

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43866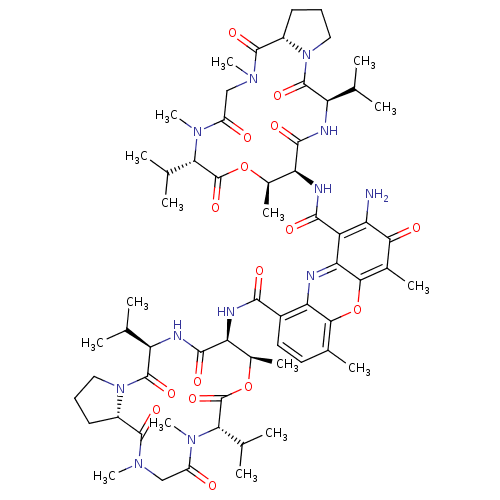 (2-amino-4,6-dimethyl-3-oxo-1-N,9-N-bis[(3R,6S,7R,1...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | Article PubMed | 41 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43865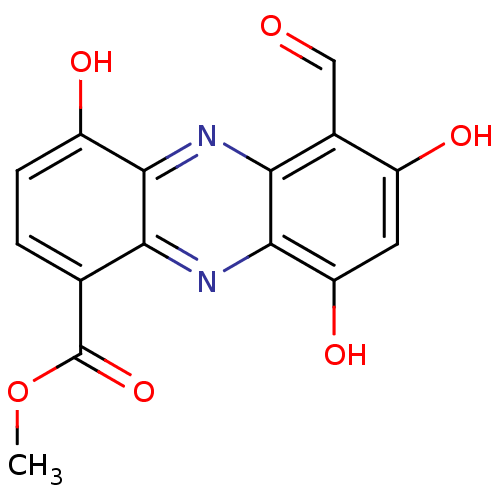 (6-formyl-4,7-dihydroxy-9-keto-5H-phenazine-1-carbo...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 62 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43863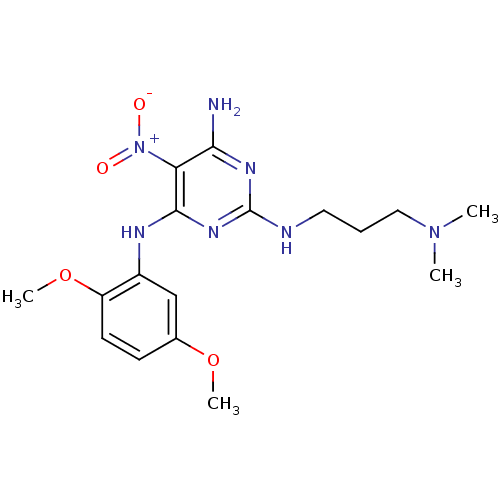 (3-[[4-amino-6-(2,5-dimethoxyanilino)-5-nitro-pyrim...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | -31.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43860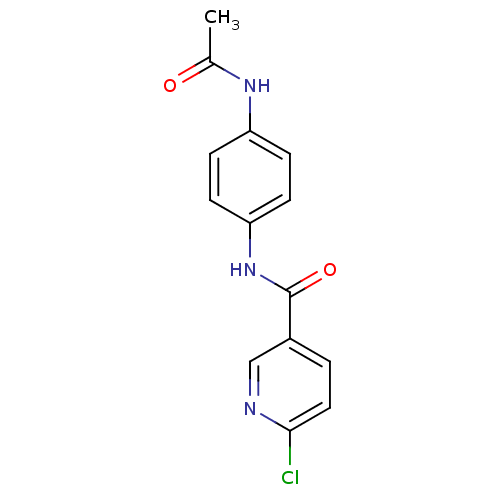 (Glycopeptide, 2 | MLS000517385 | N-(4-acetamidophe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -29.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43864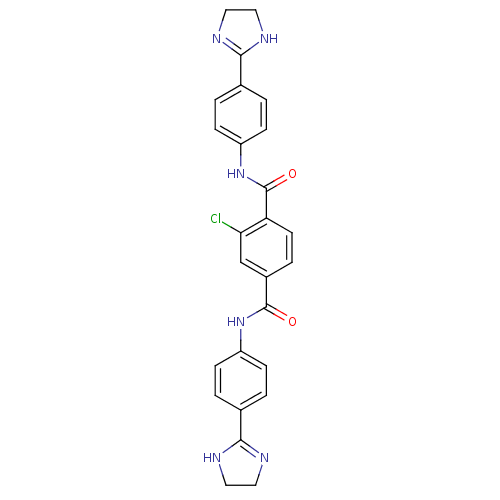 (2-chloranyl-N1,N4-bis[4-(4,5-dihydro-1H-imidazol-2...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | -28.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43861 (4-[7-[4-[diethyl(methyl)ammonio]butoxy]-9-keto-flu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | -28.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43862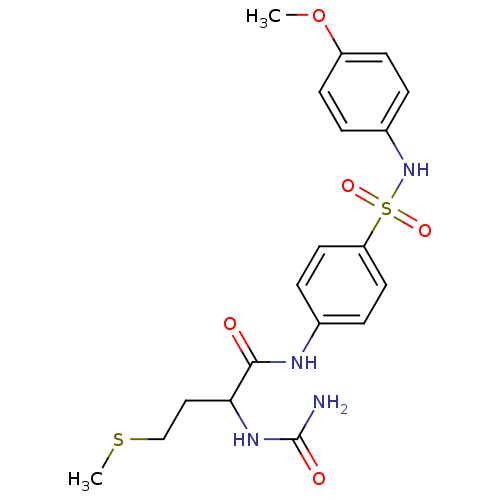 (2-(aminocarbonylamino)-N-[4-[(4-methoxyphenyl)sulf...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.40E+4 | -23.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dolichyl-diphosphooligosaccharide--protein glycosyltransferase subunit 1 (Saccharomyces cerevisiae (Baker's yeast)) | BDBM43859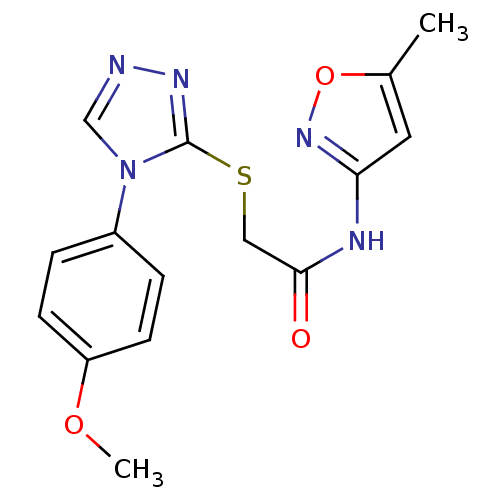 (2-[[4-(4-methoxyphenyl)-1,2,4-triazol-3-yl]sulfany...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.00E+5 | -20.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 0 |
Massachusetts Institute of Technology | Assay Description Competition assay using S. cerevisiae oligosaccharyl transferase (OT)with disaccharide donor Dol-P-P-(GlcNAc)2 and the tripeptide substrate Bz-Asn-Le... | Chem Biol 9: 1323-8 (2002) Article DOI: 10.1016/S1074-5521(02)00281-8 BindingDB Entry DOI: 10.7270/Q2736P9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM50420304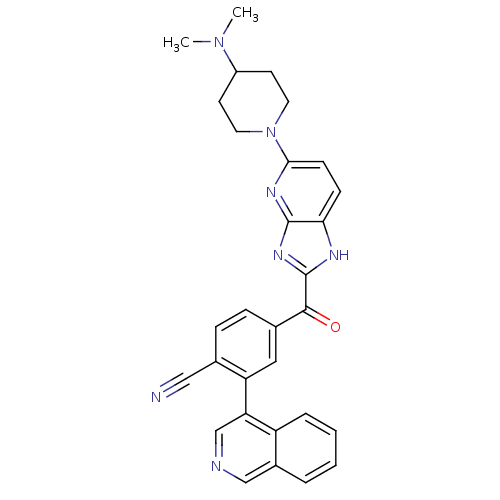 (CHEMBL2089065 | US8598217, 165) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D1 (Homo sapiens (Human)) | BDBM87119 ((1R,2R)-N-[(1S)-2-[4-(5-bromo-2-keto-3H-benzimidaz...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107751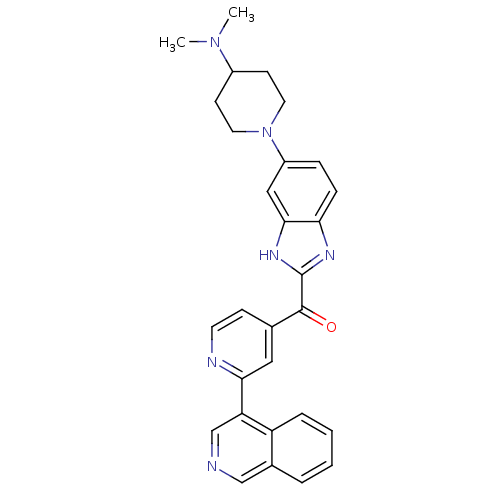 (CHEMBL2089063 | US8598217, 89) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107755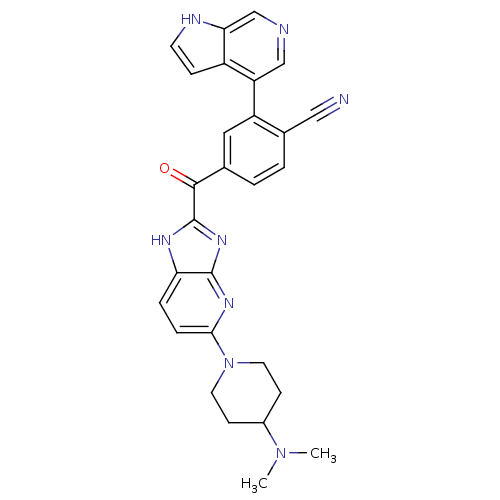 (US8598217, 140) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107761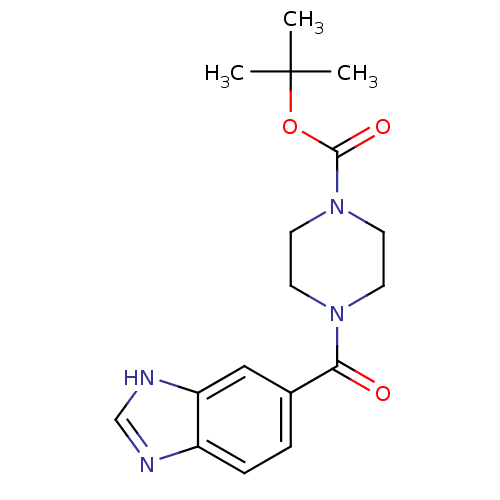 (US8598217, 174) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D1 (Homo sapiens (Human)) | BDBM154517 (ML299 (5)) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107765 (US8598217, 166) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM50420303 (CHEMBL2089064 | US8598217, 135) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM50420305 (CHEMBL2089057 | US8598217, 173) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107756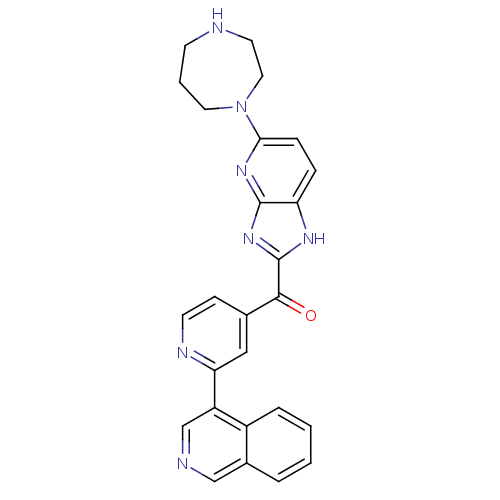 (US8598217, 163) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154517 (ML299 (5)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phospholipase D1 (Homo sapiens (Human)) | BDBM87245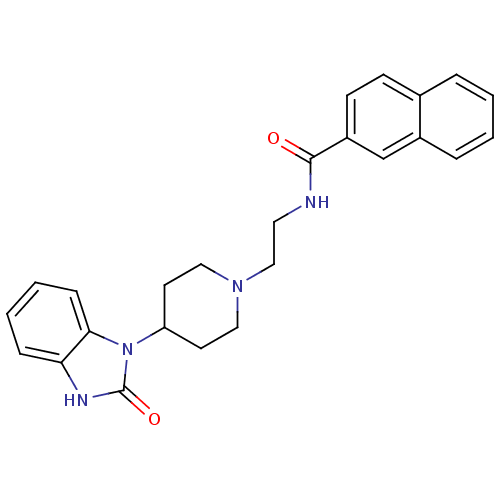 (CHEMBL492797 | ML272 | N-(2-(4-(2-Oxo-2,3-dihydro-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107757 (US8598217, 164) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107753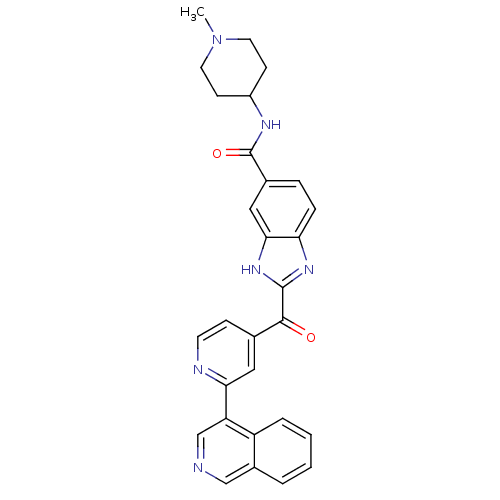 (US8598217, 99) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107750 (US8598217, 86) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107752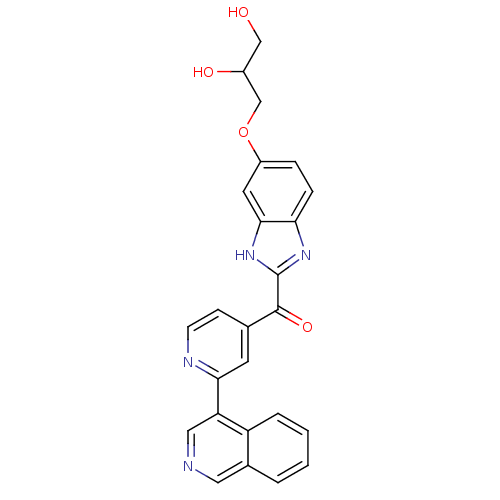 (US8598217, 98) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107749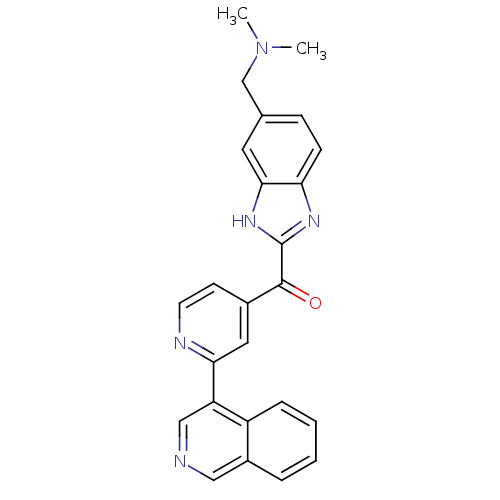 (US8598217, 84) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 57 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107754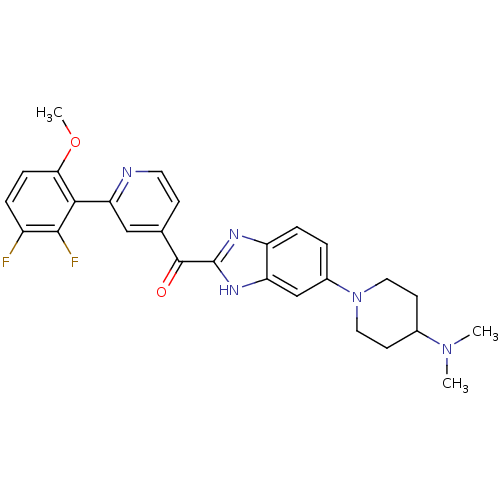 (US8598217, 103) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107764 (US8598217, 148) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 67 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107762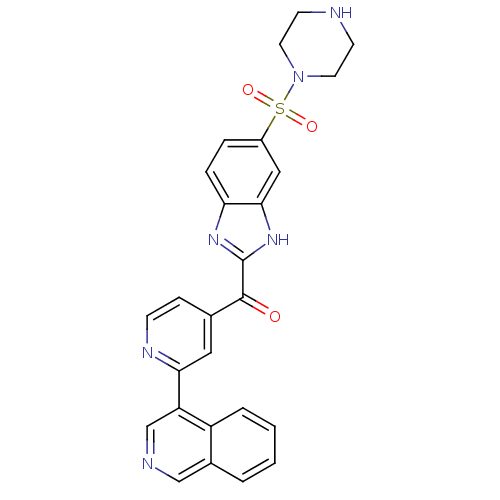 (US8598217, 182) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107763 (US8598217, 189) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 95 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107758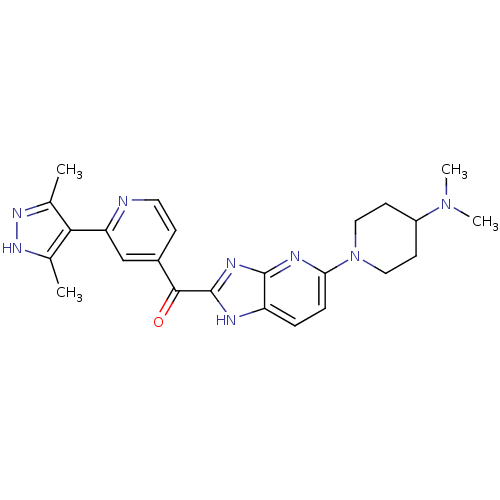 (US8598217, 167) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 195 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107759 (US8598217, 169) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM87123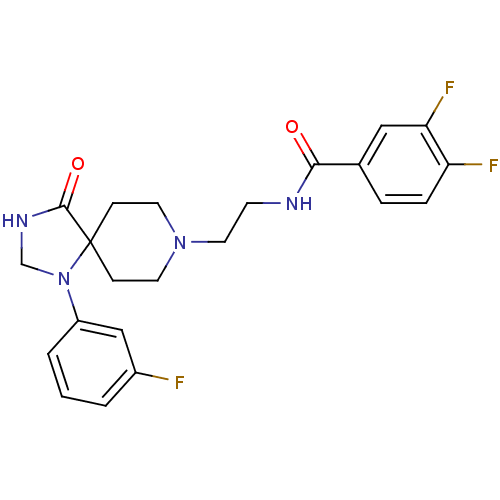 (3,4-bis(fluoranyl)-N-[2-[1-(3-fluorophenyl)-4-oxid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 355 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154518 (ML395 (6)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Oryctolagus cuniculus (Rabbit)) | BDBM107760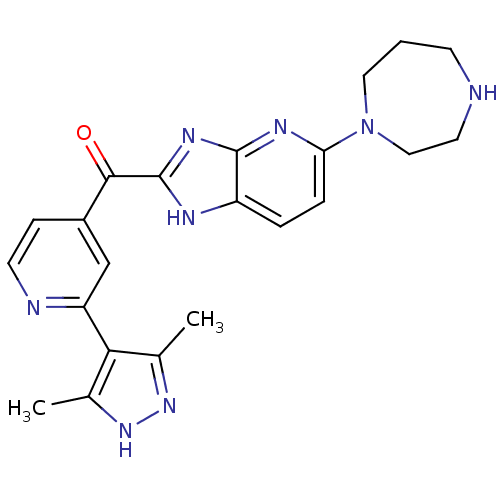 (US8598217, 170) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 548 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Ltd.; Novartis AG US Patent | Assay Description A 384-well microtiter Lance TR-FRET (time-resolved-fluorescence energy transfer) endpoint assay was used for CDK4/cyclin D1 kinase activity measureme... | US Patent US8598217 (2013) BindingDB Entry DOI: 10.7270/Q2BG2MN8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D1 (Homo sapiens (Human)) | BDBM87120 (CHEMBL1254577 | N-[2-[1-(3-fluorophenyl)-4-keto-1,...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM75441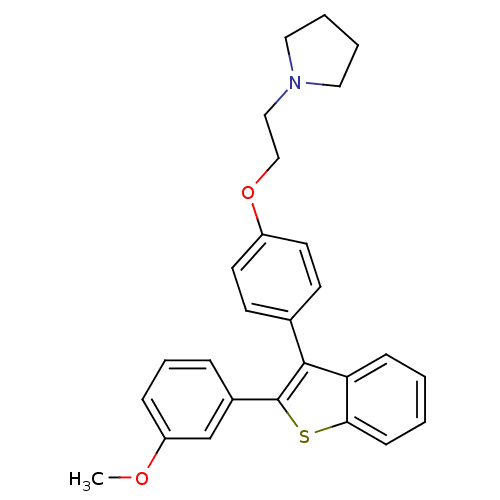 (1-[2-[4-[2-(3-methoxyphenyl)-1-benzothiophen-3-yl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM154526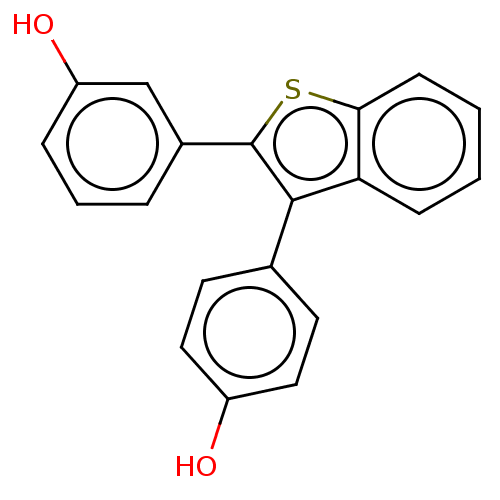 (22B1 (17i)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description In a 96-black well plate, in a final reaction volume of 200 μL, the following components were combined on ice to yield final concentrations of 5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154519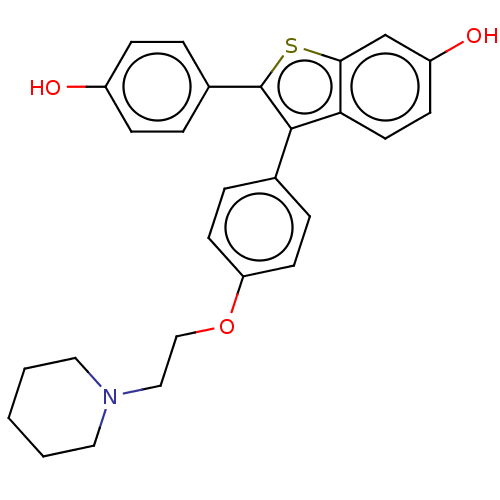 (Desketoraloxifene (10)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154519 (Desketoraloxifene (10)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D1 (Homo sapiens (Human)) | BDBM75442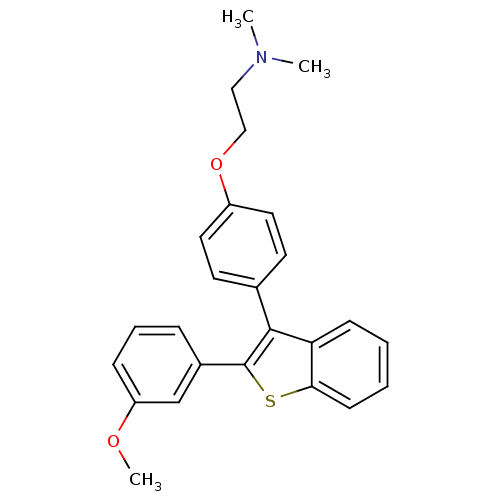 (2-[4-[2-(3-methoxyphenyl)-1-benzothiophen-3-yl]phe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D1 (Homo sapiens (Human)) | BDBM154524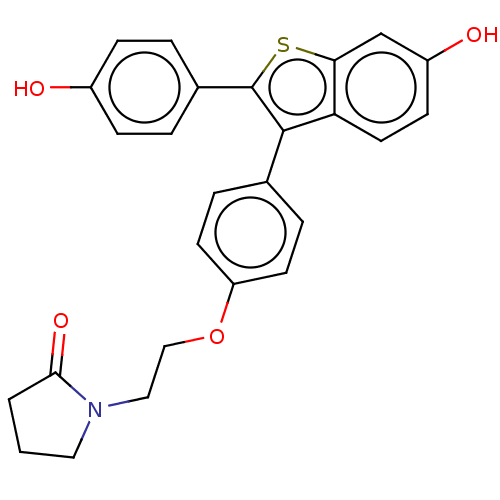 (23VK (17g)) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM154528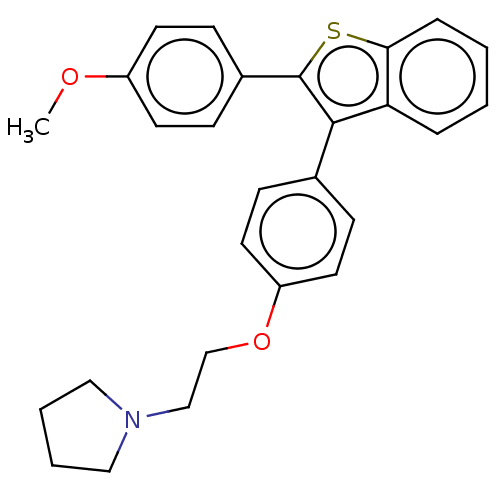 (234H (17l)) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description In a 96-black well plate, in a final reaction volume of 200 μL, the following components were combined on ice to yield final concentrations of 5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154527 (2336 (17j)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154525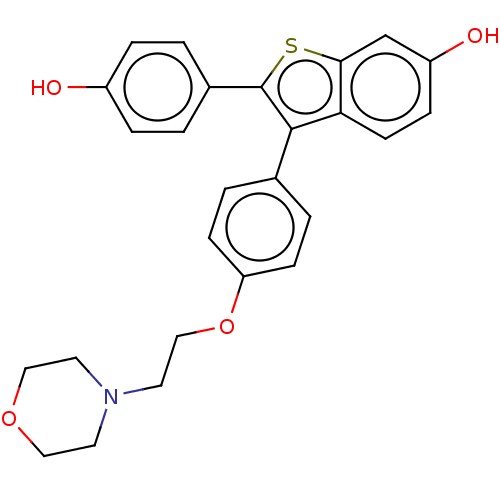 (24F0 (17h)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154524 (23VK (17g)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description In brief, 6 nM purified PldA was incubated with liposomes containing 90 μM 1,2-dioleoyl-sn-glycero-3-phospho-(1′-rac-glycerol), 10 μM... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM154523 (24DY (17f)) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D1 (Homo sapiens (Human)) | BDBM75442 (2-[4-[2-(3-methoxyphenyl)-1-benzothiophen-3-yl]phe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description Briefly, PLD activity was measured as the release of free [3H]-choline from [choline-methyl-3H] dipalmitoylphosphatidylcholine ([3H]-DPPC). 3−5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase D2 (Homo sapiens (Human)) | BDBM19441 (2-(4-hydroxyphenyl)-3-({4-[2-(piperidin-1-yl)ethox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University | Assay Description Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cell... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase (Pseudomonas aeruginosa (g-Proteobacteria)) | BDBM75443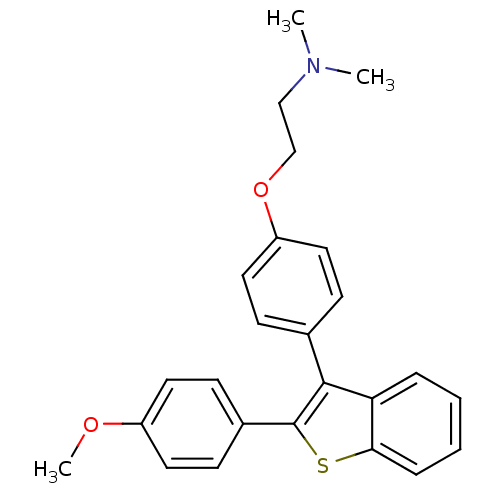 (2-[4-[2-(4-methoxyphenyl)-1-benzothiophen-3-yl]phe...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Vanderbilt University | Assay Description In a 96-black well plate, in a final reaction volume of 200 μL, the following components were combined on ice to yield final concentrations of 5... | ACS Chem Biol 10: 421-32 (2015) Article DOI: 10.1021/cb500828m BindingDB Entry DOI: 10.7270/Q2H70DK8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 105 total ) | Next | Last >> |