

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Homo sapiens (Human)) | BDBM25680 (4-[(5-bromo-1,3-thiazol-2-yl)amino]benzoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 79 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25683 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-(2-methoxyet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25681 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-methylbenzam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25684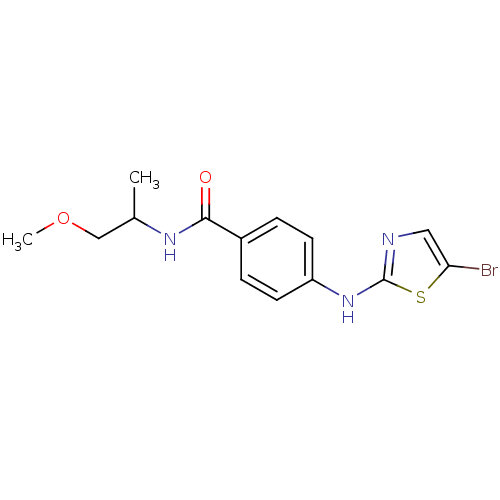 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-(1-methoxypr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25678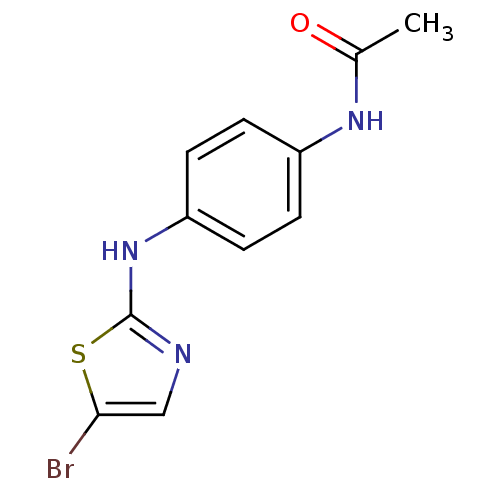 (N-{4-[(5-bromo-1,3-thiazol-2-yl)amino]phenyl}aceta...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM25658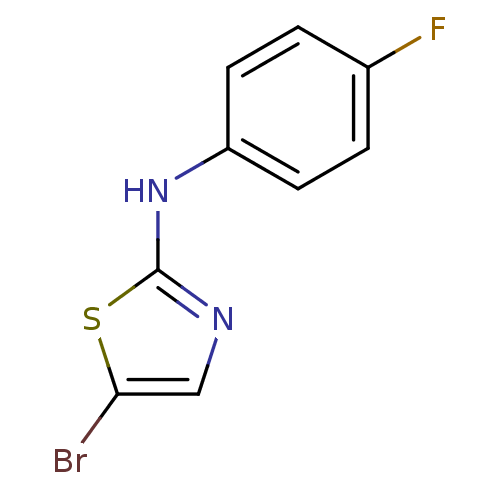 (5-bromo-N-(4-fluorophenyl)-1,3-thiazol-2-amine | a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Enzyme activity was assayed using Z-LYTE Enzymatic Kinase Assay format (Invitrogen Corp., Carlsbad, CA) in the SelectScreen Kinase Profiling Service... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25658 (5-bromo-N-(4-fluorophenyl)-1,3-thiazol-2-amine | a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM25681 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-methylbenzam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Enzyme activity was assayed using Z-LYTE Enzymatic Kinase Assay format (Invitrogen Corp., Carlsbad, CA) in the SelectScreen Kinase Profiling Service... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25697 (3-[(5-bromo-1,3-thiazol-2-yl)amino]benzoic acid | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 440 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25694 (5-bromo-N-[3-(methylsulfanyl)phenyl]-1,3-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25660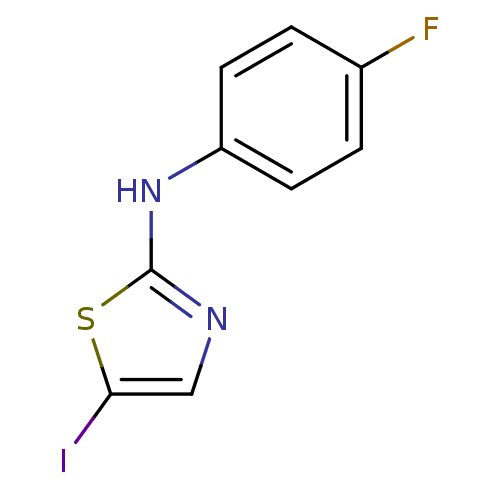 (N-(4-fluorophenyl)-5-iodo-1,3-thiazol-2-amine | am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25669 (4-[(5-bromo-1,3-thiazol-2-yl)amino]phenol | aminot...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25676 (1-N-(5-bromo-1,3-thiazol-2-yl)benzene-1,4-diamine ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25699 (3-[(5-bromo-1,3-thiazol-2-yl)amino]-N-methylbenzam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25721 (4-({6-[(5-cyano-1,3-thiazol-2-yl)amino]pyridin-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25673 (5-bromo-N-[4-(pyridin-4-yloxy)phenyl]-1,3-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25693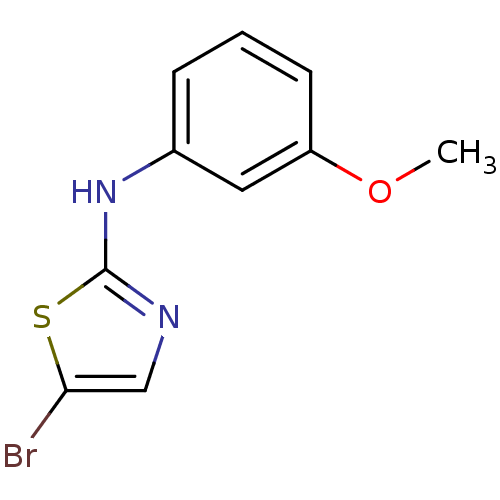 (5-bromo-N-(3-methoxyphenyl)-1,3-thiazol-2-amine | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM25680 (4-[(5-bromo-1,3-thiazol-2-yl)amino]benzoic acid | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Enzyme activity was assayed using Z-LYTE Enzymatic Kinase Assay format (Invitrogen Corp., Carlsbad, CA) in the SelectScreen Kinase Profiling Service... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25685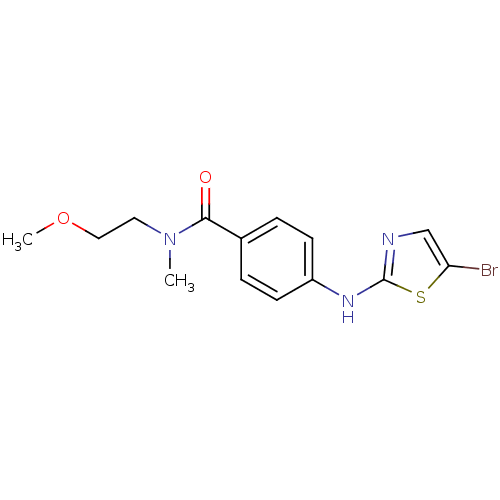 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-(2-methoxyet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25662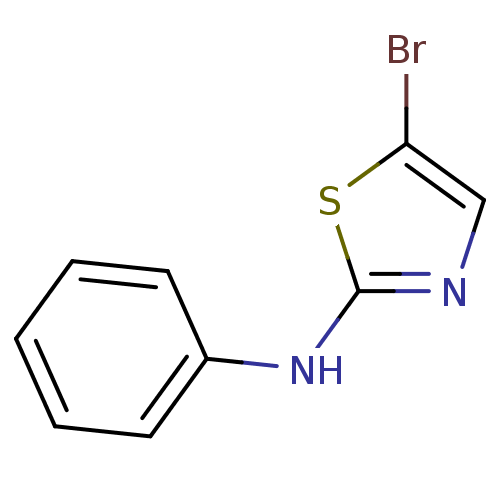 (5-bromo-N-phenyl-1,3-thiazol-2-amine | aminothiazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25657 (5-chloro-N-(4-fluorophenyl)-1,3-thiazol-2-amine | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25691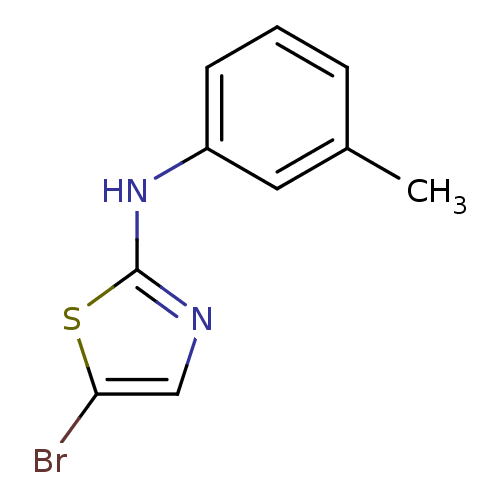 (5-bromo-N-(3-methylphenyl)-1,3-thiazol-2-amine | a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25698 (3-[(5-bromo-1,3-thiazol-2-yl)amino]-N-(2-methoxyet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25720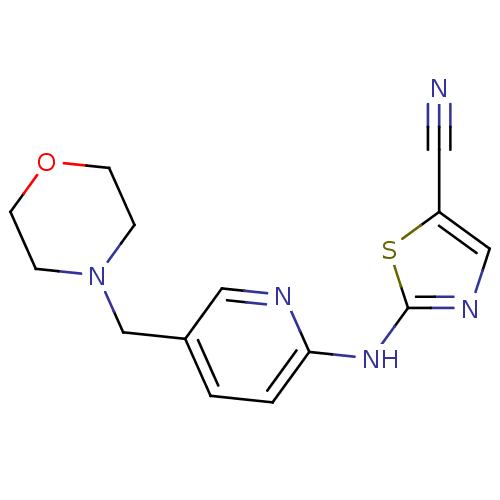 (2-{[5-(morpholin-4-ylmethyl)pyridin-2-yl]amino}-1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25672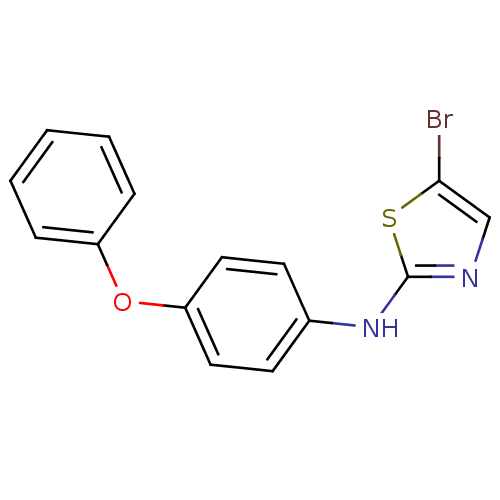 (5-bromo-N-(4-phenoxyphenyl)-1,3-thiazol-2-amine | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25682 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-(2-hydroxyet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25695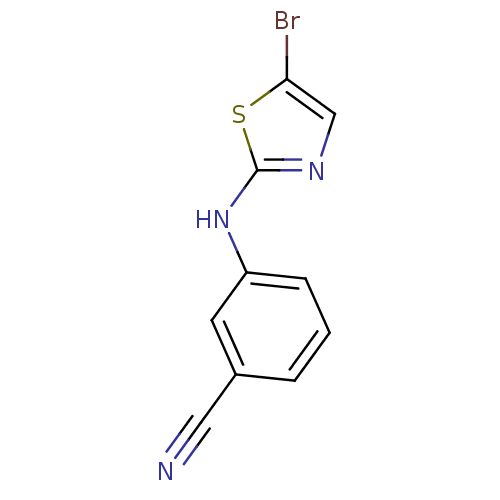 (3-[(5-bromo-1,3-thiazol-2-yl)amino]benzonitrile | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25674 (1-{4-[(5-bromo-1,3-thiazol-2-yl)amino]phenyl}ethan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25670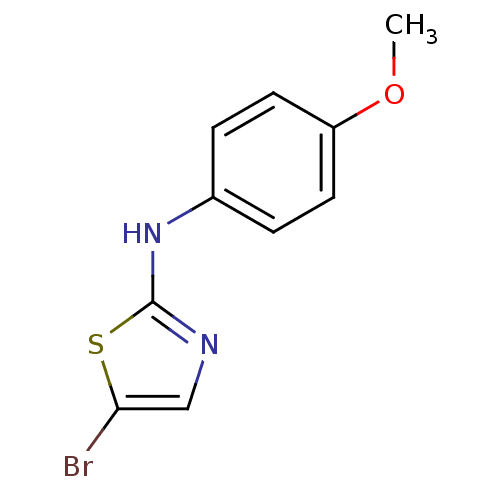 (5-bromo-N-(4-methoxyphenyl)-1,3-thiazol-2-amine | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM5359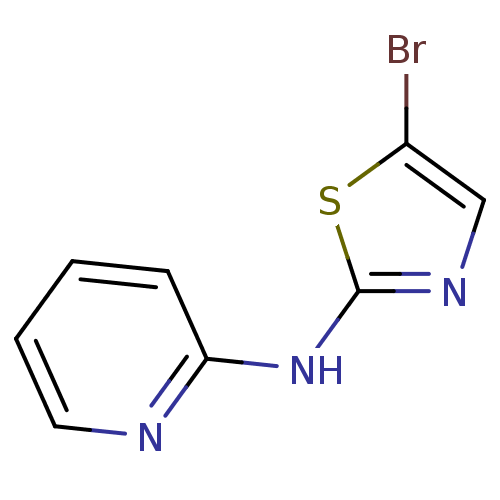 (5-bromo-N-(pyridin-2-yl)-1,3-thiazol-2-amine | ACS...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25668 (4-[(5-bromo-1,3-thiazol-2-yl)amino]benzonitrile | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25696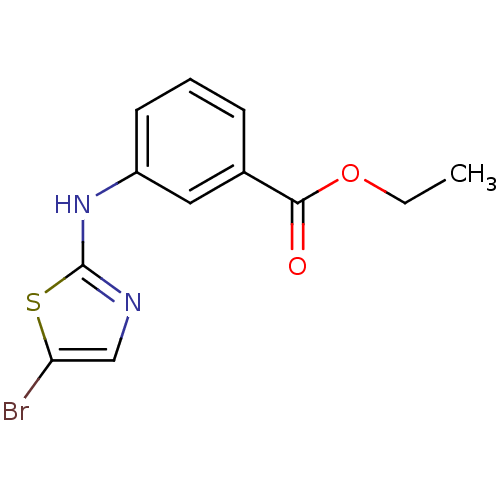 (aminothiazole analogue, 40 | ethyl 3-[(5-bromo-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25688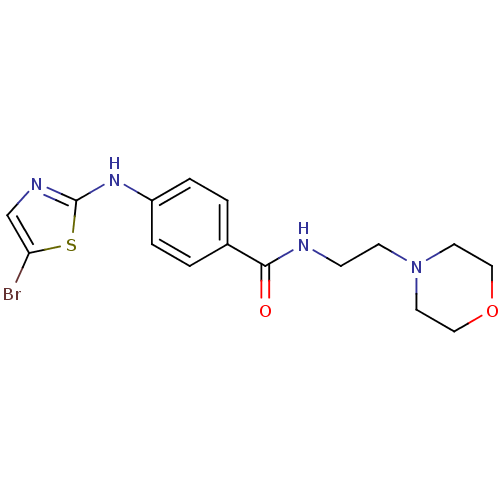 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-[2-(morpholi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25667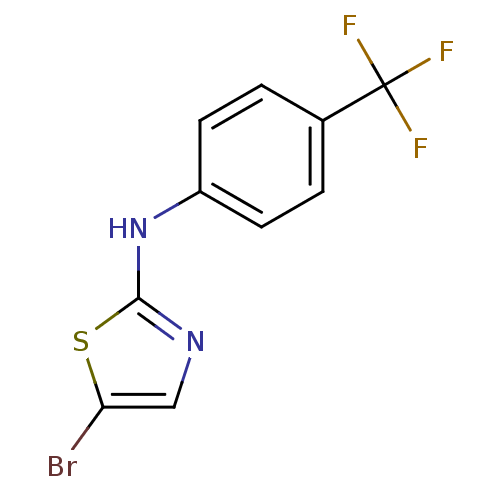 (5-bromo-N-[4-(trifluoromethyl)phenyl]-1,3-thiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM5362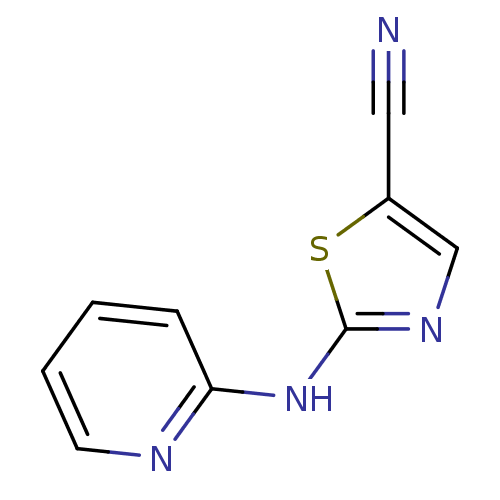 (2-(pyridin-2-ylamino)-1,3-thiazole-5-carbonitrile ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25703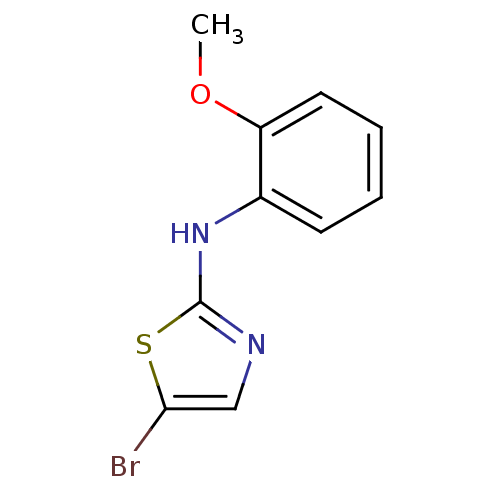 (5-bromo-N-(2-methoxyphenyl)-1,3-thiazol-2-amine | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25666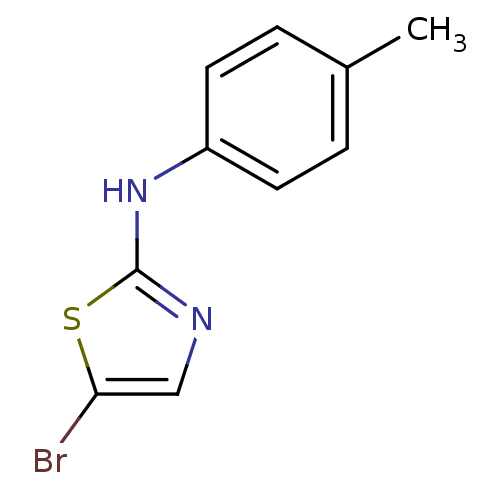 (5-bromo-N-(4-methylphenyl)-1,3-thiazol-2-amine | a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.84E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25664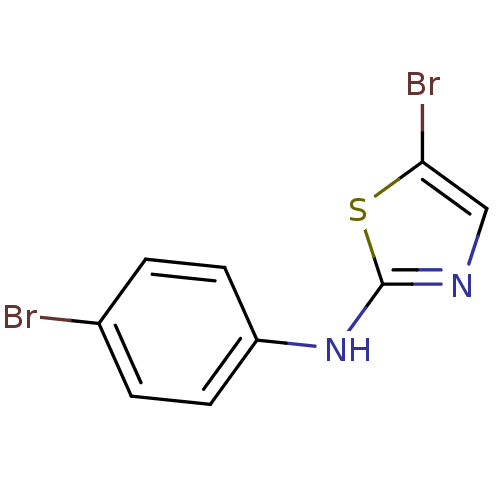 (5-bromo-N-(4-bromophenyl)-1,3-thiazol-2-amine | am...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25701 (5-bromo-N-(2-methylphenyl)-1,3-thiazol-2-amine | a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25663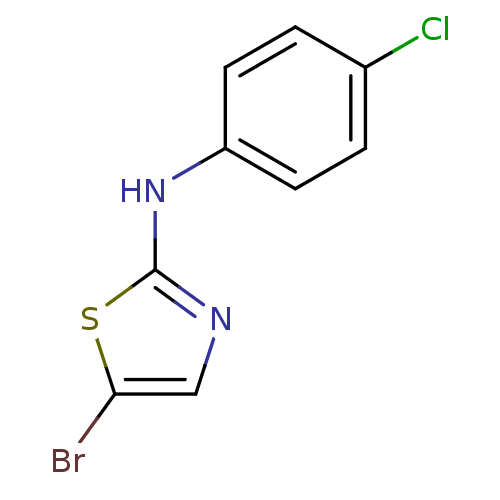 (5-bromo-N-(4-chlorophenyl)-1,3-thiazol-2-amine | a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25717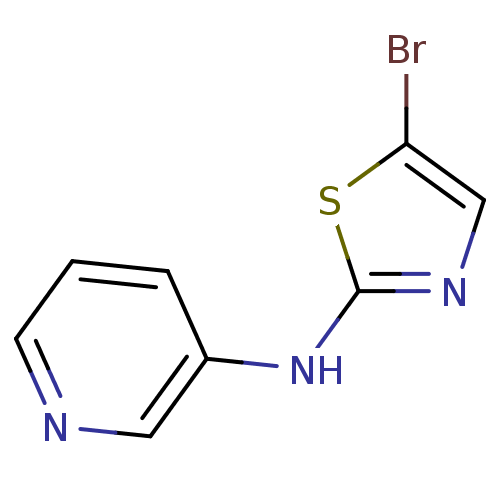 (5-bromo-N-(pyridin-3-yl)-1,3-thiazol-2-amine | ami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25722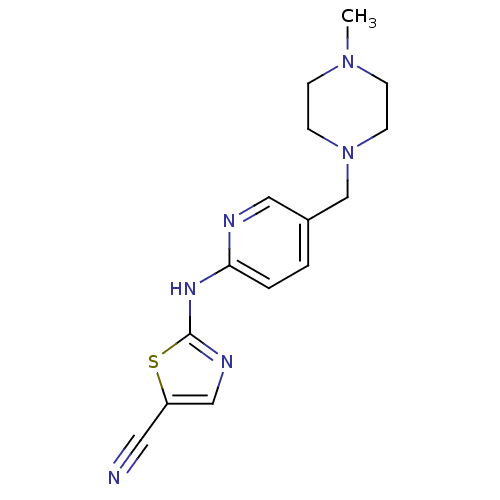 (2-({5-[(4-methylpiperazin-1-yl)methyl]pyridin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM25681 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-methylbenzam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description Enzyme activity was assayed using Z-LYTE Enzymatic Kinase Assay format (Invitrogen Corp., Carlsbad, CA) in the SelectScreen Kinase Profiling Service... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25723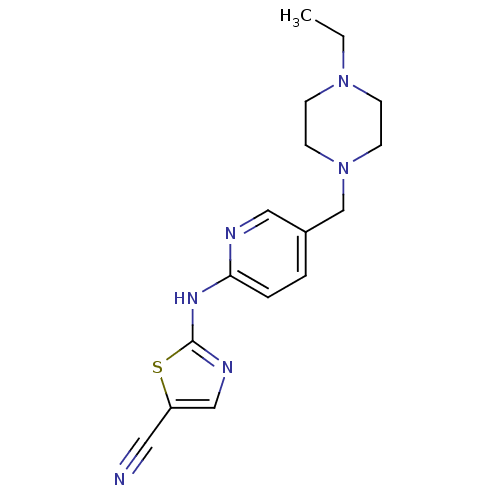 (2-({5-[(4-ethylpiperazin-1-yl)methyl]pyridin-2-yl}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25687 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-[2-(diethyla...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25715 (5-bromo-N-(naphthalen-1-yl)-1,3-thiazol-2-amine | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25686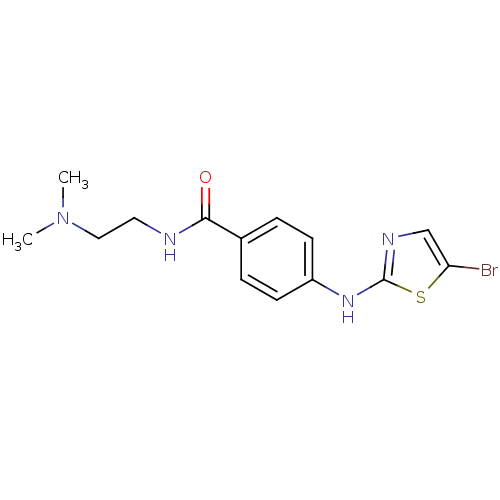 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-[2-(dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25689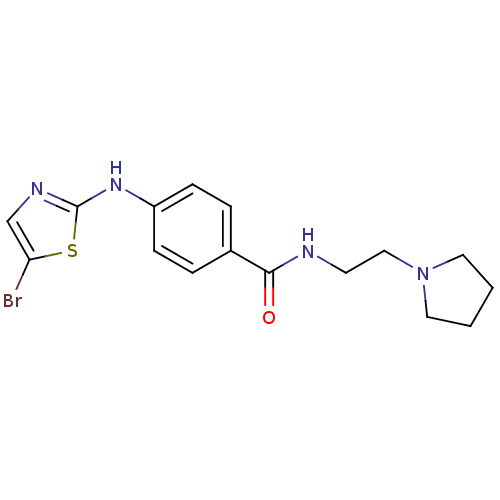 (4-[(5-bromo-1,3-thiazol-2-yl)amino]-N-[2-(pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25724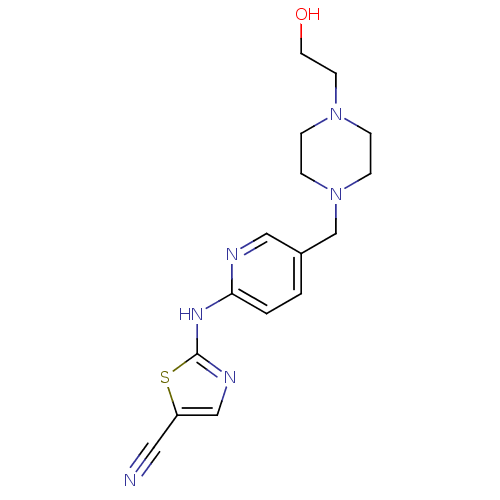 (2-[(5-{[4-(2-hydroxyethyl)piperazin-1-yl]methyl}py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM25712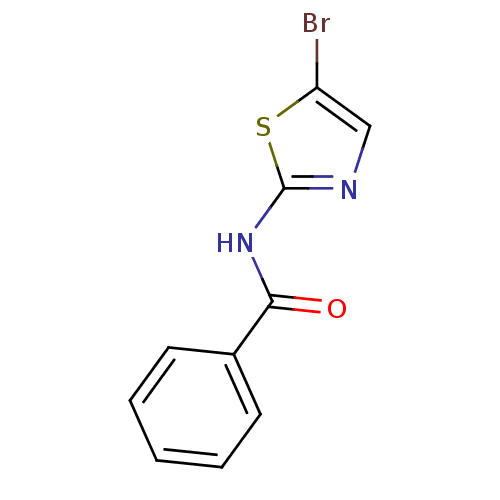 (N-(5-bromo-1,3-thiazol-2-yl)benzamide | aminothiaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis | Assay Description The biochemical activity of compounds was determined by incubation with Aurora A and substrates in the presence ATP/[gamma-33P] ATP. After incubatio... | ACS Chem Biol 3: 180-92 (2008) Article DOI: 10.1021/cb700200w BindingDB Entry DOI: 10.7270/Q2HX1B03 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 75 total ) | Next | Last >> |