 Found 473 hits with Last Name = 'nambu' and Initial = 'm'
Found 473 hits with Last Name = 'nambu' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327904

(2-amino-4-methyl-6-(1H-pyrazol-3-yl)-8-(tetrahydro...)Show SMILES Cc1nc(N)nc2n(C3CCOCC3)c(=O)c(nc12)-c1cc[nH]n1 Show InChI InChI=1S/C15H17N7O2/c1-8-11-13(20-15(16)18-8)22(9-3-6-24-7-4-9)14(23)12(19-11)10-2-5-17-21-10/h2,5,9H,3-4,6-7H2,1H3,(H,17,21)(H2,16,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327907
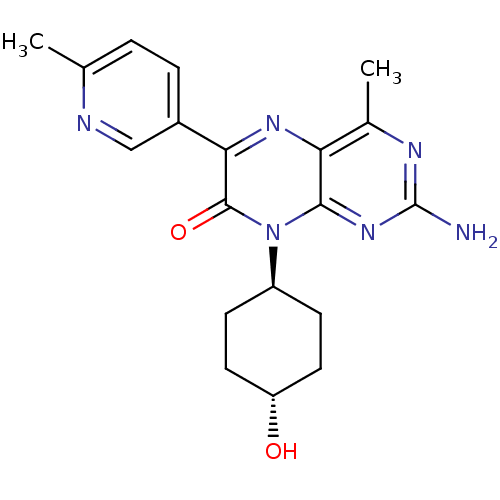
(CHEMBL1257295 | trans-2-amino-8-(4-hydroxycyclohex...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:21.23,wD:18.19,(6.09,-29.48,;4.76,-30.26,;4.77,-31.8,;3.44,-32.58,;2.1,-31.81,;2.09,-30.28,;3.42,-29.5,;.78,-32.59,;-.55,-31.83,;-1.88,-32.6,;-3.22,-31.84,;-3.22,-30.3,;-4.54,-32.61,;-4.55,-34.15,;-5.88,-34.92,;-3.21,-34.92,;-1.88,-34.14,;-.54,-34.91,;-.54,-36.45,;-1.87,-37.22,;-1.87,-38.76,;-.54,-39.53,;-.54,-41.07,;.79,-38.76,;.8,-37.22,;.79,-34.14,;2.13,-34.9,)| Show InChI InChI=1S/C19H22N6O2/c1-10-3-4-12(9-21-10)16-18(27)25(13-5-7-14(26)8-6-13)17-15(23-16)11(2)22-19(20)24-17/h3-4,9,13-14,26H,5-8H2,1-2H3,(H2,20,22,24)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396942
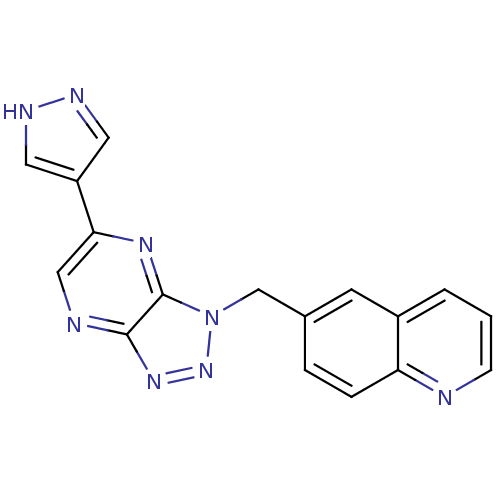
(CHEMBL2170818)Show SMILES C(c1ccc2ncccc2c1)n1nnc2ncc(nc12)-c1cn[nH]c1 Show InChI InChI=1S/C17H12N8/c1-2-12-6-11(3-4-14(12)18-5-1)10-25-17-16(23-24-25)19-9-15(22-17)13-7-20-21-8-13/h1-9H,10H2,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396976
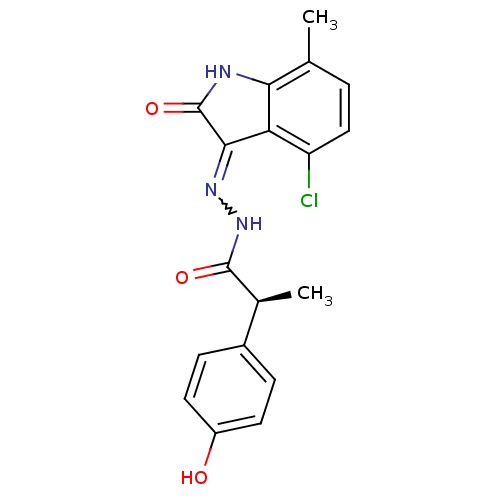
(CHEMBL2170969)Show SMILES C[C@H](C(=O)NN=C1C(=O)Nc2c1c(Cl)ccc2C)c1ccc(O)cc1 |r,w:5.4| Show InChI InChI=1S/C18H16ClN3O3/c1-9-3-8-13(19)14-15(9)20-18(25)16(14)21-22-17(24)10(2)11-4-6-12(23)7-5-11/h3-8,10,23H,1-2H3,(H,22,24)(H,20,21,25)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440904

(CHEMBL2431834)Show SMILES C[C@@H](c1ccc2ncccc2c1)n1nnc2ncc(nc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H16N8/c1-12(13-5-6-16-14(8-13)4-3-7-20-16)27-19-18(24-25-27)21-10-17(23-19)15-9-22-26(2)11-15/h3-12H,1-2H3/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50306682

((R)-3-(1-(2,6-dichloro-3-fluorophenyl)ethoxy)-5-(1...)Show SMILES C[C@@H](Oc1cc(cnc1N)-c1cnn(c1)C1CCNCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)2-3-17(24)20(19)23)30-18-8-13(9-27-21(18)25)14-10-28-29(11-14)15-4-6-26-7-5-15/h2-3,8-12,15,26H,4-7H2,1H3,(H2,25,27)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant c-MET assessed as inhibition of autophosphorylation by continuous fluorometric assay |
J Med Chem 54: 6342-63 (2011)
Article DOI: 10.1021/jm2007613
BindingDB Entry DOI: 10.7270/Q2Q52Q09 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396954
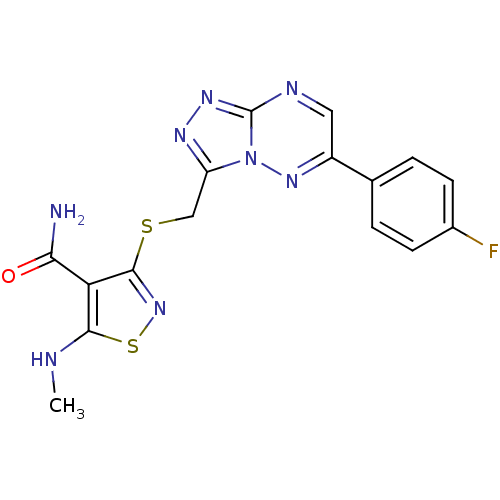
(CHEMBL2170966)Show SMILES CNc1snc(SCc2nnc3ncc(nn23)-c2ccc(F)cc2)c1C(N)=O Show InChI InChI=1S/C16H13FN8OS2/c1-19-14-12(13(18)26)15(24-28-14)27-7-11-21-22-16-20-6-10(23-25(11)16)8-2-4-9(17)5-3-8/h2-6,19H,7H2,1H3,(H2,18,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440905
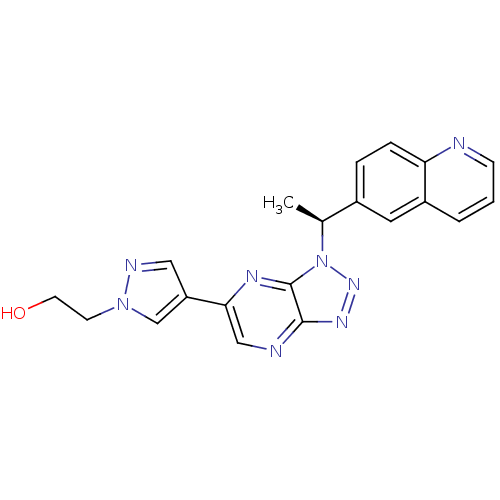
(CHEMBL2431835)Show SMILES C[C@@H](c1ccc2ncccc2c1)n1nnc2ncc(nc12)-c1cnn(CCO)c1 |r| Show InChI InChI=1S/C20H18N8O/c1-13(14-4-5-17-15(9-14)3-2-6-21-17)28-20-19(25-26-28)22-11-18(24-20)16-10-23-27(12-16)7-8-29/h2-6,9-13,29H,7-8H2,1H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440879

(CHEMBL2431824)Show SMILES COc1ccc(cc1C)-c1ccc2nnc([C@@H](C)c3ccc4ncccc4c3)n2n1 |r| Show InChI InChI=1S/C24H21N5O/c1-15-13-19(7-10-22(15)30-3)21-9-11-23-26-27-24(29(23)28-21)16(2)17-6-8-20-18(14-17)5-4-12-25-20/h4-14,16H,1-3H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440893
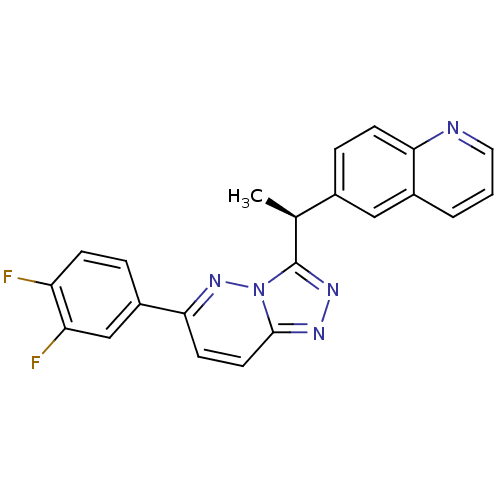
(CHEMBL2431822)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1ccc(F)c(F)c1)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C22H15F2N5/c1-13(14-5-7-19-15(11-14)3-2-10-25-19)22-27-26-21-9-8-20(28-29(21)22)16-4-6-17(23)18(24)12-16/h2-13H,1H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440899

(CHEMBL2431829)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1ccc2OCCOc2c1)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C24H19N5O2/c1-15(16-4-6-19-17(13-16)3-2-10-25-19)24-27-26-23-9-7-20(28-29(23)24)18-5-8-21-22(14-18)31-12-11-30-21/h2-10,13-15H,11-12H2,1H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396970

(CHEMBL2170954)Show InChI InChI=1S/C16H15N7/c1-2-17-14-9-19-15-16(20-14)23(22-21-15)10-11-5-6-13-12(8-11)4-3-7-18-13/h3-9H,2,10H2,1H3,(H,17,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440896
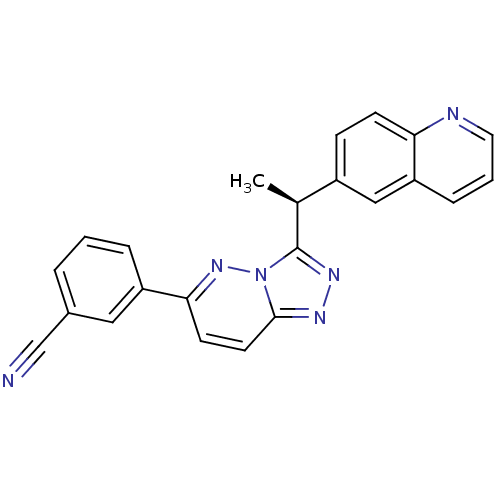
(CHEMBL2431826)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1cccc(c1)C#N)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C23H16N6/c1-15(17-7-8-20-19(13-17)6-3-11-25-20)23-27-26-22-10-9-21(28-29(22)23)18-5-2-4-16(12-18)14-24/h2-13,15H,1H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440894

(CHEMBL2431823)Show SMILES COc1ccc(cc1F)-c1ccc2nnc([C@@H](C)c3ccc4ncccc4c3)n2n1 |r| Show InChI InChI=1S/C23H18FN5O/c1-14(15-5-7-19-16(12-15)4-3-11-25-19)23-27-26-22-10-8-20(28-29(22)23)17-6-9-21(30-2)18(24)13-17/h3-14H,1-2H3/t14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396952
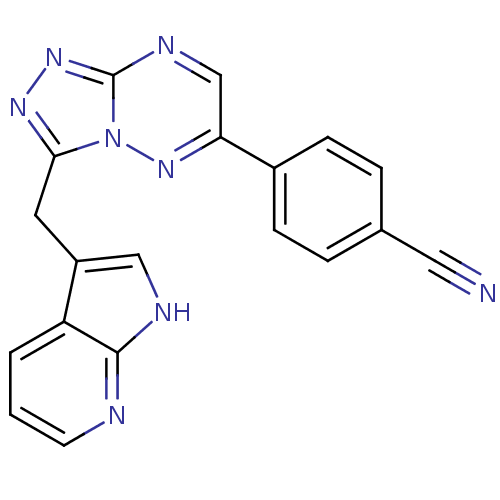
(CHEMBL2170968)Show SMILES N#Cc1ccc(cc1)-c1cnc2nnc(Cc3c[nH]c4ncccc34)n2n1 Show InChI InChI=1S/C19H12N8/c20-9-12-3-5-13(6-4-12)16-11-23-19-25-24-17(27(19)26-16)8-14-10-22-18-15(14)2-1-7-21-18/h1-7,10-11H,8H2,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396947
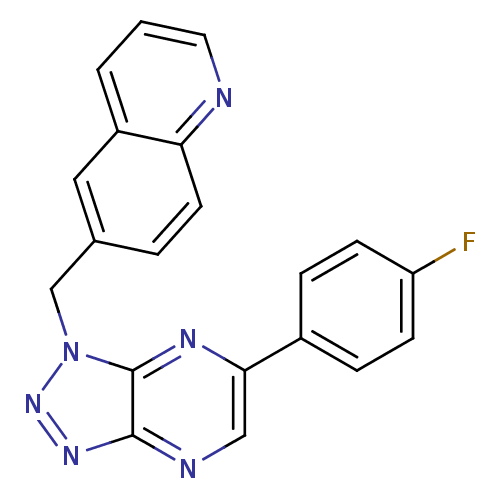
(CHEMBL2169896)Show InChI InChI=1S/C20H13FN6/c21-16-6-4-14(5-7-16)18-11-23-19-20(24-18)27(26-25-19)12-13-3-8-17-15(10-13)2-1-9-22-17/h1-11H,12H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327906
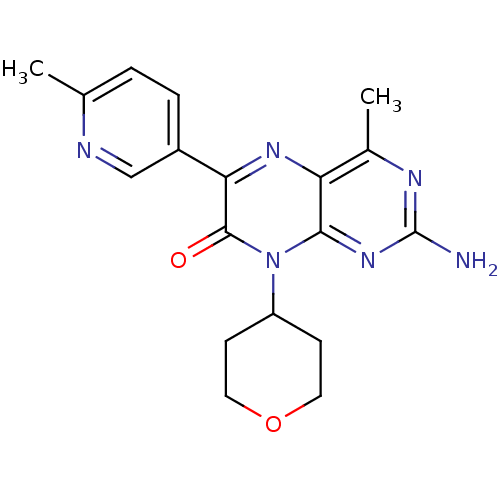
(2-amino-4-methyl-6-(6-methylpyridin-3-yl)-8-(tetra...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n(C2CCOCC2)c1=O Show InChI InChI=1S/C18H20N6O2/c1-10-3-4-12(9-20-10)15-17(25)24(13-5-7-26-8-6-13)16-14(22-15)11(2)21-18(19)23-16/h3-4,9,13H,5-8H2,1-2H3,(H2,19,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396934
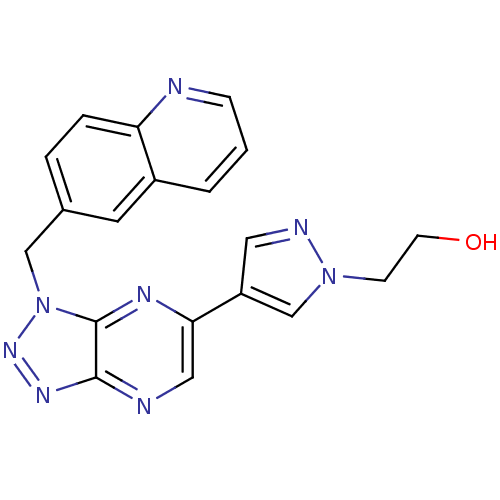
(CHEMBL2001019 | CHEMBL2170804 | US9062045, Compara...)Show SMILES OCCn1cc(cn1)-c1cnc2nnn(Cc3ccc4ncccc4c3)c2n1 Show InChI InChI=1S/C19H16N8O/c28-7-6-26-12-15(9-22-26)17-10-21-18-19(23-17)27(25-24-18)11-13-3-4-16-14(8-13)2-1-5-20-16/h1-5,8-10,12,28H,6-7,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440895

(CHEMBL2431825)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1cccc(C)c1)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C23H19N5/c1-15-5-3-6-18(13-15)21-10-11-22-25-26-23(28(22)27-21)16(2)17-8-9-20-19(14-17)7-4-12-24-20/h3-14,16H,1-2H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327894

(2-amino-8-isopropyl-6-(6-methoxypyridin-3-yl)-4-me...)Show InChI InChI=1S/C17H19N5O2/c1-9(2)22-15-12(10(3)20-17(18)21-15)7-13(16(22)23)11-5-6-14(24-4)19-8-11/h5-9H,1-4H3,(H2,18,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327903
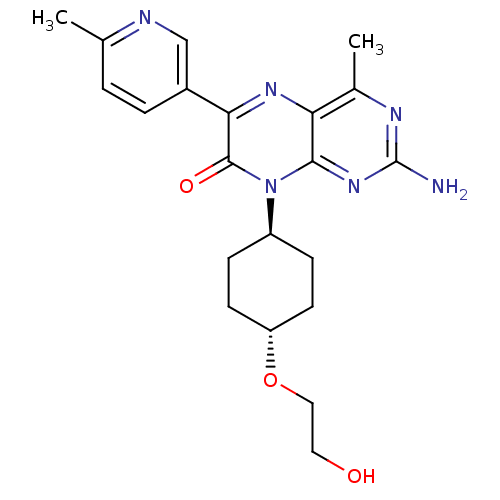
(CHEMBL1258910 | trans-2-amino-8-(4-(2-hydroxyethox...)Show SMILES Cc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@@H](CC2)OCCO)c1=O |r,wU:18.19,wD:21.26,(6.04,1.56,;4.72,.78,;4.73,-.76,;3.4,-1.53,;2.06,-.77,;2.05,.76,;3.37,1.55,;.74,-1.54,;-.6,-.78,;-1.93,-1.56,;-3.26,-.79,;-3.27,.75,;-4.59,-1.56,;-4.59,-3.11,;-5.93,-3.88,;-3.26,-3.88,;-1.93,-3.1,;-.59,-3.87,;-.59,-5.41,;.75,-6.17,;.75,-7.72,;-.59,-8.49,;-1.92,-7.71,;-1.92,-6.18,;-.59,-10.03,;-1.93,-10.8,;-1.93,-12.34,;-3.26,-13.1,;.74,-3.09,;2.08,-3.86,)| Show InChI InChI=1S/C21H26N6O3/c1-12-3-4-14(11-23-12)18-20(29)27(15-5-7-16(8-6-15)30-10-9-28)19-17(25-18)13(2)24-21(22)26-19/h3-4,11,15-16,28H,5-10H2,1-2H3,(H2,22,24,26)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396941
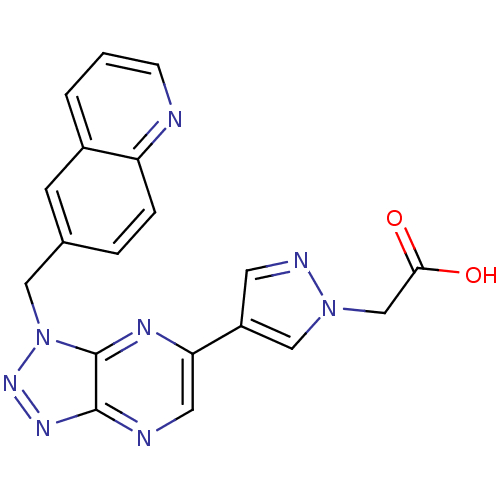
(CHEMBL2170819)Show SMILES OC(=O)Cn1cc(cn1)-c1cnc2nnn(Cc3ccc4ncccc4c3)c2n1 Show InChI InChI=1S/C19H14N8O2/c28-17(29)11-26-10-14(7-22-26)16-8-21-18-19(23-16)27(25-24-18)9-12-3-4-15-13(6-12)2-1-5-20-15/h1-8,10H,9,11H2,(H,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396943

(CHEMBL2170816)Show InChI InChI=1S/C18H14N8/c1-25-11-14(8-21-25)16-9-20-17-18(22-16)26(24-23-17)10-12-4-5-15-13(7-12)3-2-6-19-15/h2-9,11H,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396943

(CHEMBL2170816)Show InChI InChI=1S/C18H14N8/c1-25-11-14(8-21-25)16-9-20-17-18(22-16)26(24-23-17)10-12-4-5-15-13(7-12)3-2-6-19-15/h2-9,11H,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440898

(CHEMBL2431828)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1ccccc1C#N)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C23H16N6/c1-15(16-8-9-20-17(13-16)6-4-12-25-20)23-27-26-22-11-10-21(28-29(22)23)19-7-3-2-5-18(19)14-24/h2-13,15H,1H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327897
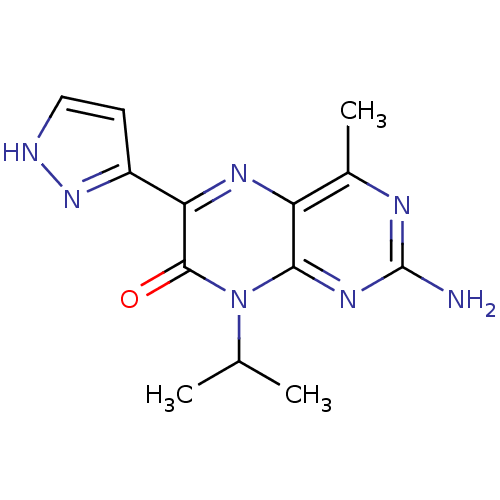
(2-amino-8-isopropyl-4-methyl-6-(1H-pyrazol-5-yl)pt...)Show InChI InChI=1S/C13H15N7O/c1-6(2)20-11-9(7(3)16-13(14)18-11)17-10(12(20)21)8-4-5-15-19-8/h4-6H,1-3H3,(H,15,19)(H2,14,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396953
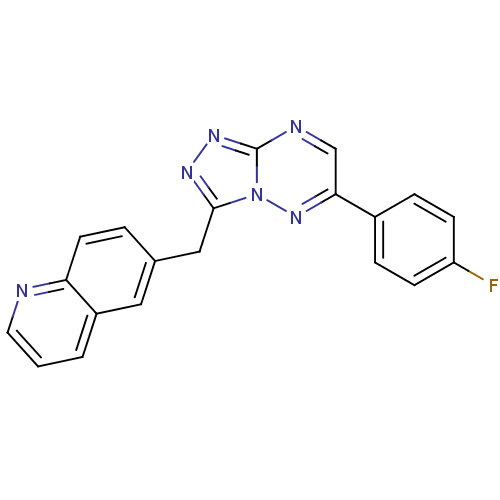
(CHEMBL2170967)Show InChI InChI=1S/C20H13FN6/c21-16-6-4-14(5-7-16)18-12-23-20-25-24-19(27(20)26-18)11-13-3-8-17-15(10-13)2-1-9-22-17/h1-10,12H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396936

(CHEMBL2170949)Show SMILES C(c1ccc2ncccc2c1)n1nnc2ncc(nc12)-c1cnn(c1)[C@@H]1CCNC1 |r| Show InChI InChI=1S/C21H19N9/c1-2-15-8-14(3-4-18(15)23-6-1)12-30-21-20(27-28-30)24-11-19(26-21)16-9-25-29(13-16)17-5-7-22-10-17/h1-4,6,8-9,11,13,17,22H,5,7,10,12H2/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327905
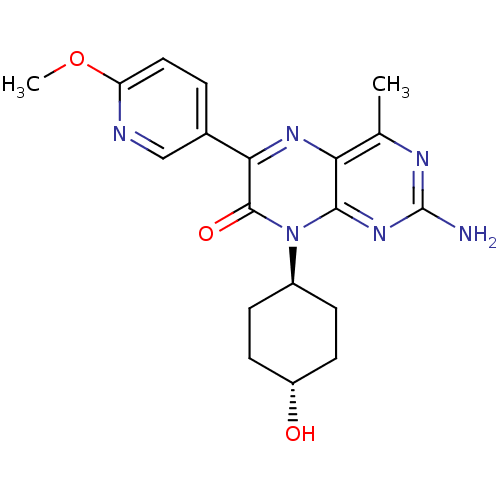
(CHEMBL1257177 | trans-2-amino-8-((1r,4r)-4-hydroxy...)Show SMILES COc1ccc(cn1)-c1nc2c(C)nc(N)nc2n([C@H]2CC[C@H](O)CC2)c1=O |r,wU:19.20,wD:22.24,(6.1,-15.1,;4.76,-14.34,;3.43,-15.12,;3.44,-16.66,;2.11,-17.44,;.77,-16.67,;.76,-15.14,;2.09,-14.36,;-.55,-17.45,;-1.88,-16.69,;-3.21,-17.46,;-4.55,-16.7,;-4.55,-15.16,;-5.87,-17.47,;-5.88,-19.01,;-7.21,-19.78,;-4.54,-19.78,;-3.21,-19,;-1.87,-19.77,;-1.87,-21.31,;-.53,-22.08,;-.54,-23.62,;-1.87,-24.39,;-1.87,-25.93,;-3.2,-23.62,;-3.2,-22.08,;-.54,-19,;.8,-19.76,)| Show InChI InChI=1S/C19H22N6O3/c1-10-15-17(24-19(20)22-10)25(12-4-6-13(26)7-5-12)18(27)16(23-15)11-3-8-14(28-2)21-9-11/h3,8-9,12-13,26H,4-7H2,1-2H3,(H2,20,22,24)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440900
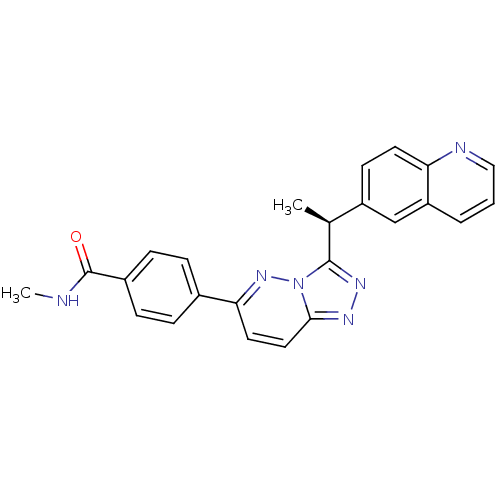
(CHEMBL2431830)Show SMILES CNC(=O)c1ccc(cc1)-c1ccc2nnc([C@@H](C)c3ccc4ncccc4c3)n2n1 |r| Show InChI InChI=1S/C24H20N6O/c1-15(18-9-10-20-19(14-18)4-3-13-26-20)23-28-27-22-12-11-21(29-30(22)23)16-5-7-17(8-6-16)24(31)25-2/h3-15H,1-2H3,(H,25,31)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396967
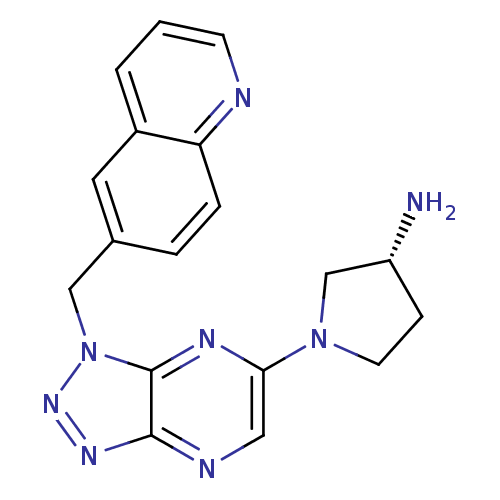
(CHEMBL2170806)Show SMILES N[C@@H]1CCN(C1)c1cnc2nnn(Cc3ccc4ncccc4c3)c2n1 |r| Show InChI InChI=1S/C18H18N8/c19-14-5-7-25(11-14)16-9-21-17-18(22-16)26(24-23-17)10-12-3-4-15-13(8-12)2-1-6-20-15/h1-4,6,8-9,14H,5,7,10-11,19H2/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440880
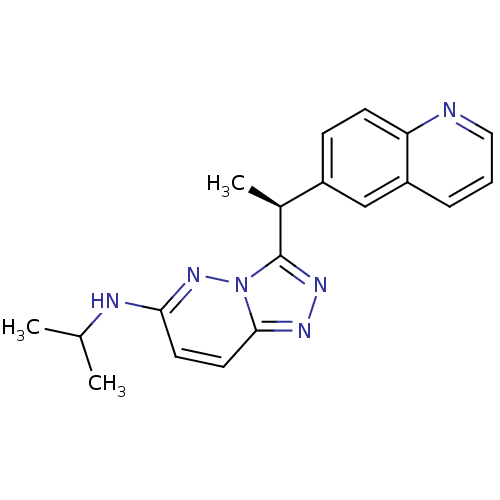
(CHEMBL2431836)Show SMILES CC(C)Nc1ccc2nnc([C@@H](C)c3ccc4ncccc4c3)n2n1 |r| Show InChI InChI=1S/C19H20N6/c1-12(2)21-17-8-9-18-22-23-19(25(18)24-17)13(3)14-6-7-16-15(11-14)5-4-10-20-16/h4-13H,1-3H3,(H,21,24)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440897

(CHEMBL2431827)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1ccc(cc1)C#N)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C23H16N6/c1-15(18-8-9-20-19(13-18)3-2-12-25-20)23-27-26-22-11-10-21(28-29(22)23)17-6-4-16(14-24)5-7-17/h2-13,15H,1H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440903

(CHEMBL2431833)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1ccc(nc1)N(C)C)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C23H21N7/c1-15(16-6-8-19-17(13-16)5-4-12-24-19)23-27-26-22-11-9-20(28-30(22)23)18-7-10-21(25-14-18)29(2)3/h4-15H,1-3H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327896
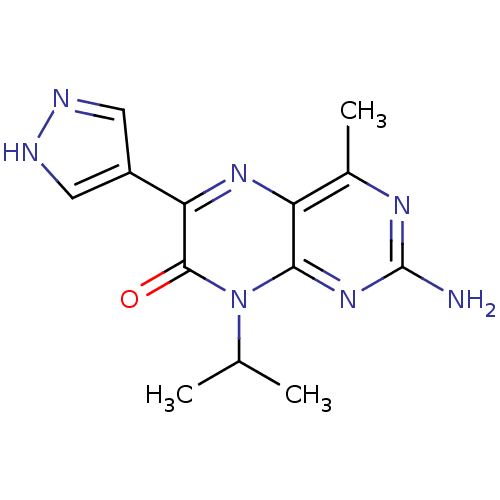
(2-amino-4-methyl-8-(1-methylethyl)-6-(1H-pyrazol-4...)Show InChI InChI=1S/C13H15N7O/c1-6(2)20-11-9(7(3)17-13(14)19-11)18-10(12(20)21)8-4-15-16-5-8/h4-6H,1-3H3,(H,15,16)(H2,14,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396969

(CHEMBL2170955)Show InChI InChI=1S/C16H15N7O/c24-7-6-18-14-9-19-15-16(20-14)23(22-21-15)10-11-3-4-13-12(8-11)2-1-5-17-13/h1-5,8-9,24H,6-7,10H2,(H,18,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440906

(CHEMBL2431819)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1cnn(C)c1)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C20H17N7/c1-13(14-5-6-17-15(10-14)4-3-9-21-17)20-24-23-19-8-7-18(25-27(19)20)16-11-22-26(2)12-16/h3-13H,1-2H3/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440884

(CHEMBL2431816)Show SMILES CC(C)Nc1ccc2nnc([C@@H](C)c3c[nH]c4ncccc34)n2n1 |r| Show InChI InChI=1S/C17H19N7/c1-10(2)20-14-6-7-15-21-22-17(24(15)23-14)11(3)13-9-19-16-12(13)5-4-8-18-16/h4-11H,1-3H3,(H,18,19)(H,20,23)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396960

(CHEMBL2170957)Show InChI InChI=1S/C17H12FN5O/c18-13-5-3-12(4-6-13)15-10-19-17-21-20-16(23(17)22-15)9-11-1-7-14(24)8-2-11/h1-8,10,24H,9H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440902
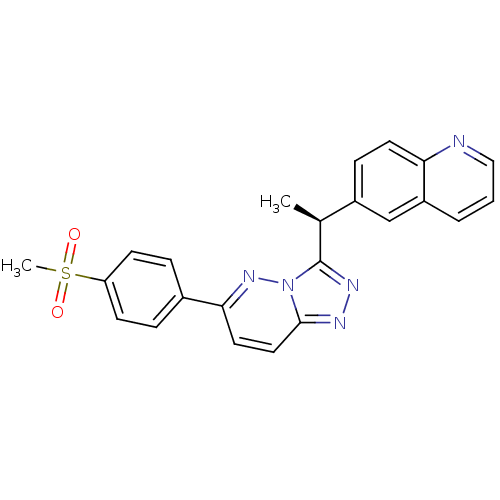
(CHEMBL2431832)Show SMILES C[C@H](c1nnc2ccc(nn12)-c1ccc(cc1)S(C)(=O)=O)c1ccc2ncccc2c1 |r| Show InChI InChI=1S/C23H19N5O2S/c1-15(17-7-10-20-18(14-17)4-3-13-24-20)23-26-25-22-12-11-21(27-28(22)23)16-5-8-19(9-6-16)31(2,29)30/h3-15H,1-2H3/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50352534
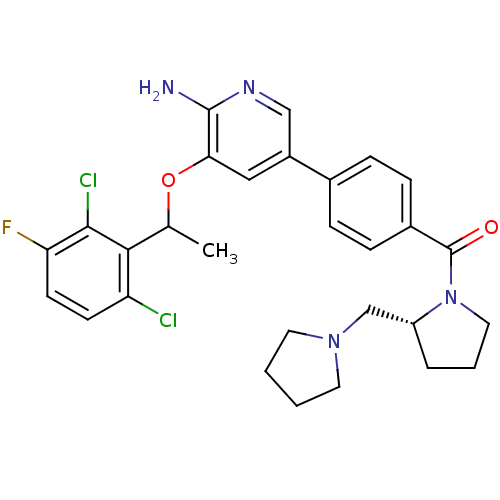
(CHEMBL1824878)Show SMILES CC(Oc1cc(cnc1N)-c1ccc(cc1)C(=O)N1CCC[C@@H]1CN1CCCC1)c1c(Cl)ccc(F)c1Cl |r| Show InChI InChI=1S/C29H31Cl2FN4O2/c1-18(26-23(30)10-11-24(32)27(26)31)38-25-15-21(16-34-28(25)33)19-6-8-20(9-7-19)29(37)36-14-4-5-22(36)17-35-12-2-3-13-35/h6-11,15-16,18,22H,2-5,12-14,17H2,1H3,(H2,33,34)/t18?,22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant c-MET assessed as inhibition of autophosphorylation by continuous fluorometric assay |
J Med Chem 54: 6342-63 (2011)
Article DOI: 10.1021/jm2007613
BindingDB Entry DOI: 10.7270/Q2Q52Q09 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440888

(CHEMBL2429888)Show SMILES N#Cc1cccc(c1)-c1ccc2nnc(Cc3ccc4ncccc4c3)n2n1 Show InChI InChI=1S/C22H14N6/c23-14-16-3-1-4-18(12-16)20-8-9-21-25-26-22(28(21)27-20)13-15-6-7-19-17(11-15)5-2-10-24-19/h1-12H,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396935
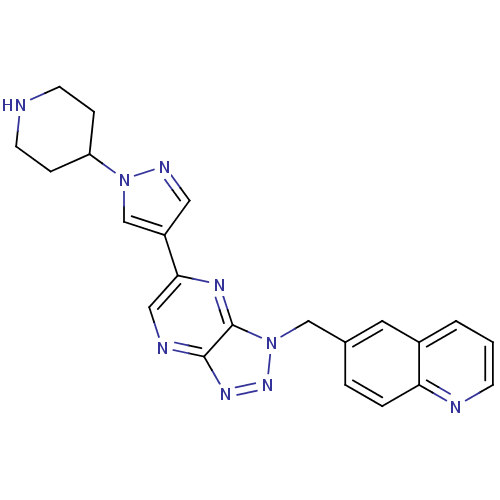
(CHEMBL2170950)Show SMILES C(c1ccc2ncccc2c1)n1nnc2ncc(nc12)-c1cnn(c1)C1CCNCC1 Show InChI InChI=1S/C22H21N9/c1-2-16-10-15(3-4-19(16)24-7-1)13-31-22-21(28-29-31)25-12-20(27-22)17-11-26-30(14-17)18-5-8-23-9-6-18/h1-4,7,10-12,14,18,23H,5-6,8-9,13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50352559
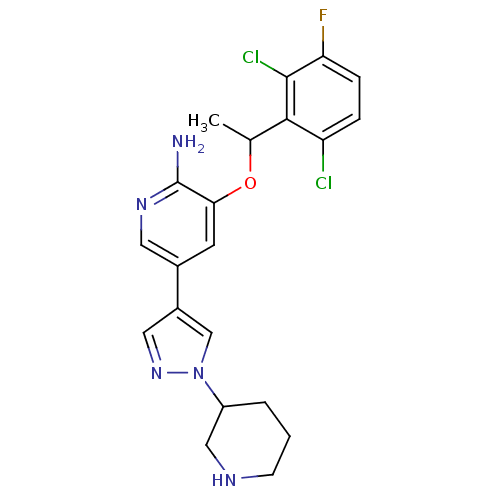
(CHEMBL1825136)Show SMILES CC(Oc1cc(cnc1N)-c1cnn(c1)C1CCCNC1)c1c(Cl)ccc(F)c1Cl Show InChI InChI=1S/C21H22Cl2FN5O/c1-12(19-16(22)4-5-17(24)20(19)23)30-18-7-13(8-27-21(18)25)14-9-28-29(11-14)15-3-2-6-26-10-15/h4-5,7-9,11-12,15,26H,2-3,6,10H2,1H3,(H2,25,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human recombinant c-MET assessed as inhibition of autophosphorylation by continuous fluorometric assay |
J Med Chem 54: 6342-63 (2011)
Article DOI: 10.1021/jm2007613
BindingDB Entry DOI: 10.7270/Q2Q52Q09 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396971

(CHEMBL2170953)Show InChI InChI=1S/C14H11N7/c15-12-7-17-13-14(18-12)21(20-19-13)8-9-3-4-11-10(6-9)2-1-5-16-11/h1-7H,8H2,(H2,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50396971

(CHEMBL2170953)Show InChI InChI=1S/C14H11N7/c15-12-7-17-13-14(18-12)21(20-19-13)8-9-3-4-11-10(6-9)2-1-5-16-11/h1-7H,8H2,(H2,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Competitive inhibition of N-terminal His6-tagged recombinant human c-MET (974 to 1390 amino acids) by spectrophotometry |
J Med Chem 55: 8091-109 (2012)
Article DOI: 10.1021/jm300967g
BindingDB Entry DOI: 10.7270/Q2Z60Q59 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440883

(CHEMBL2431815)Show SMILES C[C@@H](c1c[nH]c2ncccc12)c1nnc2ccc(nn12)-c1ccc(cc1)C#N |r| Show InChI InChI=1S/C21H15N7/c1-13(17-12-24-20-16(17)3-2-10-23-20)21-26-25-19-9-8-18(27-28(19)21)15-6-4-14(11-22)5-7-15/h2-10,12-13H,1H3,(H,23,24)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440882

(CHEMBL2431838)Show SMILES C[C@@H](c1c[nH]c2ncccc12)c1nnc2ccc(nn12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C18H16N8/c1-11(14-9-20-17-13(14)4-3-7-19-17)18-23-22-16-6-5-15(24-26(16)18)12-8-21-25(2)10-12/h3-11H,1-2H3,(H,19,20)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM50440885
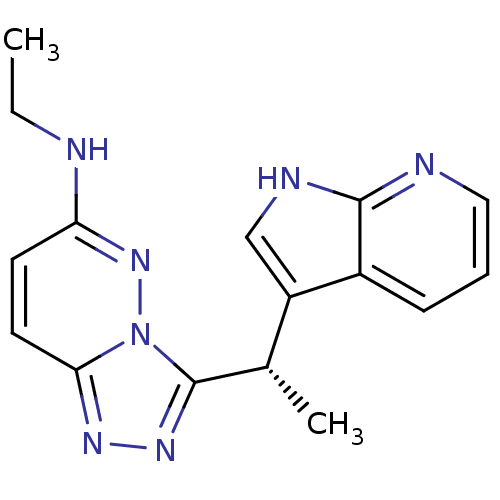
(CHEMBL2431817)Show SMILES CCNc1ccc2nnc([C@@H](C)c3c[nH]c4ncccc34)n2n1 |r| Show InChI InChI=1S/C16H17N7/c1-3-17-13-6-7-14-20-21-16(23(14)22-13)10(2)12-9-19-15-11(12)5-4-8-18-15/h4-10H,3H2,1-2H3,(H,17,22)(H,18,19)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-6-tagged human recombinant c-Met (974 to 1390) using Ac-ARDMYDKEYYSVHNK as substrate by spectrophotometric assay |
J Med Chem 56: 6651-65 (2013)
Article DOI: 10.1021/jm400926x
BindingDB Entry DOI: 10.7270/Q2HM59WR |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50327901
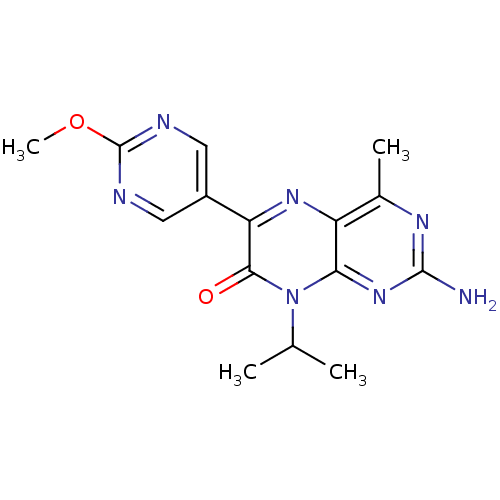
(2-amino-8-isopropyl-6-(2-methoxypyrimidin-5-yl)-4-...)Show InChI InChI=1S/C15H17N7O2/c1-7(2)22-12-10(8(3)19-14(16)21-12)20-11(13(22)23)9-5-17-15(24-4)18-6-9/h5-7H,1-4H3,(H2,16,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mTOR |
Bioorg Med Chem Lett 20: 6096-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.08.045
BindingDB Entry DOI: 10.7270/Q2ZK5GWH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data