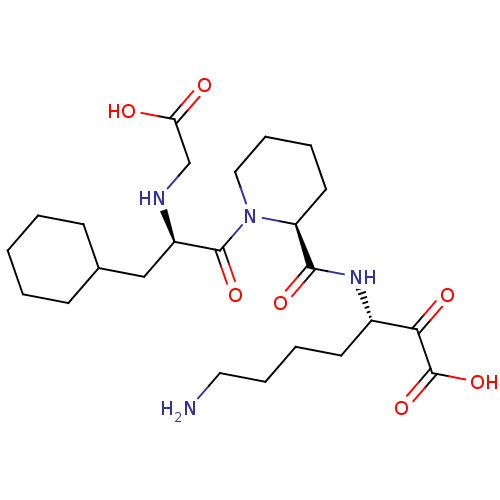
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C24H40N4O7/c25-12-6-4-10-17(21(31)24(34)35)27-22(32)19-11-5-7-13-28(19)23(33)18(26-15-20(29)30)14-16-8-2-1-3-9-16/h16-19,26H,1-15,25H2,(H,27,32)(H,29,30)(H,34,35)/t17-,18+,19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118732
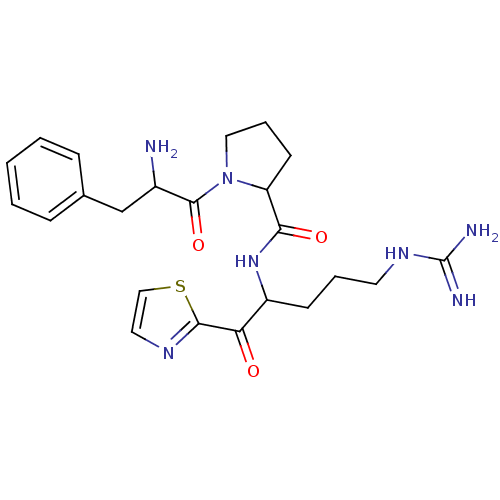
(1-(2-Amino-3-phenyl-propionyl)-pyrrolidine-2-carbo...)Show SMILES NC(Cc1ccccc1)C(=O)N1CCCC1C(=O)NC(CCCNC(N)=N)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N7O3S/c24-16(14-15-6-2-1-3-7-15)22(33)30-12-5-9-18(30)20(32)29-17(8-4-10-28-23(25)26)19(31)21-27-11-13-34-21/h1-3,6-7,11,13,16-18H,4-5,8-10,12,14,24H2,(H,29,32)(H4,25,26,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118729
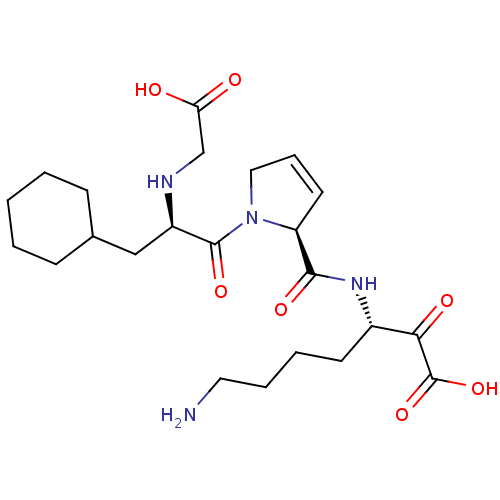
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1C=CCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O |c:10| Show InChI InChI=1S/C23H36N4O7/c24-11-5-4-9-16(20(30)23(33)34)26-21(31)18-10-6-12-27(18)22(32)17(25-14-19(28)29)13-15-7-2-1-3-8-15/h6,10,15-18,25H,1-5,7-9,11-14,24H2,(H,26,31)(H,28,29)(H,33,34)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118731
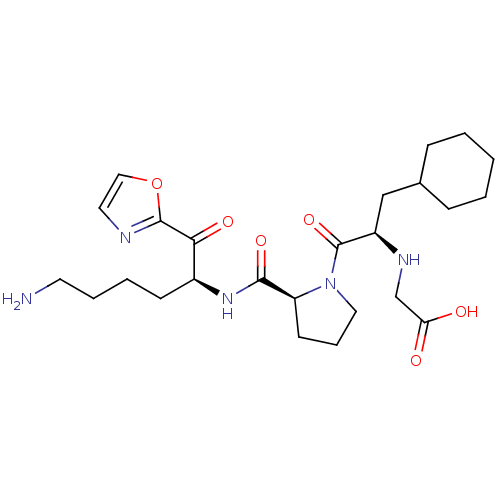
((2-{2-[5-Amino-1-(oxazole-2-carbonyl)-pentylcarbam...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)c1ncco1 Show InChI InChI=1S/C25H39N5O6/c26-11-5-4-9-18(22(33)24-27-12-14-36-24)29-23(34)20-10-6-13-30(20)25(35)19(28-16-21(31)32)15-17-7-2-1-3-8-17/h12,14,17-20,28H,1-11,13,15-16,26H2,(H,29,34)(H,31,32)/t18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118735
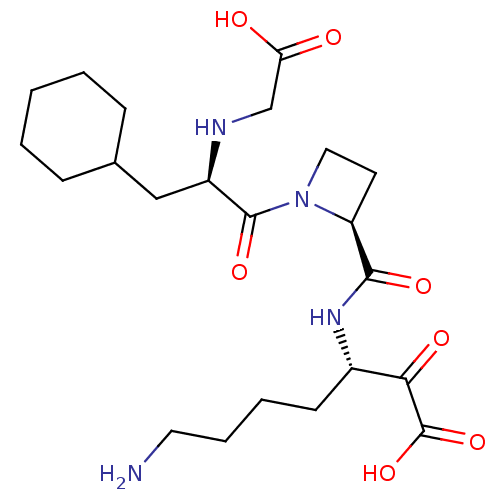
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C22H36N4O7/c23-10-5-4-8-15(19(29)22(32)33)25-20(30)17-9-11-26(17)21(31)16(24-13-18(27)28)12-14-6-2-1-3-7-14/h14-17,24H,1-13,23H2,(H,25,30)(H,27,28)(H,32,33)/t15-,16+,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313242
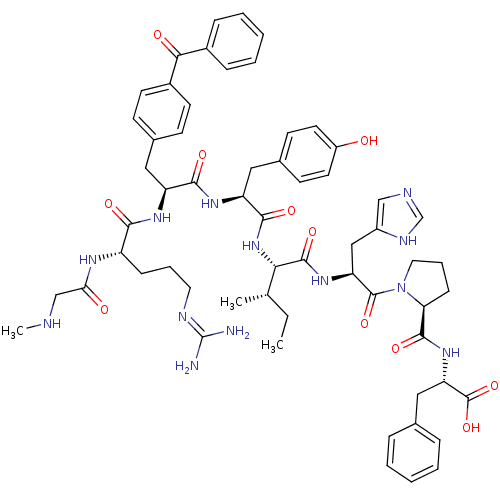
(CHEMBL1076603 | [Sar1,Bpa3]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,56.58,69.74,20.38,wD:2.2,39.49,73.77,8.17,(34.67,-36.98,;34.66,-38.57,;35.98,-39.34,;37.32,-38.56,;35.98,-40.87,;34.66,-41.63,;33.31,-40.85,;33.31,-39.33,;31.98,-41.61,;31.98,-43.17,;33.31,-43.93,;34.64,-43.18,;35.97,-43.96,;35.95,-45.5,;37.29,-46.29,;34.62,-46.26,;33.3,-45.48,;30.65,-40.84,;29.32,-41.62,;29.32,-43.17,;27.98,-40.84,;27.98,-39.31,;27.19,-37.97,;27.96,-36.62,;27.17,-35.29,;25.62,-35.3,;24.86,-36.66,;25.65,-37.99,;24.84,-33.97,;25.6,-32.63,;23.3,-33.98,;22.53,-32.66,;20.99,-32.67,;20.23,-34.01,;21.02,-35.34,;22.55,-35.32,;26.65,-41.61,;25.31,-40.84,;25.31,-39.31,;23.98,-41.61,;23.98,-43.16,;25.31,-43.92,;25.31,-45.46,;26.65,-46.23,;26.66,-47.77,;27.99,-48.53,;25.32,-48.54,;22.65,-40.82,;21.32,-41.59,;21.32,-43.14,;19.99,-40.83,;18.66,-41.59,;17.33,-40.82,;37.32,-41.64,;37.32,-43.19,;38.64,-40.88,;39.99,-41.65,;41.31,-40.89,;41.31,-39.36,;40.09,-38.45,;40.57,-36.99,;42.11,-37,;42.57,-38.47,;39.99,-43.21,;38.64,-43.98,;41.31,-43.97,;42.89,-43.43,;43.91,-44.76,;42.97,-46.14,;41.57,-45.46,;40.34,-46.39,;38.96,-45.72,;40.46,-47.92,;39.18,-48.77,;37.79,-48.1,;36.52,-48.95,;36.62,-50.49,;35.35,-51.35,;33.97,-50.68,;33.86,-49.14,;35.13,-48.28,;39.28,-50.31,;38.01,-51.17,;40.67,-50.98,)| Show InChI InChI=1S/C60H75N13O11/c1-4-36(2)51(57(81)70-47(32-42-33-64-35-66-42)58(82)73-28-12-18-49(73)56(80)71-48(59(83)84)31-37-13-7-5-8-14-37)72-55(79)46(30-39-21-25-43(74)26-22-39)69-54(78)45(29-38-19-23-41(24-20-38)52(76)40-15-9-6-10-16-40)68-53(77)44(67-50(75)34-63-3)17-11-27-65-60(61)62/h5-10,13-16,19-26,33,35-36,44-49,51,63,74H,4,11-12,17-18,27-32,34H2,1-3H3,(H,64,66)(H,67,75)(H,68,77)(H,69,78)(H,70,81)(H,71,80)(H,72,79)(H,83,84)(H4,61,62,65)/t36-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313242

(CHEMBL1076603 | [Sar1,Bpa3]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,56.58,69.74,20.38,wD:2.2,39.49,73.77,8.17,(34.67,-36.98,;34.66,-38.57,;35.98,-39.34,;37.32,-38.56,;35.98,-40.87,;34.66,-41.63,;33.31,-40.85,;33.31,-39.33,;31.98,-41.61,;31.98,-43.17,;33.31,-43.93,;34.64,-43.18,;35.97,-43.96,;35.95,-45.5,;37.29,-46.29,;34.62,-46.26,;33.3,-45.48,;30.65,-40.84,;29.32,-41.62,;29.32,-43.17,;27.98,-40.84,;27.98,-39.31,;27.19,-37.97,;27.96,-36.62,;27.17,-35.29,;25.62,-35.3,;24.86,-36.66,;25.65,-37.99,;24.84,-33.97,;25.6,-32.63,;23.3,-33.98,;22.53,-32.66,;20.99,-32.67,;20.23,-34.01,;21.02,-35.34,;22.55,-35.32,;26.65,-41.61,;25.31,-40.84,;25.31,-39.31,;23.98,-41.61,;23.98,-43.16,;25.31,-43.92,;25.31,-45.46,;26.65,-46.23,;26.66,-47.77,;27.99,-48.53,;25.32,-48.54,;22.65,-40.82,;21.32,-41.59,;21.32,-43.14,;19.99,-40.83,;18.66,-41.59,;17.33,-40.82,;37.32,-41.64,;37.32,-43.19,;38.64,-40.88,;39.99,-41.65,;41.31,-40.89,;41.31,-39.36,;40.09,-38.45,;40.57,-36.99,;42.11,-37,;42.57,-38.47,;39.99,-43.21,;38.64,-43.98,;41.31,-43.97,;42.89,-43.43,;43.91,-44.76,;42.97,-46.14,;41.57,-45.46,;40.34,-46.39,;38.96,-45.72,;40.46,-47.92,;39.18,-48.77,;37.79,-48.1,;36.52,-48.95,;36.62,-50.49,;35.35,-51.35,;33.97,-50.68,;33.86,-49.14,;35.13,-48.28,;39.28,-50.31,;38.01,-51.17,;40.67,-50.98,)| Show InChI InChI=1S/C60H75N13O11/c1-4-36(2)51(57(81)70-47(32-42-33-64-35-66-42)58(82)73-28-12-18-49(73)56(80)71-48(59(83)84)31-37-13-7-5-8-14-37)72-55(79)46(30-39-21-25-43(74)26-22-39)69-54(78)45(29-38-19-23-41(24-20-38)52(76)40-15-9-6-10-16-40)68-53(77)44(67-50(75)34-63-3)17-11-27-65-60(61)62/h5-10,13-16,19-26,33,35-36,44-49,51,63,74H,4,11-12,17-18,27-32,34H2,1-3H3,(H,64,66)(H,67,75)(H,68,77)(H,69,78)(H,70,81)(H,71,80)(H,72,79)(H,83,84)(H4,61,62,65)/t36-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313247

(CHEMBL1076632 | [Sar1,Bpa8]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(cc1)C(=O)c1ccccc1)C(O)=O |r,wU:44.44,57.60,4.4,20.21,wD:2.2,24.32,8.17,61.63,(38.66,-15.79,;38.59,-17.33,;39.9,-18.15,;41.26,-17.44,;39.84,-19.68,;38.49,-20.39,;37.17,-19.56,;37.23,-18.03,;35.82,-20.27,;35.76,-21.82,;37.05,-22.64,;38.41,-21.94,;39.71,-22.77,;39.63,-24.31,;40.93,-25.15,;38.27,-25.02,;36.97,-24.19,;34.51,-19.45,;33.15,-20.17,;33.09,-21.73,;31.84,-19.34,;30.47,-20.06,;29.17,-19.24,;29.23,-17.71,;27.81,-19.95,;27.75,-21.5,;29.05,-22.32,;28.99,-23.86,;30.3,-24.67,;30.24,-26.21,;31.55,-27.03,;28.88,-26.93,;26.52,-19.12,;25.16,-19.84,;25.1,-21.38,;23.85,-19.02,;22.5,-19.73,;21.2,-18.91,;31.9,-17.81,;33.26,-17.1,;30.59,-16.99,;41.15,-20.51,;41.09,-22.06,;42.5,-19.8,;43.86,-20.56,;45.17,-19.78,;45.16,-18.24,;46.4,-17.34,;45.91,-15.88,;44.37,-15.89,;43.91,-17.36,;43.87,-22.12,;42.53,-22.91,;45.21,-22.86,;46.59,-22.21,;47.62,-23.33,;46.89,-24.66,;45.39,-24.37,;44.12,-25.24,;42.77,-24.5,;44.15,-26.78,;45.26,-27.84,;44.88,-29.33,;45.98,-30.41,;45.6,-31.9,;46.71,-32.97,;48.19,-32.55,;48.57,-31.06,;47.47,-29.99,;49.3,-33.63,;50.78,-33.21,;48.92,-35.12,;47.43,-35.53,;47.06,-37.03,;48.16,-38.1,;49.65,-37.68,;50.02,-36.18,;46.74,-27.42,;47.85,-28.5,;47.12,-25.93,)| Show InChI InChI=1S/C56H75N13O11/c1-6-33(4)47(68-50(74)41(26-35-18-22-39(70)23-19-35)64-52(76)46(32(2)3)67-49(73)40(63-45(71)30-59-5)14-10-24-61-56(57)58)53(77)65-42(28-38-29-60-31-62-38)54(78)69-25-11-15-44(69)51(75)66-43(55(79)80)27-34-16-20-37(21-17-34)48(72)36-12-8-7-9-13-36/h7-9,12-13,16-23,29,31-33,40-44,46-47,59,70H,6,10-11,14-15,24-28,30H2,1-5H3,(H,60,62)(H,63,71)(H,64,76)(H,65,77)(H,66,75)(H,67,73)(H,68,74)(H,79,80)(H4,57,58,61)/t33-,40-,41-,42-,43-,44-,46-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313247

(CHEMBL1076632 | [Sar1,Bpa8]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(cc1)C(=O)c1ccccc1)C(O)=O |r,wU:44.44,57.60,4.4,20.21,wD:2.2,24.32,8.17,61.63,(38.66,-15.79,;38.59,-17.33,;39.9,-18.15,;41.26,-17.44,;39.84,-19.68,;38.49,-20.39,;37.17,-19.56,;37.23,-18.03,;35.82,-20.27,;35.76,-21.82,;37.05,-22.64,;38.41,-21.94,;39.71,-22.77,;39.63,-24.31,;40.93,-25.15,;38.27,-25.02,;36.97,-24.19,;34.51,-19.45,;33.15,-20.17,;33.09,-21.73,;31.84,-19.34,;30.47,-20.06,;29.17,-19.24,;29.23,-17.71,;27.81,-19.95,;27.75,-21.5,;29.05,-22.32,;28.99,-23.86,;30.3,-24.67,;30.24,-26.21,;31.55,-27.03,;28.88,-26.93,;26.52,-19.12,;25.16,-19.84,;25.1,-21.38,;23.85,-19.02,;22.5,-19.73,;21.2,-18.91,;31.9,-17.81,;33.26,-17.1,;30.59,-16.99,;41.15,-20.51,;41.09,-22.06,;42.5,-19.8,;43.86,-20.56,;45.17,-19.78,;45.16,-18.24,;46.4,-17.34,;45.91,-15.88,;44.37,-15.89,;43.91,-17.36,;43.87,-22.12,;42.53,-22.91,;45.21,-22.86,;46.59,-22.21,;47.62,-23.33,;46.89,-24.66,;45.39,-24.37,;44.12,-25.24,;42.77,-24.5,;44.15,-26.78,;45.26,-27.84,;44.88,-29.33,;45.98,-30.41,;45.6,-31.9,;46.71,-32.97,;48.19,-32.55,;48.57,-31.06,;47.47,-29.99,;49.3,-33.63,;50.78,-33.21,;48.92,-35.12,;47.43,-35.53,;47.06,-37.03,;48.16,-38.1,;49.65,-37.68,;50.02,-36.18,;46.74,-27.42,;47.85,-28.5,;47.12,-25.93,)| Show InChI InChI=1S/C56H75N13O11/c1-6-33(4)47(68-50(74)41(26-35-18-22-39(70)23-19-35)64-52(76)46(32(2)3)67-49(73)40(63-45(71)30-59-5)14-10-24-61-56(57)58)53(77)65-42(28-38-29-60-31-62-38)54(78)69-25-11-15-44(69)51(75)66-43(55(79)80)27-34-16-20-37(21-17-34)48(72)36-12-8-7-9-13-36/h7-9,12-13,16-23,29,31-33,40-44,46-47,59,70H,6,10-11,14-15,24-28,30H2,1-5H3,(H,60,62)(H,63,71)(H,64,76)(H,65,77)(H,66,75)(H,67,73)(H,68,74)(H,79,80)(H4,57,58,61)/t33-,40-,41-,42-,43-,44-,46-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118738
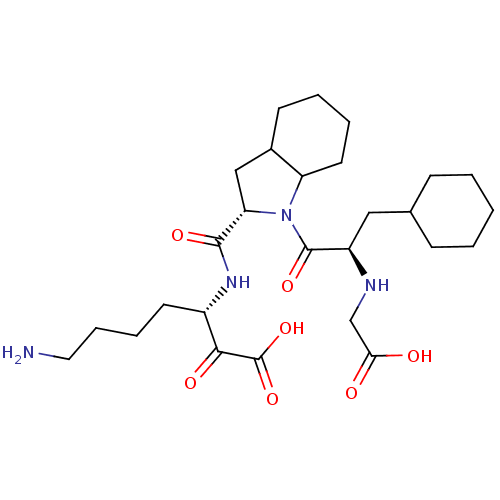
(7-Amino-3-({1-[2-(carboxymethyl-amino)-3-cyclohexy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CC2CCCCC2N1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(O)=O Show InChI InChI=1S/C27H44N4O7/c28-13-7-6-11-19(24(34)27(37)38)30-25(35)22-15-18-10-4-5-12-21(18)31(22)26(36)20(29-16-23(32)33)14-17-8-2-1-3-9-17/h17-22,29H,1-16,28H2,(H,30,35)(H,32,33)(H,37,38)/t18?,19-,20+,21?,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118718
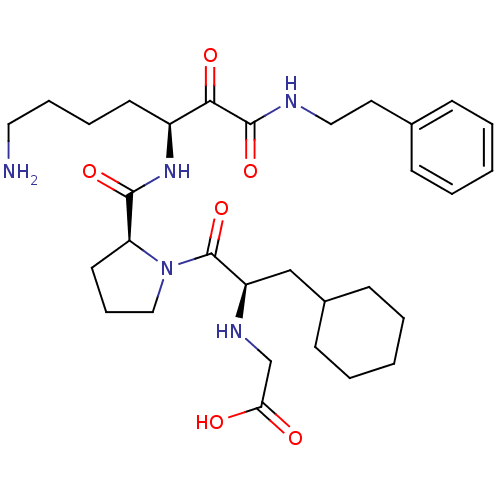
(CHEMBL343804 | {2-[2-(5-Amino-1-phenethylaminooxal...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C31H47N5O6/c32-17-8-7-14-24(28(39)30(41)33-18-16-22-10-3-1-4-11-22)35-29(40)26-15-9-19-36(26)31(42)25(34-21-27(37)38)20-23-12-5-2-6-13-23/h1,3-4,10-11,23-26,34H,2,5-9,12-21,32H2,(H,33,41)(H,35,40)(H,37,38)/t24-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313240

(CHEMBL1076633 | [Sar1,Tdf8]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)C(O)=O |r,wU:44.44,57.60,4.4,20.21,wD:2.2,24.32,8.17,61.63,c:74,(7.79,3.66,;7.73,2.12,;9.03,1.3,;10.39,2.02,;8.97,-.22,;7.62,-.93,;6.31,-.11,;6.37,1.42,;4.95,-.81,;4.89,-2.36,;6.19,-3.18,;7.54,-2.48,;8.84,-3.32,;8.76,-4.86,;10.06,-5.69,;7.41,-5.56,;6.11,-4.73,;3.65,0,;2.28,-.72,;2.22,-2.27,;.97,.11,;-.39,-.61,;-1.69,.22,;-1.63,1.75,;-3.05,-.49,;-3.12,-2.04,;-1.82,-2.86,;-1.87,-4.4,;-.57,-5.22,;-.62,-6.75,;.68,-7.57,;-1.98,-7.48,;-4.35,.34,;-5.71,-.38,;-5.77,-1.92,;-7.01,.43,;-8.36,-.28,;-9.67,.55,;1.03,1.64,;2.39,2.36,;-.27,2.46,;10.28,-1.05,;10.22,-2.6,;11.63,-.34,;12.99,-1.1,;14.3,-.33,;14.29,1.21,;15.53,2.12,;15.04,3.58,;13.5,3.57,;13.04,2.1,;13,-2.66,;11.66,-3.45,;14.34,-3.4,;15.72,-2.76,;16.75,-3.87,;16.02,-5.2,;14.52,-4.91,;13.25,-5.78,;11.9,-5.04,;13.28,-7.32,;14.39,-8.38,;14.01,-9.87,;15.11,-10.95,;14.73,-12.44,;15.84,-13.51,;17.32,-13.09,;17.7,-11.6,;16.6,-10.53,;18.43,-14.17,;18.85,-15.65,;19.92,-14.54,;19.76,-13.38,;21.1,-14.14,;19.74,-11.84,;20.85,-12.29,;15.87,-7.97,;16.97,-9.04,;16.25,-6.48,)| Show InChI InChI=1S/C51H70F3N15O10/c1-6-28(4)41(66-43(73)35(21-30-13-17-33(70)18-14-30)62-45(75)40(27(2)3)65-42(72)34(61-39(71)25-57-5)9-7-19-59-49(55)56)46(76)63-36(23-32-24-58-26-60-32)47(77)69-20-8-10-38(69)44(74)64-37(48(78)79)22-29-11-15-31(16-12-29)50(67-68-50)51(52,53)54/h11-18,24,26-28,34-38,40-41,57,70H,6-10,19-23,25H2,1-5H3,(H,58,60)(H,61,71)(H,62,75)(H,63,76)(H,64,74)(H,65,72)(H,66,73)(H,78,79)(H4,55,56,59)/t28-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313240

(CHEMBL1076633 | [Sar1,Tdf8]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)C(O)=O |r,wU:44.44,57.60,4.4,20.21,wD:2.2,24.32,8.17,61.63,c:74,(7.79,3.66,;7.73,2.12,;9.03,1.3,;10.39,2.02,;8.97,-.22,;7.62,-.93,;6.31,-.11,;6.37,1.42,;4.95,-.81,;4.89,-2.36,;6.19,-3.18,;7.54,-2.48,;8.84,-3.32,;8.76,-4.86,;10.06,-5.69,;7.41,-5.56,;6.11,-4.73,;3.65,0,;2.28,-.72,;2.22,-2.27,;.97,.11,;-.39,-.61,;-1.69,.22,;-1.63,1.75,;-3.05,-.49,;-3.12,-2.04,;-1.82,-2.86,;-1.87,-4.4,;-.57,-5.22,;-.62,-6.75,;.68,-7.57,;-1.98,-7.48,;-4.35,.34,;-5.71,-.38,;-5.77,-1.92,;-7.01,.43,;-8.36,-.28,;-9.67,.55,;1.03,1.64,;2.39,2.36,;-.27,2.46,;10.28,-1.05,;10.22,-2.6,;11.63,-.34,;12.99,-1.1,;14.3,-.33,;14.29,1.21,;15.53,2.12,;15.04,3.58,;13.5,3.57,;13.04,2.1,;13,-2.66,;11.66,-3.45,;14.34,-3.4,;15.72,-2.76,;16.75,-3.87,;16.02,-5.2,;14.52,-4.91,;13.25,-5.78,;11.9,-5.04,;13.28,-7.32,;14.39,-8.38,;14.01,-9.87,;15.11,-10.95,;14.73,-12.44,;15.84,-13.51,;17.32,-13.09,;17.7,-11.6,;16.6,-10.53,;18.43,-14.17,;18.85,-15.65,;19.92,-14.54,;19.76,-13.38,;21.1,-14.14,;19.74,-11.84,;20.85,-12.29,;15.87,-7.97,;16.97,-9.04,;16.25,-6.48,)| Show InChI InChI=1S/C51H70F3N15O10/c1-6-28(4)41(66-43(73)35(21-30-13-17-33(70)18-14-30)62-45(75)40(27(2)3)65-42(72)34(61-39(71)25-57-5)9-7-19-59-49(55)56)46(76)63-36(23-32-24-58-26-60-32)47(77)69-20-8-10-38(69)44(74)64-37(48(78)79)22-29-11-15-31(16-12-29)50(67-68-50)51(52,53)54/h11-18,24,26-28,34-38,40-41,57,70H,6-10,19-23,25H2,1-5H3,(H,58,60)(H,61,71)(H,62,75)(H,63,76)(H,64,74)(H,65,72)(H,66,73)(H,78,79)(H4,55,56,59)/t28-,34-,35-,36-,37-,38-,40-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118723
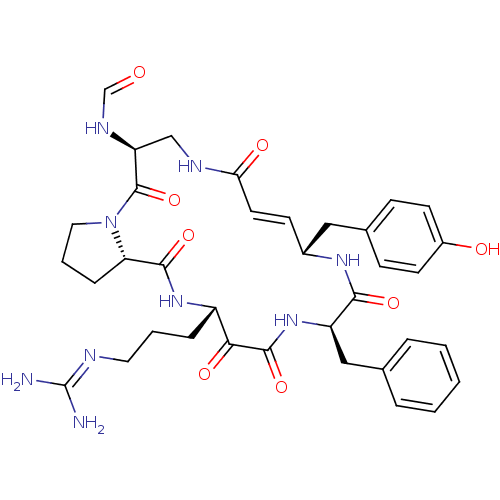
(CHEMBL342672 | CYCLOTHEONAMIDE A | N-[14-Benzyl-18...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H]-1-[#7]-[#6](=O)-[#6@@H]-2-[#6]-[#6]-[#6]-[#7]-2-[#6](=O)-[#6@H](-[#6]-[#7]-[#6](=O)\[#6]=[#6]\[#6@H](-[#6]-c2ccc(-[#8])cc2)-[#7]-[#6](=O)-[#6@@H](-[#6]-c2ccccc2)-[#7]-[#6](=O)-[#6]-1=O)-[#7]-[#6]=O |r,t:24| Show InChI InChI=1S/C36H45N9O8/c37-36(38)39-16-4-8-26-31(49)34(52)44-27(19-22-6-2-1-3-7-22)32(50)42-24(18-23-10-13-25(47)14-11-23)12-15-30(48)40-20-28(41-21-46)35(53)45-17-5-9-29(45)33(51)43-26/h1-3,6-7,10-15,21,24,26-29,47H,4-5,8-9,16-20H2,(H,40,48)(H,41,46)(H,42,50)(H,43,51)(H,44,52)(H4,37,38,39)/b15-12+/t24-,26+,27-,28+,29+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50007400

(4-Phenyl-1-(4-phenyl-butyl)-piperidine | 4-Phenyl-...)Show InChI InChI=1S/C21H27N/c1-3-9-19(10-4-1)11-7-8-16-22-17-14-21(15-18-22)20-12-5-2-6-13-20/h1-6,9-10,12-13,21H,7-8,11,14-18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]-haloperidol in the presence of 25 nM unlabeled spiperone |
J Med Chem 36: 3923-8 (1994)
BindingDB Entry DOI: 10.7270/Q2H13122 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118727
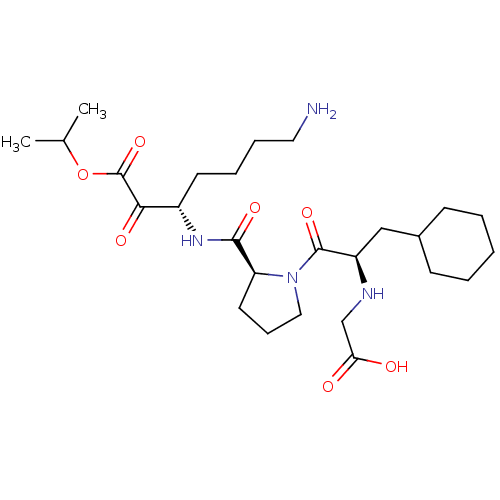
(2-((R)-1-((S)-2-(((S)-7-amino-1-isopropoxy-1,2-dio...)Show SMILES CC(C)OC(=O)C(=O)[C@H](CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O Show InChI InChI=1S/C26H44N4O7/c1-17(2)37-26(36)23(33)19(11-6-7-13-27)29-24(34)21-12-8-14-30(21)25(35)20(28-16-22(31)32)15-18-9-4-3-5-10-18/h17-21,28H,3-16,27H2,1-2H3,(H,29,34)(H,31,32)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313241

(CHEMBL1076604 | [Sar1,Tdf3]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,55.57,68.73,20.37,wD:2.2,38.48,72.76,8.17,c:31,(7.37,.5,;7.36,-1.08,;8.67,-1.85,;10.02,-1.07,;8.67,-3.38,;7.36,-4.13,;6.01,-3.36,;6.01,-1.84,;4.68,-4.12,;4.68,-5.67,;6.01,-6.44,;7.34,-5.69,;8.67,-6.47,;8.65,-8.01,;9.98,-8.8,;7.32,-8.76,;5.99,-7.99,;3.35,-3.35,;2.02,-4.12,;2.02,-5.68,;.68,-3.35,;.68,-1.82,;-.11,-.48,;.66,.87,;-.13,2.2,;-1.68,2.19,;-2.44,.83,;-1.65,-.5,;-2.47,3.52,;-2.48,5.06,;-3.81,4.27,;-3.81,2.76,;-5.13,3.53,;-3.82,1.22,;-5.14,1.99,;-.65,-4.12,;-1.99,-3.35,;-1.99,-1.82,;-3.32,-4.12,;-3.32,-5.67,;-1.99,-6.43,;-1.99,-7.97,;-.65,-8.73,;-.65,-10.27,;.69,-11.04,;-1.98,-11.05,;-4.65,-3.33,;-5.98,-4.1,;-5.98,-5.64,;-7.31,-3.34,;-8.63,-4.1,;-9.97,-3.33,;10.02,-4.15,;10.02,-5.7,;11.34,-3.39,;12.68,-4.16,;14.01,-3.4,;14.01,-1.87,;12.78,-.96,;13.27,.5,;14.81,.49,;15.27,-.98,;12.68,-5.72,;11.34,-6.49,;14.01,-6.48,;15.59,-5.94,;16.61,-7.27,;15.66,-8.65,;14.27,-7.97,;13.04,-8.9,;11.66,-8.22,;13.16,-10.43,;11.87,-11.28,;10.49,-10.61,;9.22,-11.46,;9.32,-13,;8.05,-13.86,;6.67,-13.19,;6.56,-11.65,;7.83,-10.79,;11.98,-12.81,;10.71,-13.68,;13.36,-13.49,)| Show InChI InChI=1S/C55H70F3N15O10/c1-4-31(2)45(50(80)68-41(27-36-28-62-30-64-36)51(81)73-23-9-13-43(73)49(79)69-42(52(82)83)26-32-10-6-5-7-11-32)70-48(78)40(25-34-16-20-37(74)21-17-34)67-47(77)39(24-33-14-18-35(19-15-33)54(71-72-54)55(56,57)58)66-46(76)38(65-44(75)29-61-3)12-8-22-63-53(59)60/h5-7,10-11,14-21,28,30-31,38-43,45,61,74H,4,8-9,12-13,22-27,29H2,1-3H3,(H,62,64)(H,65,75)(H,66,76)(H,67,77)(H,68,80)(H,69,79)(H,70,78)(H,82,83)(H4,59,60,63)/t31-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313241

(CHEMBL1076604 | [Sar1,Tdf3]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)NC(=O)[C@H](CCCN=C(N)N)NC(=O)CNC)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,55.57,68.73,20.37,wD:2.2,38.48,72.76,8.17,c:31,(7.37,.5,;7.36,-1.08,;8.67,-1.85,;10.02,-1.07,;8.67,-3.38,;7.36,-4.13,;6.01,-3.36,;6.01,-1.84,;4.68,-4.12,;4.68,-5.67,;6.01,-6.44,;7.34,-5.69,;8.67,-6.47,;8.65,-8.01,;9.98,-8.8,;7.32,-8.76,;5.99,-7.99,;3.35,-3.35,;2.02,-4.12,;2.02,-5.68,;.68,-3.35,;.68,-1.82,;-.11,-.48,;.66,.87,;-.13,2.2,;-1.68,2.19,;-2.44,.83,;-1.65,-.5,;-2.47,3.52,;-2.48,5.06,;-3.81,4.27,;-3.81,2.76,;-5.13,3.53,;-3.82,1.22,;-5.14,1.99,;-.65,-4.12,;-1.99,-3.35,;-1.99,-1.82,;-3.32,-4.12,;-3.32,-5.67,;-1.99,-6.43,;-1.99,-7.97,;-.65,-8.73,;-.65,-10.27,;.69,-11.04,;-1.98,-11.05,;-4.65,-3.33,;-5.98,-4.1,;-5.98,-5.64,;-7.31,-3.34,;-8.63,-4.1,;-9.97,-3.33,;10.02,-4.15,;10.02,-5.7,;11.34,-3.39,;12.68,-4.16,;14.01,-3.4,;14.01,-1.87,;12.78,-.96,;13.27,.5,;14.81,.49,;15.27,-.98,;12.68,-5.72,;11.34,-6.49,;14.01,-6.48,;15.59,-5.94,;16.61,-7.27,;15.66,-8.65,;14.27,-7.97,;13.04,-8.9,;11.66,-8.22,;13.16,-10.43,;11.87,-11.28,;10.49,-10.61,;9.22,-11.46,;9.32,-13,;8.05,-13.86,;6.67,-13.19,;6.56,-11.65,;7.83,-10.79,;11.98,-12.81,;10.71,-13.68,;13.36,-13.49,)| Show InChI InChI=1S/C55H70F3N15O10/c1-4-31(2)45(50(80)68-41(27-36-28-62-30-64-36)51(81)73-23-9-13-43(73)49(79)69-42(52(82)83)26-32-10-6-5-7-11-32)70-48(78)40(25-34-16-20-37(74)21-17-34)67-47(77)39(24-33-14-18-35(19-15-33)54(71-72-54)55(56,57)58)66-46(76)38(65-44(75)29-61-3)12-8-22-63-53(59)60/h5-7,10-11,14-21,28,30-31,38-43,45,61,74H,4,8-9,12-13,22-27,29H2,1-3H3,(H,62,64)(H,65,75)(H,66,76)(H,67,77)(H,68,80)(H,69,79)(H,70,78)(H,82,83)(H4,59,60,63)/t31-,38-,39-,40-,41-,42-,43-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118720
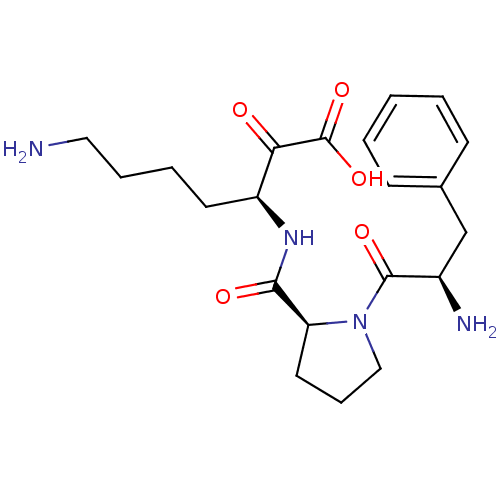
(7-Amino-3-{[1-(2-amino-3-phenyl-propionyl)-pyrroli...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)C(O)=O Show InChI InChI=1S/C21H30N4O5/c22-11-5-4-9-16(18(26)21(29)30)24-19(27)17-10-6-12-25(17)20(28)15(23)13-14-7-2-1-3-8-14/h1-3,7-8,15-17H,4-6,9-13,22-23H2,(H,24,27)(H,29,30)/t15-,16+,17+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM29388
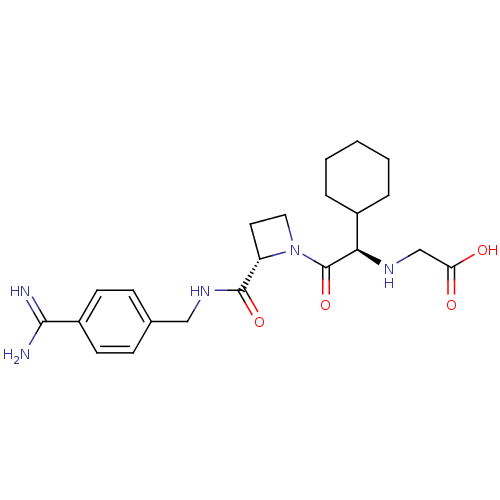
(Exanta | Melagatran | US11584714, Compound 999)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2CCN2C(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C22H31N5O4/c23-20(24)16-8-6-14(7-9-16)12-26-21(30)17-10-11-27(17)22(31)19(25-13-18(28)29)15-4-2-1-3-5-15/h6-9,15,17,19,25H,1-5,10-13H2,(H3,23,24)(H,26,30)(H,28,29)/t17-,19+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50118717
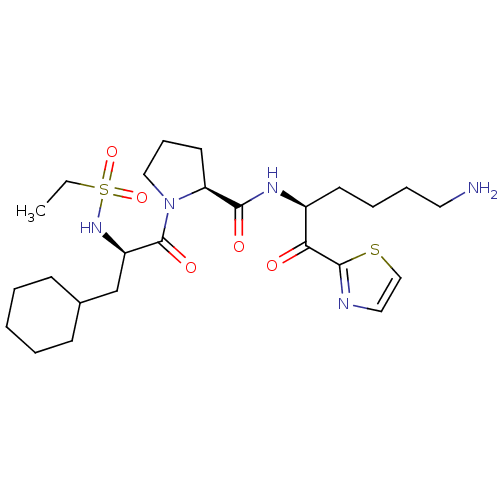
((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...)Show SMILES CCS(=O)(=O)N[C@H](CC1CCCCC1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)c1nccs1 Show InChI InChI=1S/C25H41N5O5S2/c1-2-37(34,35)29-20(17-18-9-4-3-5-10-18)25(33)30-15-8-12-21(30)23(32)28-19(11-6-7-13-26)22(31)24-27-14-16-36-24/h14,16,18-21,29H,2-13,15,17,26H2,1H3,(H,28,32)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
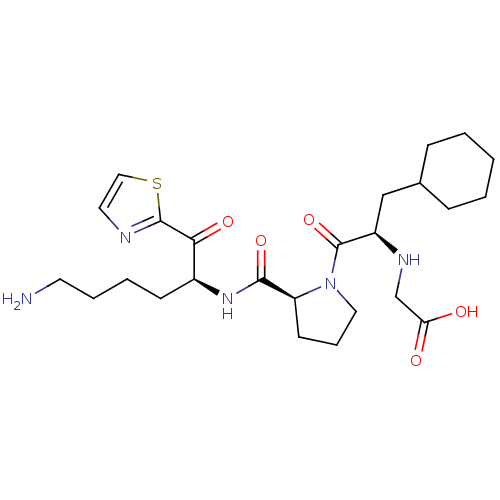
Prothrombin
(Homo sapiens (Human)) | BDBM50118737
((2-{2-[5-Amino-1-(thiazole-2-carbonyl)-pentylcarba...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)c1nccs1 Show InChI InChI=1S/C25H39N5O5S/c26-11-5-4-9-18(22(33)24-27-12-14-36-24)29-23(34)20-10-6-13-30(20)25(35)19(28-16-21(31)32)15-17-7-2-1-3-8-17/h12,14,17-20,28H,1-11,13,15-16,26H2,(H,29,34)(H,31,32)/t18-,19+,20-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118721
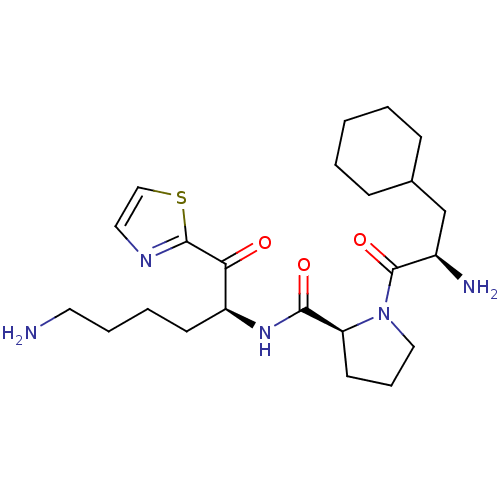
((S)-N-((S)-6-amino-1-oxo-1-(thiazol-2-yl)hexan-2-y...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)CC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C23H37N5O3S/c24-11-5-4-9-18(20(29)22-26-12-14-32-22)27-21(30)19-10-6-13-28(19)23(31)17(25)15-16-7-2-1-3-8-16/h12,14,16-19H,1-11,13,15,24-25H2,(H,27,30)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50002245

(4-[2-(1-Cyclopropylmethyl-piperidin-4-yl)-acetyl]-...)Show InChI InChI=1S/C18H22N2O/c19-12-15-3-5-17(6-4-15)18(21)11-14-7-9-20(10-8-14)13-16-1-2-16/h3-6,14,16H,1-2,7-11,13H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]- haloperidol |
J Med Chem 36: 3923-8 (1994)
BindingDB Entry DOI: 10.7270/Q2H13122 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50002218

(2-(1-Cyclopropylmethyl-piperidin-4-yl)-1-(4-fluoro...)Show InChI InChI=1S/C17H22FNO/c18-16-5-3-15(4-6-16)17(20)11-13-7-9-19(10-8-13)12-14-1-2-14/h3-6,13-14H,1-2,7-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
New York University
Curated by ChEMBL
| Assay Description
Binding affinity against sigma receptor in bovine cerebellum using 2.0 nM [3H]- haloperidol |
J Med Chem 36: 3923-8 (1994)
BindingDB Entry DOI: 10.7270/Q2H13122 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118722

(2-Amino-N-{[5-amino-1-(thiazole-2-carbonyl)-pentyl...)Show SMILES NCCCC[C@H](NC(=O)CN(C1CC1)C(=O)[C@H](N)CC1CCCCC1)C(=O)c1nccs1 Show InChI InChI=1S/C23H37N5O3S/c24-11-5-4-8-19(21(30)22-26-12-13-32-22)27-20(29)15-28(17-9-10-17)23(31)18(25)14-16-6-2-1-3-7-16/h12-13,16-19H,1-11,14-15,24-25H2,(H,27,29)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118725
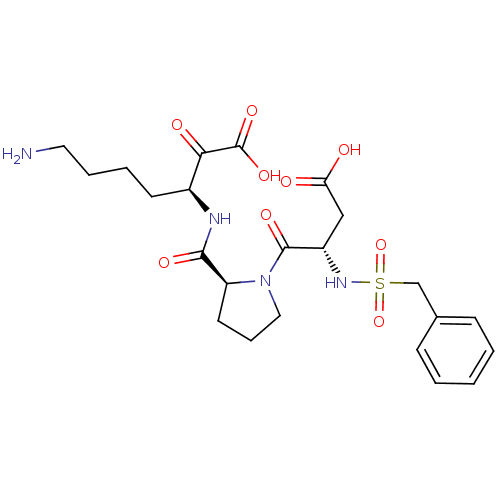
(7-Amino-3-{[1-(3-carboxy-2-phenylmethanesulfonylam...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](CC(O)=O)NS(=O)(=O)Cc1ccccc1)C(=O)C(O)=O Show InChI InChI=1S/C23H32N4O9S/c24-11-5-4-9-16(20(30)23(33)34)25-21(31)18-10-6-12-27(18)22(32)17(13-19(28)29)26-37(35,36)14-15-7-2-1-3-8-15/h1-3,7-8,16-18,26H,4-6,9-14,24H2,(H,25,31)(H,28,29)(H,33,34)/t16-,17-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313244

(CHEMBL1076601 | [Sar1,Bpa2]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C59H72N10O11/c1-6-36(4)51(57(77)65-46(31-42-32-61-34-62-42)58(78)69-27-13-18-48(69)55(75)66-47(59(79)80)30-37-14-9-7-10-15-37)68-54(74)45(29-39-21-25-43(70)26-22-39)64-56(76)50(35(2)3)67-53(73)44(63-49(71)33-60-5)28-38-19-23-41(24-20-38)52(72)40-16-11-8-12-17-40/h7-12,14-17,19-26,32,34-36,44-48,50-51,60,70H,6,13,18,27-31,33H2,1-5H3,(H,61,62)(H,63,71)(H,64,76)(H,65,77)(H,66,75)(H,67,73)(H,68,74)(H,79,80)/t36-,44-,45-,46-,47-,48-,50-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313244

(CHEMBL1076601 | [Sar1,Bpa2]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(cc1)C(=O)c1ccccc1)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r| Show InChI InChI=1S/C59H72N10O11/c1-6-36(4)51(57(77)65-46(31-42-32-61-34-62-42)58(78)69-27-13-18-48(69)55(75)66-47(59(79)80)30-37-14-9-7-10-15-37)68-54(74)45(29-39-21-25-43(70)26-22-39)64-56(76)50(35(2)3)67-53(73)44(63-49(71)33-60-5)28-38-19-23-41(24-20-38)52(72)40-16-11-8-12-17-40/h7-12,14-17,19-26,32,34-36,44-48,50-51,60,70H,6,13,18,27-31,33H2,1-5H3,(H,61,62)(H,63,71)(H,64,76)(H,65,77)(H,66,75)(H,67,73)(H,68,74)(H,79,80)/t36-,44-,45-,46-,47-,48-,50-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50038001
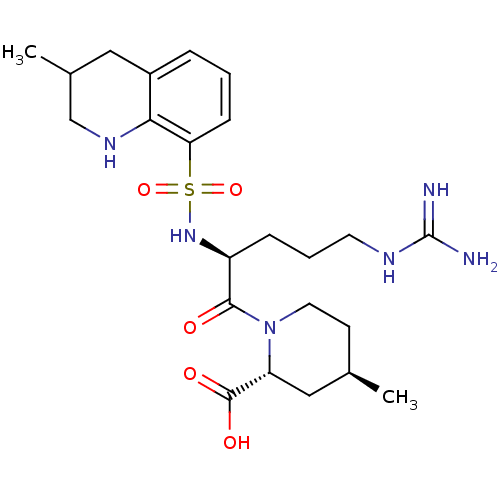
((2R,4R)-1-((S)-5-(diaminomethyleneamino)-2-(3-meth...)Show SMILES C[C@@H]1CCN([C@H](C1)C(O)=O)C(=O)[C@H](CCCNC(N)=N)NS(=O)(=O)c1cccc2CC(C)CNc12 Show InChI InChI=1S/C23H36N6O5S/c1-14-8-10-29(18(12-14)22(31)32)21(30)17(6-4-9-26-23(24)25)28-35(33,34)19-7-3-5-16-11-15(2)13-27-20(16)19/h3,5,7,14-15,17-18,27-28H,4,6,8-13H2,1-2H3,(H,31,32)(H4,24,25,26)/t14-,15?,17+,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313246
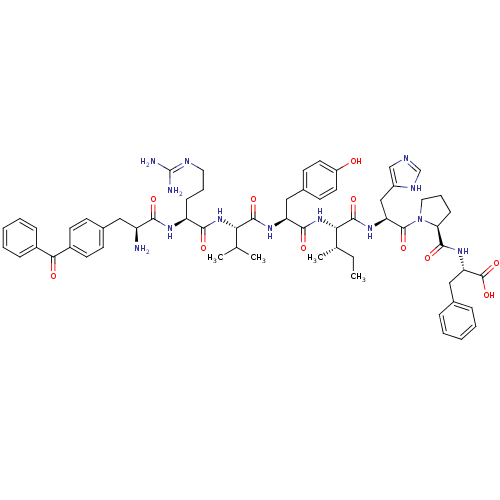
(CHEMBL1076612 | [Bpa1]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1ccc(cc1)C(=O)c1ccccc1)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,58.60,71.76,35.36,20.21,wD:2.2,24.32,75.79,8.17,(34.53,1.09,;34.53,-.46,;35.84,-1.23,;37.19,-.45,;35.84,-2.75,;34.53,-3.51,;33.18,-2.74,;33.18,-1.21,;31.85,-3.5,;31.85,-5.05,;33.18,-5.82,;34.51,-5.07,;35.84,-5.85,;35.82,-7.39,;37.15,-8.18,;34.49,-8.14,;33.16,-7.37,;30.52,-2.73,;29.19,-3.5,;29.19,-5.06,;27.84,-2.72,;26.51,-3.5,;25.18,-2.73,;25.18,-1.2,;23.85,-3.49,;23.85,-5.05,;25.18,-5.81,;25.18,-7.36,;26.5,-8.13,;26.5,-9.68,;25.15,-10.45,;27.82,-10.44,;22.52,-2.71,;21.19,-3.48,;21.19,-5.02,;19.86,-2.72,;18.53,-3.48,;19.86,-1.16,;21.2,-.39,;22.55,-1.16,;23.89,-.39,;23.89,1.17,;22.53,1.94,;21.2,1.16,;25.22,1.94,;26.55,1.18,;25.21,3.48,;26.55,4.25,;26.54,5.78,;25.21,6.55,;23.87,5.77,;23.88,4.23,;27.84,-1.2,;29.19,-.42,;26.53,-.44,;37.19,-3.53,;37.19,-5.08,;38.51,-2.77,;39.86,-3.54,;41.18,-2.78,;41.18,-1.25,;39.96,-.34,;40.44,1.12,;41.98,1.11,;42.44,-.36,;39.86,-5.1,;38.51,-5.87,;41.18,-5.86,;42.76,-5.32,;43.78,-6.65,;42.83,-8.03,;41.44,-7.35,;40.21,-8.28,;38.83,-7.61,;40.33,-9.81,;39.04,-10.66,;37.66,-9.99,;36.39,-10.84,;36.49,-12.38,;35.22,-13.24,;33.84,-12.57,;33.73,-11.03,;35,-10.17,;39.15,-12.2,;37.88,-13.06,;40.53,-12.87,)| Show InChI InChI=1S/C62H79N13O11/c1-5-37(4)52(59(83)71-48(33-43-34-66-35-68-43)60(84)75-29-13-19-50(75)57(81)72-49(61(85)86)32-38-14-8-6-9-15-38)74-56(80)47(31-40-22-26-44(76)27-23-40)70-58(82)51(36(2)3)73-55(79)46(18-12-28-67-62(64)65)69-54(78)45(63)30-39-20-24-42(25-21-39)53(77)41-16-10-7-11-17-41/h6-11,14-17,20-27,34-37,45-52,76H,5,12-13,18-19,28-33,63H2,1-4H3,(H,66,68)(H,69,78)(H,70,82)(H,71,83)(H,72,81)(H,73,79)(H,74,80)(H,85,86)(H4,64,65,67)/t37-,45-,46-,47-,48-,49-,50-,51-,52-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313246

(CHEMBL1076612 | [Bpa1]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1ccc(cc1)C(=O)c1ccccc1)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,58.60,71.76,35.36,20.21,wD:2.2,24.32,75.79,8.17,(34.53,1.09,;34.53,-.46,;35.84,-1.23,;37.19,-.45,;35.84,-2.75,;34.53,-3.51,;33.18,-2.74,;33.18,-1.21,;31.85,-3.5,;31.85,-5.05,;33.18,-5.82,;34.51,-5.07,;35.84,-5.85,;35.82,-7.39,;37.15,-8.18,;34.49,-8.14,;33.16,-7.37,;30.52,-2.73,;29.19,-3.5,;29.19,-5.06,;27.84,-2.72,;26.51,-3.5,;25.18,-2.73,;25.18,-1.2,;23.85,-3.49,;23.85,-5.05,;25.18,-5.81,;25.18,-7.36,;26.5,-8.13,;26.5,-9.68,;25.15,-10.45,;27.82,-10.44,;22.52,-2.71,;21.19,-3.48,;21.19,-5.02,;19.86,-2.72,;18.53,-3.48,;19.86,-1.16,;21.2,-.39,;22.55,-1.16,;23.89,-.39,;23.89,1.17,;22.53,1.94,;21.2,1.16,;25.22,1.94,;26.55,1.18,;25.21,3.48,;26.55,4.25,;26.54,5.78,;25.21,6.55,;23.87,5.77,;23.88,4.23,;27.84,-1.2,;29.19,-.42,;26.53,-.44,;37.19,-3.53,;37.19,-5.08,;38.51,-2.77,;39.86,-3.54,;41.18,-2.78,;41.18,-1.25,;39.96,-.34,;40.44,1.12,;41.98,1.11,;42.44,-.36,;39.86,-5.1,;38.51,-5.87,;41.18,-5.86,;42.76,-5.32,;43.78,-6.65,;42.83,-8.03,;41.44,-7.35,;40.21,-8.28,;38.83,-7.61,;40.33,-9.81,;39.04,-10.66,;37.66,-9.99,;36.39,-10.84,;36.49,-12.38,;35.22,-13.24,;33.84,-12.57,;33.73,-11.03,;35,-10.17,;39.15,-12.2,;37.88,-13.06,;40.53,-12.87,)| Show InChI InChI=1S/C62H79N13O11/c1-5-37(4)52(59(83)71-48(33-43-34-66-35-68-43)60(84)75-29-13-19-50(75)57(81)72-49(61(85)86)32-38-14-8-6-9-15-38)74-56(80)47(31-40-22-26-44(76)27-23-40)70-58(82)51(36(2)3)73-55(79)46(18-12-28-67-62(64)65)69-54(78)45(63)30-39-20-24-42(25-21-39)53(77)41-16-10-7-11-17-41/h6-11,14-17,20-27,34-37,45-52,76H,5,12-13,18-19,28-33,63H2,1-4H3,(H,66,68)(H,69,78)(H,70,82)(H,71,83)(H,72,81)(H,73,79)(H,74,80)(H,85,86)(H4,64,65,67)/t37-,45-,46-,47-,48-,49-,50-,51-,52-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313243

(CHEMBL1076602 | [Sar1,Tdf2]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,c:35| Show InChI InChI=1S/C54H67F3N12O10/c1-6-31(4)45(50(76)63-40(26-36-27-59-29-60-36)51(77)69-22-10-13-42(69)48(74)64-41(52(78)79)25-32-11-8-7-9-12-32)66-47(73)39(24-34-16-20-37(70)21-17-34)62-49(75)44(30(2)3)65-46(72)38(61-43(71)28-58-5)23-33-14-18-35(19-15-33)53(67-68-53)54(55,56)57/h7-9,11-12,14-21,27,29-31,38-42,44-45,58,70H,6,10,13,22-26,28H2,1-5H3,(H,59,60)(H,61,71)(H,62,75)(H,63,76)(H,64,74)(H,65,72)(H,66,73)(H,78,79)/t31-,38-,39-,40-,41-,42-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313243

(CHEMBL1076602 | [Sar1,Tdf2]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(cc1)C1(N=N1)C(F)(F)F)NC(=O)CNC)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,c:35| Show InChI InChI=1S/C54H67F3N12O10/c1-6-31(4)45(50(76)63-40(26-36-27-59-29-60-36)51(77)69-22-10-13-42(69)48(74)64-41(52(78)79)25-32-11-8-7-9-12-32)66-47(73)39(24-34-16-20-37(70)21-17-34)62-49(75)44(30(2)3)65-46(72)38(61-43(71)28-58-5)23-33-14-18-35(19-15-33)53(67-68-53)54(55,56)57/h7-9,11-12,14-21,27,29-31,38-42,44-45,58,70H,6,10,13,22-26,28H2,1-5H3,(H,59,60)(H,61,71)(H,62,75)(H,63,76)(H,64,74)(H,65,72)(H,66,73)(H,78,79)/t31-,38-,39-,40-,41-,42-,44-,45-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313245
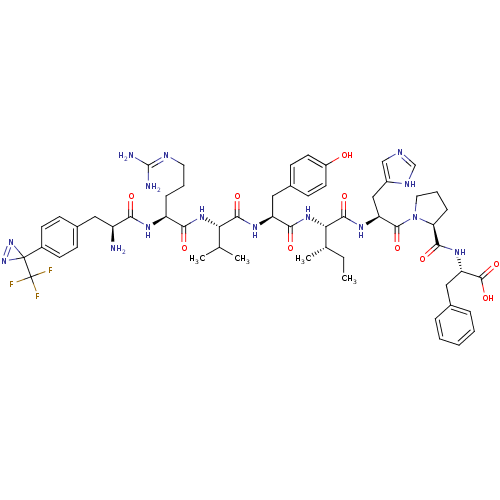
(CHEMBL1076613 | [Tdf1]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1ccc(cc1)C1(N=N1)C(F)(F)F)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,57.59,70.75,35.36,20.21,wD:2.2,24.32,74.78,8.17,c:47,(6.95,-16.43,;6.95,-17.98,;8.26,-18.74,;9.61,-17.96,;8.26,-20.27,;6.95,-21.03,;5.6,-20.25,;5.6,-18.73,;4.28,-21.01,;4.28,-22.56,;5.6,-23.33,;6.93,-22.58,;8.25,-23.35,;8.24,-24.9,;9.57,-25.69,;6.91,-25.65,;5.59,-24.88,;2.94,-20.24,;1.61,-21.02,;1.61,-22.57,;.27,-20.24,;-1.06,-21.02,;-2.39,-20.25,;-2.39,-18.72,;-3.72,-21.01,;-3.72,-22.56,;-2.4,-23.32,;-2.4,-24.87,;-1.08,-25.64,;-1.08,-27.19,;-2.42,-27.96,;.25,-27.94,;-5.05,-20.22,;-6.38,-20.99,;-6.38,-22.53,;-7.71,-20.23,;-9.04,-20.99,;-7.71,-18.68,;-6.37,-17.9,;-5.03,-18.68,;-3.69,-17.9,;-3.69,-16.35,;-5.04,-15.58,;-6.38,-16.36,;-2.35,-15.57,;-.82,-15.57,;-1.59,-14.24,;-3.45,-14.47,;-3.05,-12.99,;-4.94,-14.86,;-4.54,-13.37,;.27,-18.71,;1.61,-17.94,;-1.05,-17.95,;9.61,-21.04,;9.61,-22.59,;10.92,-20.28,;12.27,-21.05,;13.59,-20.29,;13.59,-18.77,;12.37,-17.86,;12.85,-16.4,;14.39,-16.41,;14.85,-17.88,;12.27,-22.61,;10.92,-23.38,;13.59,-23.37,;15.17,-22.83,;16.19,-24.16,;15.24,-25.54,;13.85,-24.86,;12.62,-25.78,;11.24,-25.11,;12.74,-27.32,;11.45,-28.17,;10.07,-27.5,;8.8,-28.35,;8.9,-29.89,;7.63,-30.74,;6.26,-30.07,;6.15,-28.54,;7.42,-27.68,;11.56,-29.7,;10.29,-30.56,;12.94,-30.37,)| Show InChI InChI=1S/C57H74F3N15O10/c1-5-32(4)46(52(82)69-42(28-37-29-64-30-66-37)53(83)75-24-10-14-44(75)50(80)70-43(54(84)85)27-33-11-7-6-8-12-33)72-49(79)41(26-35-17-21-38(76)22-18-35)68-51(81)45(31(2)3)71-48(78)40(13-9-23-65-55(62)63)67-47(77)39(61)25-34-15-19-36(20-16-34)56(73-74-56)57(58,59)60/h6-8,11-12,15-22,29-32,39-46,76H,5,9-10,13-14,23-28,61H2,1-4H3,(H,64,66)(H,67,77)(H,68,81)(H,69,82)(H,70,80)(H,71,78)(H,72,79)(H,84,85)(H4,62,63,65)/t32-,39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Type-1 angiotensin II receptor
(Homo sapiens (Human)) | BDBM50313245

(CHEMBL1076613 | [Tdf1]AngII)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)Cc1ccc(cc1)C1(N=N1)C(F)(F)F)C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](Cc1ccccc1)C(O)=O |r,wU:4.4,57.59,70.75,35.36,20.21,wD:2.2,24.32,74.78,8.17,c:47,(6.95,-16.43,;6.95,-17.98,;8.26,-18.74,;9.61,-17.96,;8.26,-20.27,;6.95,-21.03,;5.6,-20.25,;5.6,-18.73,;4.28,-21.01,;4.28,-22.56,;5.6,-23.33,;6.93,-22.58,;8.25,-23.35,;8.24,-24.9,;9.57,-25.69,;6.91,-25.65,;5.59,-24.88,;2.94,-20.24,;1.61,-21.02,;1.61,-22.57,;.27,-20.24,;-1.06,-21.02,;-2.39,-20.25,;-2.39,-18.72,;-3.72,-21.01,;-3.72,-22.56,;-2.4,-23.32,;-2.4,-24.87,;-1.08,-25.64,;-1.08,-27.19,;-2.42,-27.96,;.25,-27.94,;-5.05,-20.22,;-6.38,-20.99,;-6.38,-22.53,;-7.71,-20.23,;-9.04,-20.99,;-7.71,-18.68,;-6.37,-17.9,;-5.03,-18.68,;-3.69,-17.9,;-3.69,-16.35,;-5.04,-15.58,;-6.38,-16.36,;-2.35,-15.57,;-.82,-15.57,;-1.59,-14.24,;-3.45,-14.47,;-3.05,-12.99,;-4.94,-14.86,;-4.54,-13.37,;.27,-18.71,;1.61,-17.94,;-1.05,-17.95,;9.61,-21.04,;9.61,-22.59,;10.92,-20.28,;12.27,-21.05,;13.59,-20.29,;13.59,-18.77,;12.37,-17.86,;12.85,-16.4,;14.39,-16.41,;14.85,-17.88,;12.27,-22.61,;10.92,-23.38,;13.59,-23.37,;15.17,-22.83,;16.19,-24.16,;15.24,-25.54,;13.85,-24.86,;12.62,-25.78,;11.24,-25.11,;12.74,-27.32,;11.45,-28.17,;10.07,-27.5,;8.8,-28.35,;8.9,-29.89,;7.63,-30.74,;6.26,-30.07,;6.15,-28.54,;7.42,-27.68,;11.56,-29.7,;10.29,-30.56,;12.94,-30.37,)| Show InChI InChI=1S/C57H74F3N15O10/c1-5-32(4)46(52(82)69-42(28-37-29-64-30-66-37)53(83)75-24-10-14-44(75)50(80)70-43(54(84)85)27-33-11-7-6-8-12-33)72-49(79)41(26-35-17-21-38(76)22-18-35)68-51(81)45(31(2)3)71-48(78)40(13-9-23-65-55(62)63)67-47(77)39(61)25-34-15-19-36(20-16-34)56(73-74-56)57(58,59)60/h6-8,11-12,15-22,29-32,39-46,76H,5,9-10,13-14,23-28,61H2,1-4H3,(H,64,66)(H,67,77)(H,68,81)(H,69,82)(H,70,80)(H,71,78)(H,72,79)(H,84,85)(H4,62,63,65)/t32-,39-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universite de Sherbrooke
Curated by ChEMBL
| Assay Description
Displacement of [125I][Sar1,Ile8]Ang2 from wild-type human AT1 receptor expressed in CHO cells by gamma counting |
J Med Chem 53: 2063-75 (2010)
Article DOI: 10.1021/jm9015747
BindingDB Entry DOI: 10.7270/Q2XD11TP |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118734
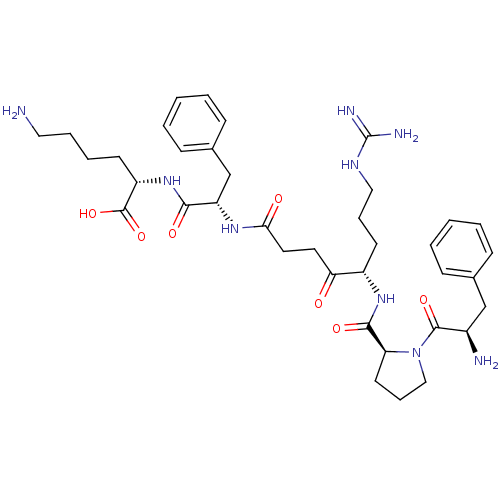
(6-Amino-2-[2-(5-{[1-(2-amino-3-phenyl-propionyl)-p...)Show SMILES NCCCC[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CCC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C38H55N9O7/c39-20-8-7-15-29(37(53)54)46-34(50)30(24-26-13-5-2-6-14-26)44-33(49)19-18-32(48)28(16-9-21-43-38(41)42)45-35(51)31-17-10-22-47(31)36(52)27(40)23-25-11-3-1-4-12-25/h1-6,11-14,27-31H,7-10,15-24,39-40H2,(H,44,49)(H,45,51)(H,46,50)(H,53,54)(H4,41,42,43)/t27-,28+,29+,30+,31+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86564

(Azanium analog, 56)Show InChI InChI=1S/C15H17Cl2NO2/c1-15(2,9-19)18-8-13-3-4-14(20-13)10-5-11(16)7-12(17)6-10/h3-7,18-19H,8-9H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 80 | -40.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86569

(Azanium analog, 61)Show InChI InChI=1S/C13H13Cl2NO/c1-2-16-8-10-4-6-13(17-10)11-7-9(14)3-5-12(11)15/h3-7,16H,2,8H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86568
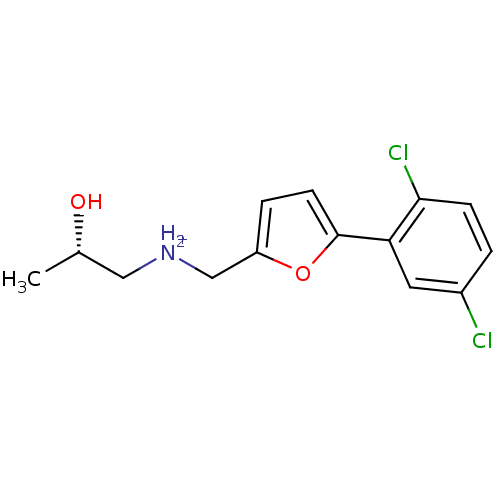
(Azanium analog, 60)Show SMILES C[C@H](O)C[NH2+]Cc1ccc(o1)-c1cc(Cl)ccc1Cl |r| Show InChI InChI=1S/C14H15Cl2NO2/c1-9(18)7-17-8-11-3-5-14(19-11)12-6-10(15)2-4-13(12)16/h2-6,9,17-18H,7-8H2,1H3/p+1/t9-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86570

(Azanium analog, 62)Show InChI InChI=1S/C14H15Cl2NO/c1-9(2)17-8-11-4-6-14(18-11)12-7-10(15)3-5-13(12)16/h3-7,9,17H,8H2,1-2H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | -39.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50118724
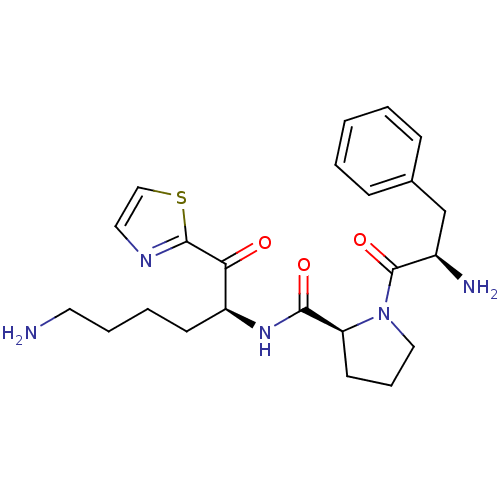
((S)-1-((R)-2-Amino-3-phenyl-propionyl)-pyrrolidine...)Show SMILES NCCCC[C@H](NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1)C(=O)c1nccs1 Show InChI InChI=1S/C23H31N5O3S/c24-11-5-4-9-18(20(29)22-26-12-14-32-22)27-21(30)19-10-6-13-28(19)23(31)17(25)15-16-7-2-1-3-8-16/h1-3,7-8,12,14,17-19H,4-6,9-11,13,15,24-25H2,(H,27,30)/t17-,18+,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin. |
J Med Chem 45: 4419-32 (2002)
BindingDB Entry DOI: 10.7270/Q2SX6DZV |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86552

(Piperidin-1-ium analog, 4)Show SMILES O[C@@H](C[NH+]1CCN(CC1)c1nsc2ccccc12)c1cccs1 |r| Show InChI InChI=1S/C17H19N3OS2/c21-14(16-6-3-11-22-16)12-19-7-9-20(10-8-19)17-13-4-1-2-5-15(13)23-18-17/h1-6,11,14,21H,7-10,12H2/p+1/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 200 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86561
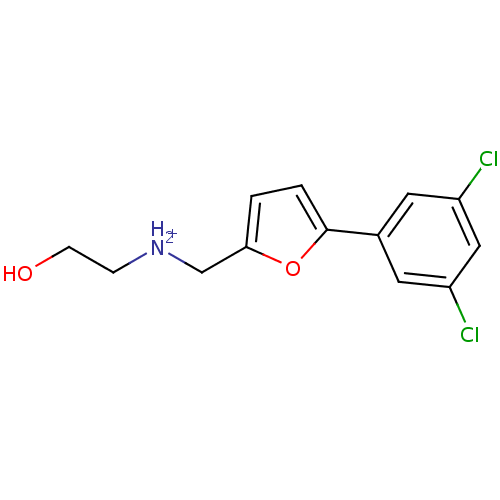
(Azanium analog, 53)Show InChI InChI=1S/C13H13Cl2NO2/c14-10-5-9(6-11(15)7-10)13-2-1-12(18-13)8-16-3-4-17/h1-2,5-7,16-17H,3-4,8H2/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86562

(Azanium analog, 54)Show InChI InChI=1S/C14H15Cl2NO2/c1-18-5-4-17-9-13-2-3-14(19-13)10-6-11(15)8-12(16)7-10/h2-3,6-8,17H,4-5,9H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM86563
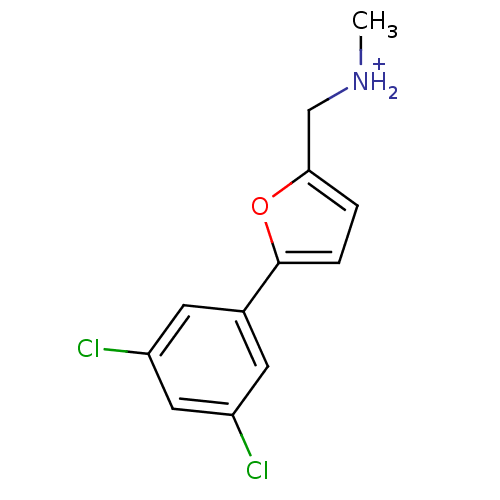
(Azanium analog, 55)Show InChI InChI=1S/C12H11Cl2NO/c1-15-7-11-2-3-12(16-11)8-4-9(13)6-10(14)5-8/h2-6,15H,7H2,1H3/p+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM86562

(Azanium analog, 54)Show InChI InChI=1S/C14H15Cl2NO2/c1-18-5-4-17-9-13-2-3-14(19-13)10-6-11(15)8-12(16)7-10/h2-3,6-8,17H,4-5,9H2,1H3/p+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | -37.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
University of California San Francisco
| Assay Description
Affinities for D3-dopaminergic, D2-dopaminergic and beta2-adrenergic receptors were determined by radioligand competition binding at the National Ins... |
Nat Chem Biol 7: 769-78 (2011)
Article DOI: 10.1038/nchembio.662
BindingDB Entry DOI: 10.7270/Q2TM78P6 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data


















































