 Found 178 hits with Last Name = 'riviello' and Initial = 'cm'
Found 178 hits with Last Name = 'riviello' and Initial = 'cm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033318
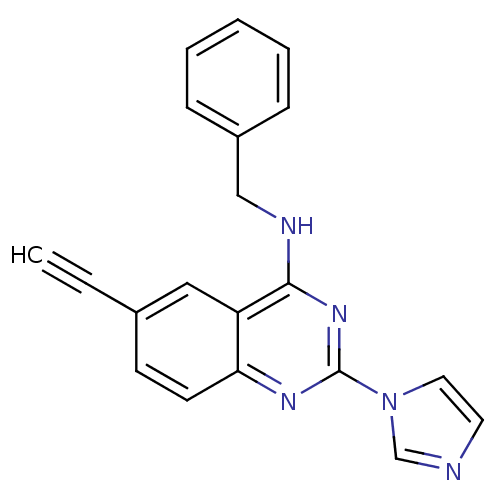
(Benzyl-(6-ethynyl-2-imidazol-1-yl-quinazolin-4-yl)...)Show InChI InChI=1S/C20H15N5/c1-2-15-8-9-18-17(12-15)19(22-13-16-6-4-3-5-7-16)24-20(23-18)25-11-10-21-14-25/h1,3-12,14H,13H2,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311657
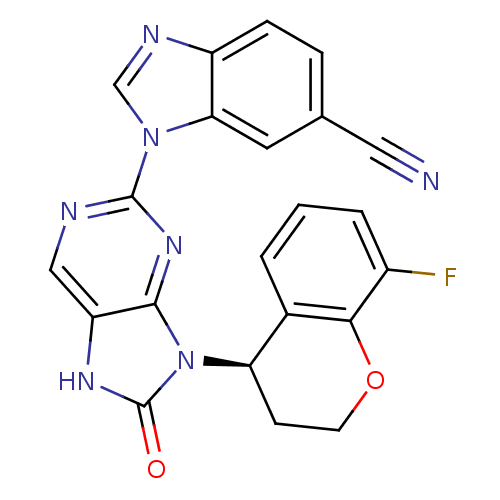
(1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...)Show SMILES Fc1cccc2[C@@H](CCOc12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccc(cc12)C#N |r| Show InChI InChI=1S/C22H14FN7O2/c23-14-3-1-2-13-17(6-7-32-19(13)14)30-20-16(27-22(30)31)10-25-21(28-20)29-11-26-15-5-4-12(9-24)8-18(15)29/h1-5,8,10-11,17H,6-7H2,(H,27,31)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033343

(Benzyl-(6-bromo-2-imidazol-1-yl-quinazolin-4-yl)-a...)Show InChI InChI=1S/C18H14BrN5/c19-14-6-7-16-15(10-14)17(21-11-13-4-2-1-3-5-13)23-18(22-16)24-9-8-20-12-24/h1-10,12H,11H2,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033313

(Benzyl-(2-imidazol-1-yl-6-nitro-quinazolin-4-yl)-a...)Show SMILES [O-][N+](=O)c1ccc2nc(nc(NCc3ccccc3)c2c1)-n1ccnc1 Show InChI InChI=1S/C18H14N6O2/c25-24(26)14-6-7-16-15(10-14)17(20-11-13-4-2-1-3-5-13)22-18(21-16)23-9-8-19-12-23/h1-10,12H,11H2,(H,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033312
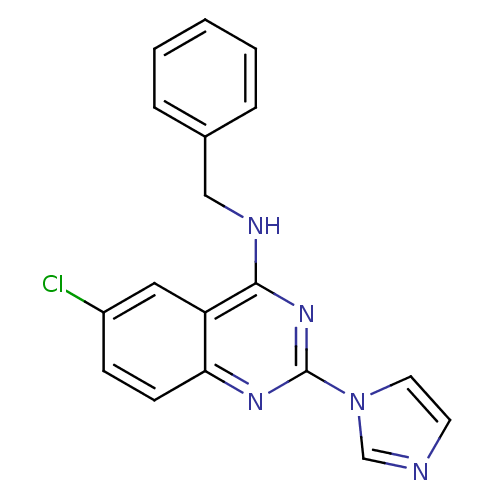
(Benzyl-(6-chloro-2-imidazol-1-yl-quinazolin-4-yl)-...)Show InChI InChI=1S/C18H14ClN5/c19-14-6-7-16-15(10-14)17(21-11-13-4-2-1-3-5-13)23-18(22-16)24-9-8-20-12-24/h1-10,12H,11H2,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325890
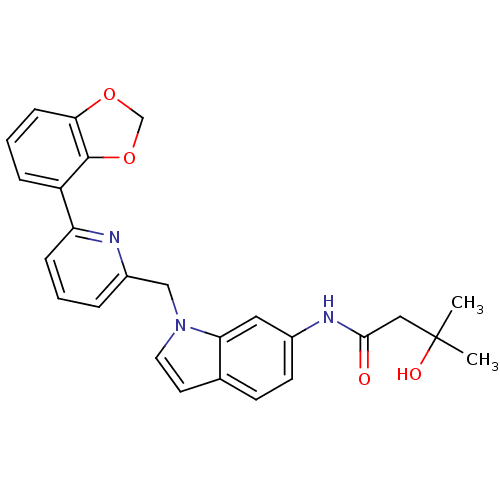
(CHEMBL1224536 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...)Show SMILES CC(C)(O)CC(=O)Nc1ccc2ccn(Cc3cccc(n3)-c3cccc4OCOc34)c2c1 Show InChI InChI=1S/C26H25N3O4/c1-26(2,31)14-24(30)28-18-10-9-17-11-12-29(22(17)13-18)15-19-5-3-7-21(27-19)20-6-4-8-23-25(20)33-16-32-23/h3-13,31H,14-16H2,1-2H3,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311656
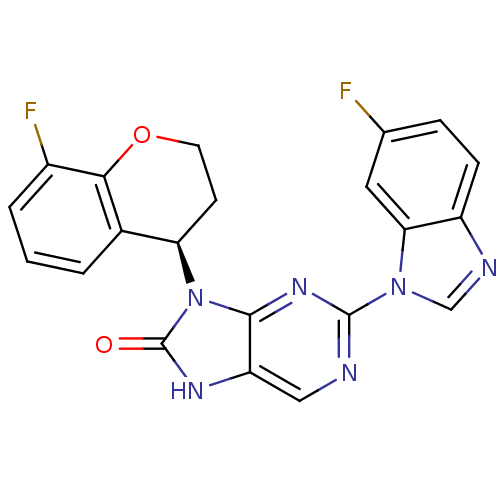
(2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...)Show SMILES Fc1ccc2ncn(-c3ncc4[nH]c(=O)n([C@@H]5CCOc6c(F)cccc56)c4n3)c2c1 |r| Show InChI InChI=1S/C21H14F2N6O2/c22-11-4-5-14-17(8-11)28(10-25-14)20-24-9-15-19(27-20)29(21(30)26-15)16-6-7-31-18-12(16)2-1-3-13(18)23/h1-5,8-10,16H,6-7H2,(H,26,30)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325894
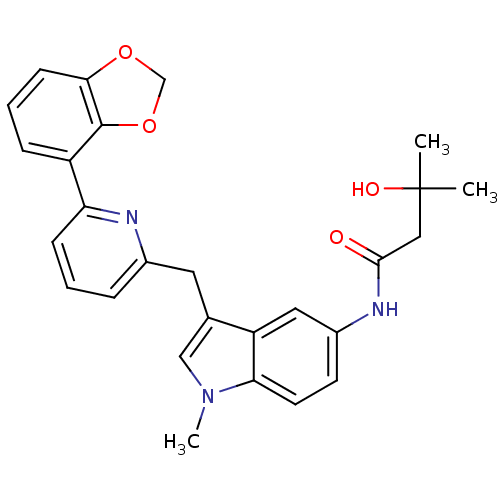
(CHEMBL1224615 | N-(3-((6-(benzo[d][1,3]dioxol-4-yl...)Show SMILES Cn1cc(Cc2cccc(n2)-c2cccc3OCOc23)c2cc(NC(=O)CC(C)(C)O)ccc12 Show InChI InChI=1S/C27H27N3O4/c1-27(2,32)14-25(31)29-19-10-11-23-21(13-19)17(15-30(23)3)12-18-6-4-8-22(28-18)20-7-5-9-24-26(20)34-16-33-24/h4-11,13,15,32H,12,14,16H2,1-3H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50311657

(1-(9-(8-fluorochroman-4-yl)-8-oxo-8,9-dihydro-7H-p...)Show SMILES Fc1cccc2[C@@H](CCOc12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccc(cc12)C#N |r| Show InChI InChI=1S/C22H14FN7O2/c23-14-3-1-2-13-17(6-7-32-19(13)14)30-20-16(27-22(30)31)10-25-21(28-20)29-11-26-15-5-4-12(9-24)8-18(15)29/h1-5,8,10-11,17H,6-7H2,(H,27,31)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311644
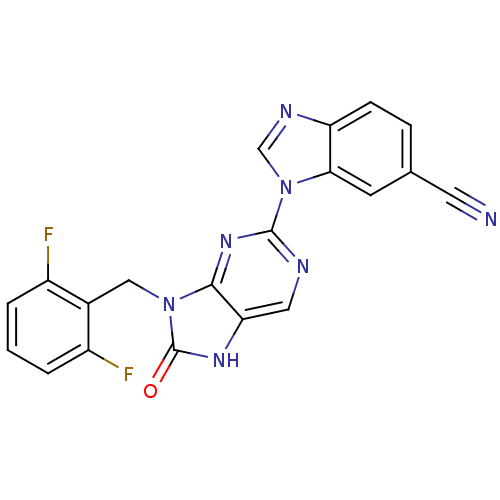
(1-(9-(2,6-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...)Show SMILES Fc1cccc(F)c1Cn1c2nc(ncc2[nH]c1=O)-n1cnc2ccc(cc12)C#N Show InChI InChI=1S/C20H11F2N7O/c21-13-2-1-3-14(22)12(13)9-28-18-16(26-20(28)30)8-24-19(27-18)29-10-25-15-5-4-11(7-23)6-17(15)29/h1-6,8,10H,9H2,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325892
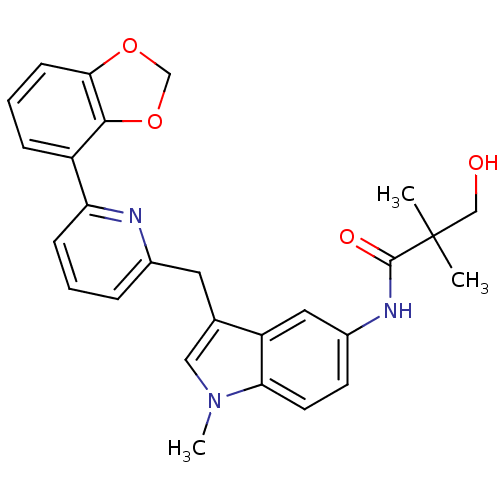
(CHEMBL1224613 | N-(3-((6-(benzo[d][1,3]dioxol-4-yl...)Show SMILES Cn1cc(Cc2cccc(n2)-c2cccc3OCOc23)c2cc(NC(=O)C(C)(C)CO)ccc12 Show InChI InChI=1S/C27H27N3O4/c1-27(2,15-31)26(32)29-19-10-11-23-21(13-19)17(14-30(23)3)12-18-6-4-8-22(28-18)20-7-5-9-24-25(20)34-16-33-24/h4-11,13-14,31H,12,15-16H2,1-3H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325888
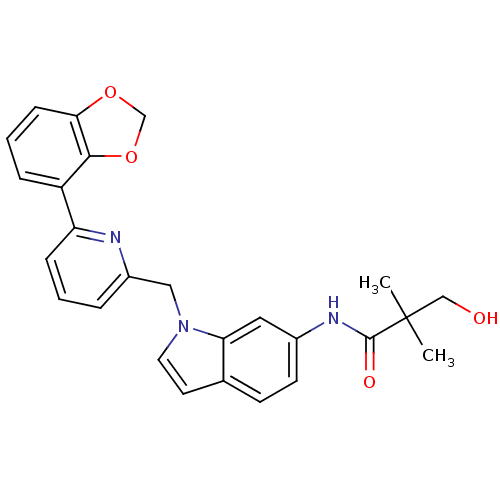
(CHEMBL1224534 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...)Show SMILES CC(C)(CO)C(=O)Nc1ccc2ccn(Cc3cccc(n3)-c3cccc4OCOc34)c2c1 Show InChI InChI=1S/C26H25N3O4/c1-26(2,15-30)25(31)28-18-10-9-17-11-12-29(22(17)13-18)14-19-5-3-7-21(27-19)20-6-4-8-23-24(20)33-16-32-23/h3-13,30H,14-16H2,1-2H3,(H,28,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311640
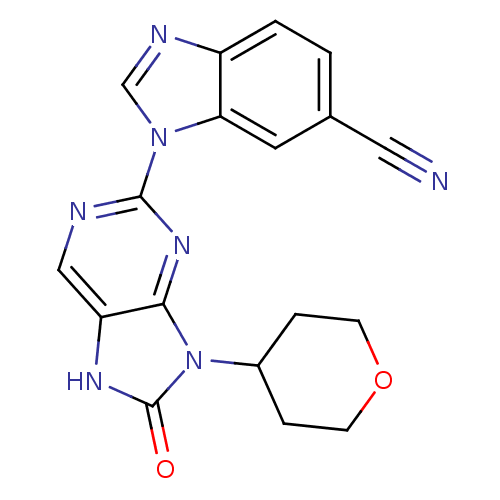
(1-(8-oxo-9-(tetrahydro-2H-pyran-4-yl)-8,9-dihydro-...)Show SMILES O=c1[nH]c2cnc(nc2n1C1CCOCC1)-n1cnc2ccc(cc12)C#N Show InChI InChI=1S/C18H15N7O2/c19-8-11-1-2-13-15(7-11)24(10-21-13)17-20-9-14-16(23-17)25(18(26)22-14)12-3-5-27-6-4-12/h1-2,7,9-10,12H,3-6H2,(H,22,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033306
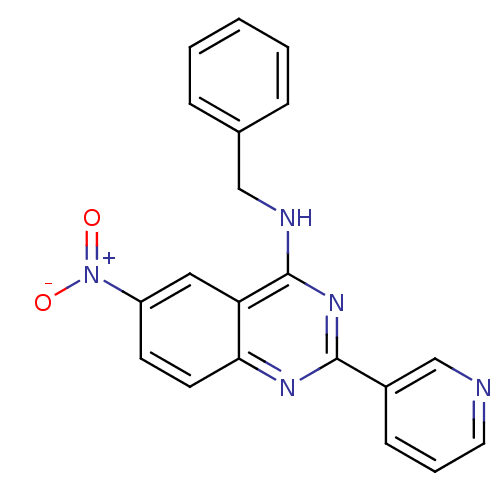
(Benzyl-(6-nitro-2-pyridin-3-yl-quinazolin-4-yl)-am...)Show SMILES [O-][N+](=O)c1ccc2nc(nc(NCc3ccccc3)c2c1)-c1cccnc1 Show InChI InChI=1S/C20H15N5O2/c26-25(27)16-8-9-18-17(11-16)20(22-12-14-5-2-1-3-6-14)24-19(23-18)15-7-4-10-21-13-15/h1-11,13H,12H2,(H,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033328
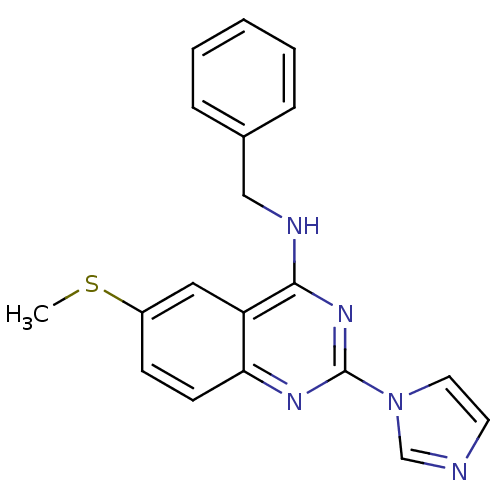
(Benzyl-(2-imidazol-1-yl-6-methylsulfanyl-quinazoli...)Show InChI InChI=1S/C19H17N5S/c1-25-15-7-8-17-16(11-15)18(21-12-14-5-3-2-4-6-14)23-19(22-17)24-10-9-20-13-24/h2-11,13H,12H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311639
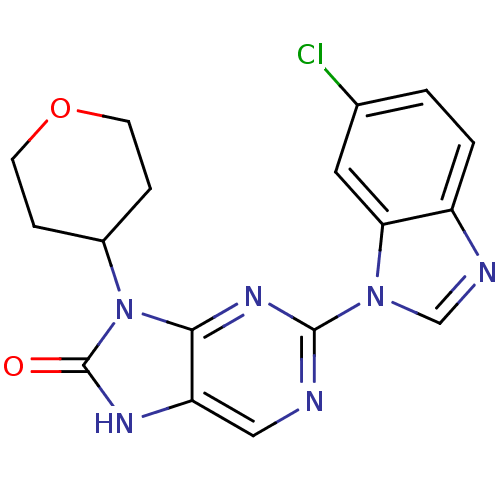
(2-(6-chloro-1H-benzo[d]imidazol-1-yl)-9-(tetrahydr...)Show SMILES Clc1ccc2ncn(-c3ncc4[nH]c(=O)n(C5CCOCC5)c4n3)c2c1 Show InChI InChI=1S/C17H15ClN6O2/c18-10-1-2-12-14(7-10)23(9-20-12)16-19-8-13-15(22-16)24(17(25)21-13)11-3-5-26-6-4-11/h1-2,7-9,11H,3-6H2,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311655
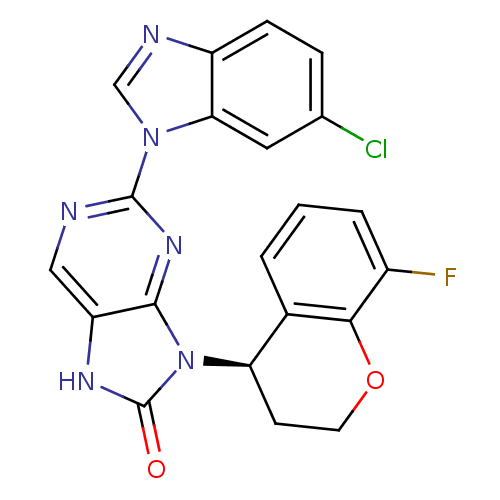
(2-(6-chloro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...)Show SMILES Fc1cccc2[C@@H](CCOc12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccc(Cl)cc12 |r| Show InChI InChI=1S/C21H14ClFN6O2/c22-11-4-5-14-17(8-11)28(10-25-14)20-24-9-15-19(27-20)29(21(30)26-15)16-6-7-31-18-12(16)2-1-3-13(18)23/h1-5,8-10,16H,6-7H2,(H,26,30)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033293

(Benzyl-(2-imidazol-1-yl-6-methoxy-quinazolin-4-yl)...)Show InChI InChI=1S/C19H17N5O/c1-25-15-7-8-17-16(11-15)18(21-12-14-5-3-2-4-6-14)23-19(22-17)24-10-9-20-13-24/h2-11,13H,12H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311642
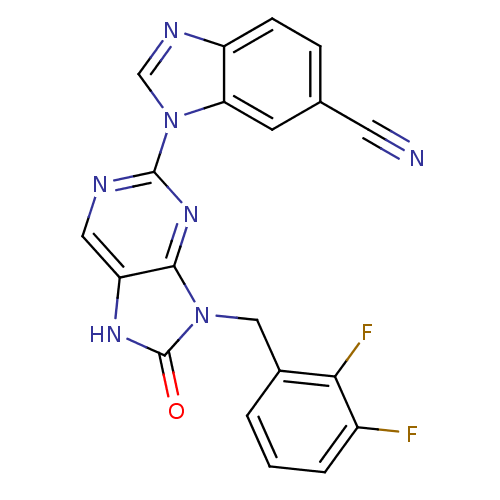
(1-(9-(2,3-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...)Show SMILES Fc1cccc(Cn2c3nc(ncc3[nH]c2=O)-n2cnc3ccc(cc23)C#N)c1F Show InChI InChI=1S/C20H11F2N7O/c21-13-3-1-2-12(17(13)22)9-28-18-15(26-20(28)30)8-24-19(27-18)29-10-25-14-5-4-11(7-23)6-16(14)29/h1-6,8,10H,9H2,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325895
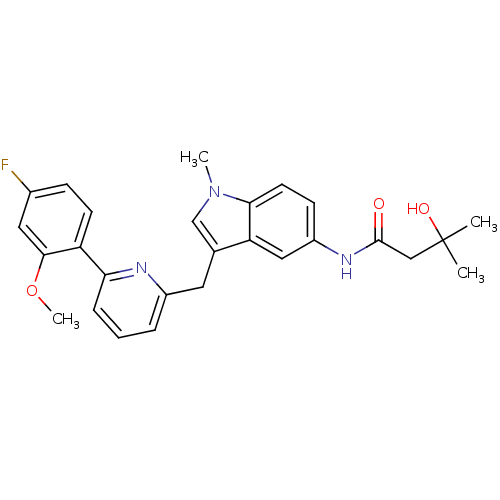
(CHEMBL1224667 | N-(3-((6-(4-fluoro-2-methoxyphenyl...)Show SMILES COc1cc(F)ccc1-c1cccc(Cc2cn(C)c3ccc(NC(=O)CC(C)(C)O)cc23)n1 Show InChI InChI=1S/C27H28FN3O3/c1-27(2,33)15-26(32)30-20-9-11-24-22(14-20)17(16-31(24)3)12-19-6-5-7-23(29-19)21-10-8-18(28)13-25(21)34-4/h5-11,13-14,16,33H,12,15H2,1-4H3,(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325882

(CHEMBL1224373 | N-(1-((6-(benzo[d][1,3]dioxol-4-yl...)Show SMILES CC(C)(C)C(=O)Nc1ccc2ncn(Cc3cccc(n3)-c3cccc4OCOc34)c2c1 Show InChI InChI=1S/C25H24N4O3/c1-25(2,3)24(30)28-16-10-11-20-21(12-16)29(14-26-20)13-17-6-4-8-19(27-17)18-7-5-9-22-23(18)32-15-31-22/h4-12,14H,13,15H2,1-3H3,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325891
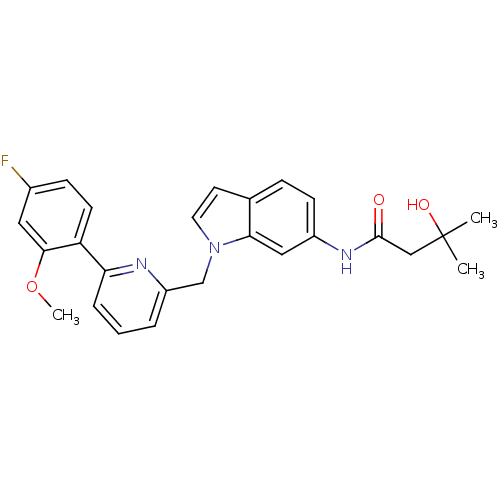
(CHEMBL1224612 | N-(1-((6-(4-fluoro-2-methoxyphenyl...)Show SMILES COc1cc(F)ccc1-c1cccc(Cn2ccc3ccc(NC(=O)CC(C)(C)O)cc23)n1 Show InChI InChI=1S/C26H26FN3O3/c1-26(2,32)15-25(31)29-19-9-7-17-11-12-30(23(17)14-19)16-20-5-4-6-22(28-20)21-10-8-18(27)13-24(21)33-3/h4-14,32H,15-16H2,1-3H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033287
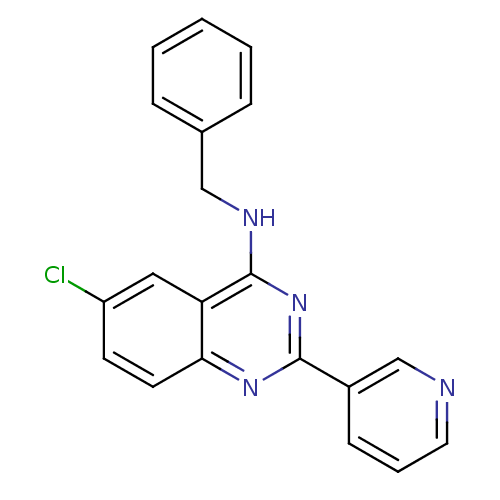
(Benzyl-(6-chloro-2-pyridin-3-yl-quinazolin-4-yl)-a...)Show InChI InChI=1S/C20H15ClN4/c21-16-8-9-18-17(11-16)20(23-12-14-5-2-1-3-6-14)25-19(24-18)15-7-4-10-22-13-15/h1-11,13H,12H2,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50311656

(2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...)Show SMILES Fc1ccc2ncn(-c3ncc4[nH]c(=O)n([C@@H]5CCOc6c(F)cccc56)c4n3)c2c1 |r| Show InChI InChI=1S/C21H14F2N6O2/c22-11-4-5-14-17(8-11)28(10-25-14)20-24-9-15-19(27-20)29(21(30)26-15)16-6-7-31-18-12(16)2-1-3-13(18)23/h1-5,8-10,16H,6-7H2,(H,26,30)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325893
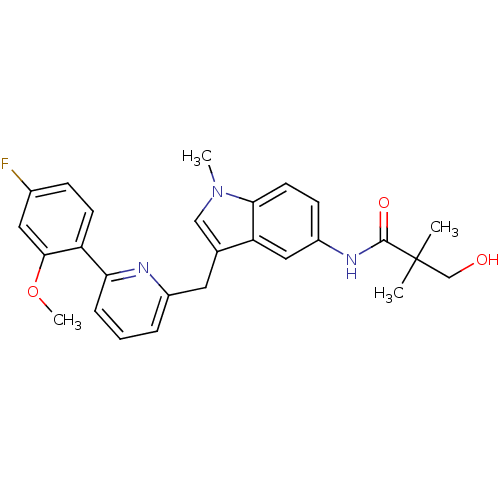
(CHEMBL1224614 | N-(3-((6-(4-fluoro-2-methoxyphenyl...)Show SMILES COc1cc(F)ccc1-c1cccc(Cc2cn(C)c3ccc(NC(=O)C(C)(C)CO)cc23)n1 Show InChI InChI=1S/C27H28FN3O3/c1-27(2,16-32)26(33)30-20-9-11-24-22(14-20)17(15-31(24)3)12-19-6-5-7-23(29-19)21-10-8-18(28)13-25(21)34-4/h5-11,13-15,32H,12,16H2,1-4H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311653
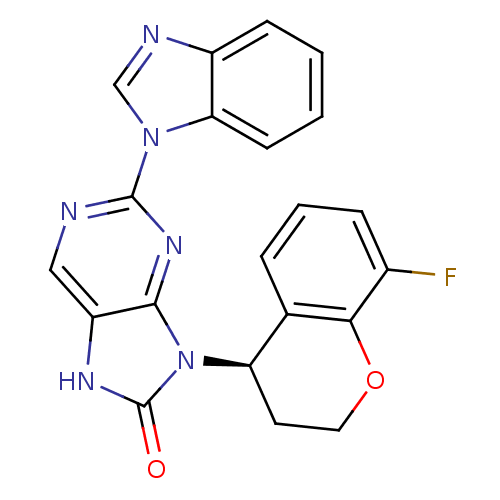
((R)-2-(1H-benzo[d]imidazol-1-yl)-9-(8-fluorochroma...)Show SMILES Fc1cccc2[C@@H](CCOc12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccccc12 |r| Show InChI InChI=1S/C21H15FN6O2/c22-13-5-3-4-12-16(8-9-30-18(12)13)28-19-15(25-21(28)29)10-23-20(26-19)27-11-24-14-6-1-2-7-17(14)27/h1-7,10-11,16H,8-9H2,(H,25,29)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311648
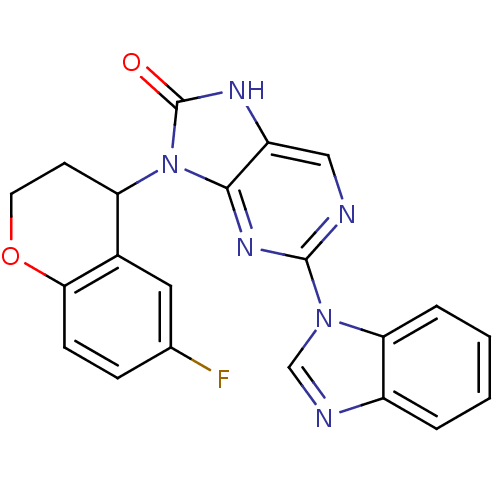
((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(6-fluorochro...)Show SMILES Fc1ccc2OCCC(c2c1)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccccc12 Show InChI InChI=1S/C21H15FN6O2/c22-12-5-6-18-13(9-12)16(7-8-30-18)28-19-15(25-21(28)29)10-23-20(26-19)27-11-24-14-3-1-2-4-17(14)27/h1-6,9-11,16H,7-8H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50311644

(1-(9-(2,6-difluorobenzyl)-8-oxo-8,9-dihydro-7H-pur...)Show SMILES Fc1cccc(F)c1Cn1c2nc(ncc2[nH]c1=O)-n1cnc2ccc(cc12)C#N Show InChI InChI=1S/C20H11F2N7O/c21-13-2-1-3-14(22)12(13)9-28-18-16(26-20(28)30)8-24-19(27-18)29-10-25-15-5-4-11(7-23)6-17(15)29/h1-6,8,10H,9H2,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311651
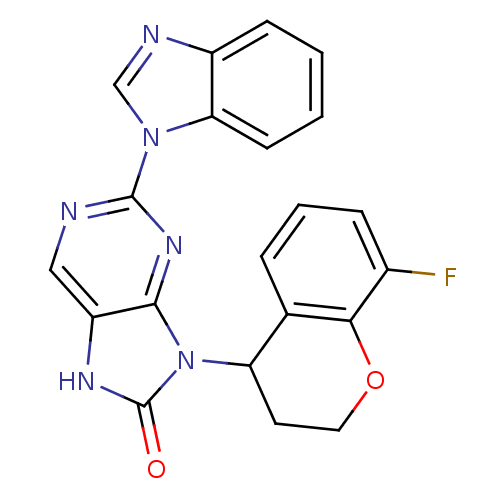
((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(8-fluorochro...)Show SMILES Fc1cccc2C(CCOc12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccccc12 Show InChI InChI=1S/C21H15FN6O2/c22-13-5-3-4-12-16(8-9-30-18(12)13)28-19-15(25-21(28)29)10-23-20(26-19)27-11-24-14-6-1-2-7-17(14)27/h1-7,10-11,16H,8-9H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325886
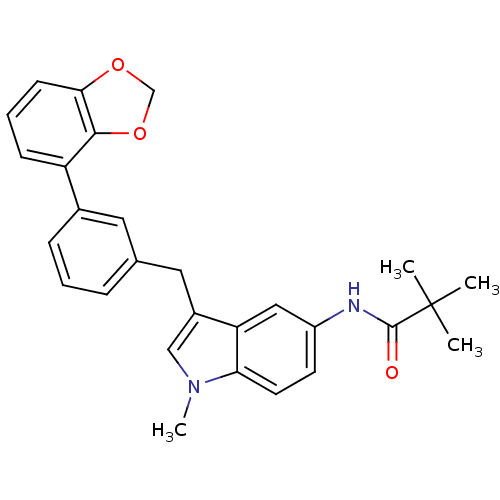
(CHEMBL1224460 | N-(3-(3-(benzo[d][1,3]dioxol-4-yl)...)Show SMILES Cn1cc(Cc2cccc(c2)-c2cccc3OCOc23)c2cc(NC(=O)C(C)(C)C)ccc12 Show InChI InChI=1S/C28H28N2O3/c1-28(2,3)27(31)29-21-11-12-24-23(15-21)20(16-30(24)4)14-18-7-5-8-19(13-18)22-9-6-10-25-26(22)33-17-32-25/h5-13,15-16H,14,17H2,1-4H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311638
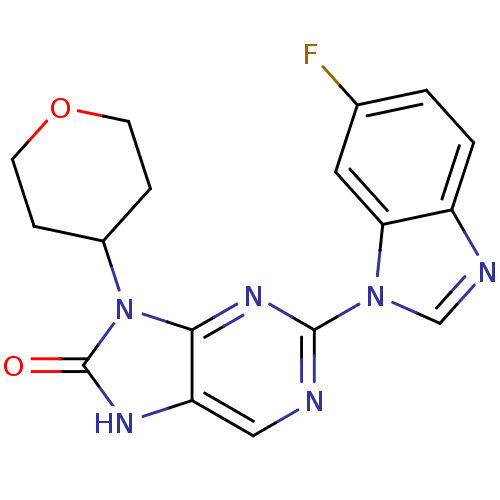
(2-(6-fluoro-1H-benzo[d]imidazol-1-yl)-9-(tetrahydr...)Show SMILES Fc1ccc2ncn(-c3ncc4[nH]c(=O)n(C5CCOCC5)c4n3)c2c1 Show InChI InChI=1S/C17H15FN6O2/c18-10-1-2-12-14(7-10)23(9-20-12)16-19-8-13-15(22-16)24(17(25)21-13)11-3-5-26-6-4-11/h1-2,7-9,11H,3-6H2,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325889

(CHEMBL1224535 | N-(1-((6-(4-fluoro-2-methoxyphenyl...)Show SMILES COc1cc(F)ccc1-c1cccc(Cn2ccc3ccc(NC(=O)C(C)(C)CO)cc23)n1 Show InChI InChI=1S/C26H26FN3O3/c1-26(2,16-31)25(32)29-19-9-7-17-11-12-30(23(17)14-19)15-20-5-4-6-22(28-20)21-10-8-18(27)13-24(21)33-3/h4-14,31H,15-16H2,1-3H3,(H,29,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325887
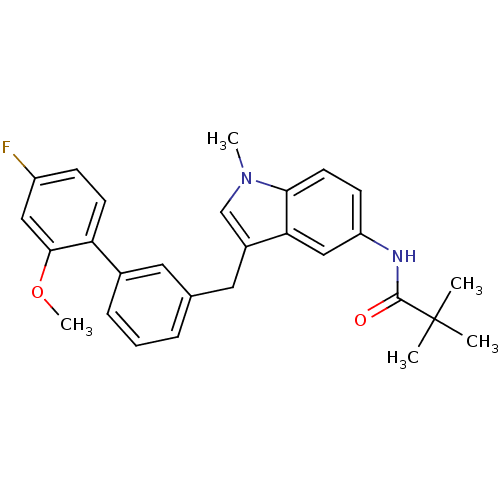
(CHEMBL1224533 | N-(3-((4'-fluoro-2'-methoxybipheny...)Show SMILES COc1cc(F)ccc1-c1cccc(Cc2cn(C)c3ccc(NC(=O)C(C)(C)C)cc23)c1 Show InChI InChI=1S/C28H29FN2O2/c1-28(2,3)27(32)30-22-10-12-25-24(16-22)20(17-31(25)4)14-18-7-6-8-19(13-18)23-11-9-21(29)15-26(23)33-5/h6-13,15-17H,14H2,1-5H3,(H,30,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311647
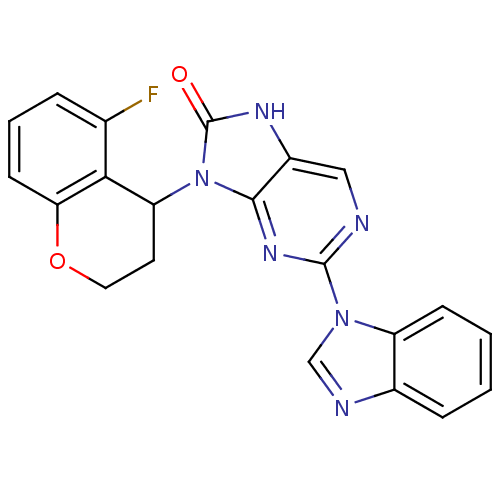
((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(5-fluorochro...)Show SMILES Fc1cccc2OCCC(c12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccccc12 Show InChI InChI=1S/C21H15FN6O2/c22-12-4-3-7-17-18(12)16(8-9-30-17)28-19-14(25-21(28)29)10-23-20(26-19)27-11-24-13-5-1-2-6-15(13)27/h1-7,10-11,16H,8-9H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325884

(CHEMBL1224458 | N-(1-(3-(benzo[d][1,3]dioxol-4-yl)...)Show SMILES CC(C)(C)C(=O)Nc1ccc2ccn(Cc3cccc(c3)-c3cccc4OCOc34)c2c1 Show InChI InChI=1S/C27H26N2O3/c1-27(2,3)26(30)28-21-11-10-19-12-13-29(23(19)15-21)16-18-6-4-7-20(14-18)22-8-5-9-24-25(22)32-17-31-24/h4-15H,16-17H2,1-3H3,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50311655

(2-(6-chloro-1H-benzo[d]imidazol-1-yl)-9-(8-fluoroc...)Show SMILES Fc1cccc2[C@@H](CCOc12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccc(Cl)cc12 |r| Show InChI InChI=1S/C21H14ClFN6O2/c22-11-4-5-14-17(8-11)28(10-25-14)20-24-9-15-19(27-20)29(21(30)26-15)16-6-7-31-18-12(16)2-1-3-13(18)23/h1-5,8-10,16H,6-7H2,(H,26,30)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325885
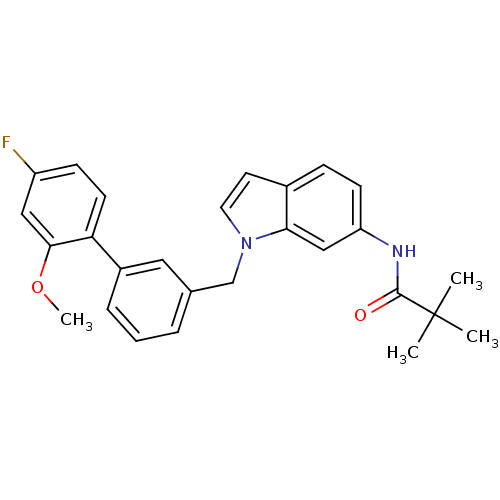
(CHEMBL1224459 | N-(1-((4'-fluoro-2'-methoxybipheny...)Show SMILES COc1cc(F)ccc1-c1cccc(Cn2ccc3ccc(NC(=O)C(C)(C)C)cc23)c1 Show InChI InChI=1S/C27H27FN2O2/c1-27(2,3)26(31)29-22-10-8-19-12-13-30(24(19)16-22)17-18-6-5-7-20(14-18)23-11-9-21(28)15-25(23)32-4/h5-16H,17H2,1-4H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325880

(CHEMBL1224371 | N-(1-(3-(benzo[d][1,3]dioxol-4-yl)...)Show SMILES CC(C)(C)C(=O)Nc1ccc2ncn(Cc3cccc(c3)-c3cccc4OCOc34)c2c1 Show InChI InChI=1S/C26H25N3O3/c1-26(2,3)25(30)28-19-10-11-21-22(13-19)29(15-27-21)14-17-6-4-7-18(12-17)20-8-5-9-23-24(20)32-16-31-23/h4-13,15H,14,16H2,1-3H3,(H,28,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311637
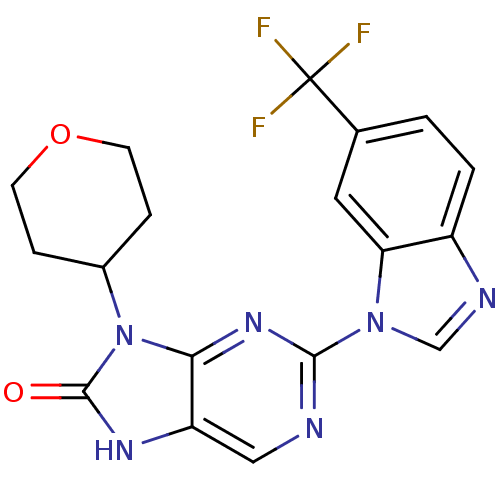
(9-(tetrahydro-2H-pyran-4-yl)-2-(6-(trifluoromethyl...)Show SMILES FC(F)(F)c1ccc2ncn(-c3ncc4[nH]c(=O)n(C5CCOCC5)c4n3)c2c1 Show InChI InChI=1S/C18H15F3N6O2/c19-18(20,21)10-1-2-12-14(7-10)26(9-23-12)16-22-8-13-15(25-16)27(17(28)24-13)11-3-5-29-6-4-11/h1-2,7-9,11H,3-6H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311643

(9-(2,6-difluorobenzyl)-2-(6-fluoro-1H-benzo[d]imid...)Show SMILES Fc1ccc2ncn(-c3ncc4[nH]c(=O)n(Cc5c(F)cccc5F)c4n3)c2c1 Show InChI InChI=1S/C19H11F3N6O/c20-10-4-5-14-16(6-10)28(9-24-14)18-23-7-15-17(26-18)27(19(29)25-15)8-11-12(21)2-1-3-13(11)22/h1-7,9H,8H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033334
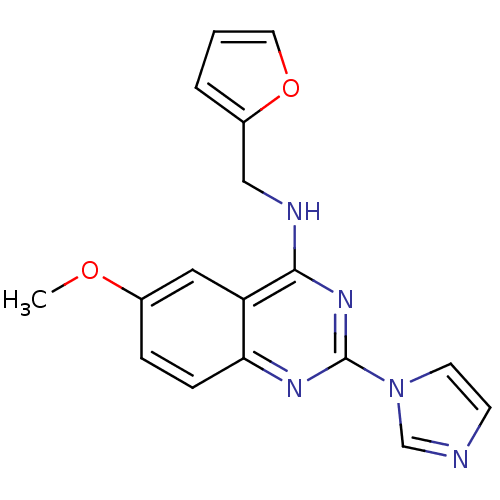
(CHEMBL542707 | Furan-2-ylmethyl-(2-imidazol-1-yl-6...)Show InChI InChI=1S/C17H15N5O2/c1-23-12-4-5-15-14(9-12)16(19-10-13-3-2-8-24-13)21-17(20-15)22-7-6-18-11-22/h2-9,11H,10H2,1H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325883
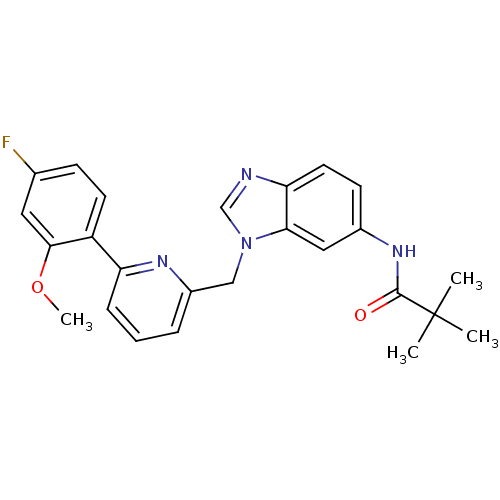
(CHEMBL1224457 | N-(1-((6-(4-fluoro-2-methoxyphenyl...)Show SMILES COc1cc(F)ccc1-c1cccc(Cn2cnc3ccc(NC(=O)C(C)(C)C)cc23)n1 Show InChI InChI=1S/C25H25FN4O2/c1-25(2,3)24(31)29-17-9-11-21-22(13-17)30(15-27-21)14-18-6-5-7-20(28-18)19-10-8-16(26)12-23(19)32-4/h5-13,15H,14H2,1-4H3,(H,29,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50311648

((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(6-fluorochro...)Show SMILES Fc1ccc2OCCC(c2c1)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccccc12 Show InChI InChI=1S/C21H15FN6O2/c22-12-5-6-18-13(9-12)16(7-8-30-18)28-19-15(25-21(28)29)10-23-20(26-19)27-11-24-14-3-1-2-4-17(14)27/h1-6,9-11,16H,7-8H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM50311646
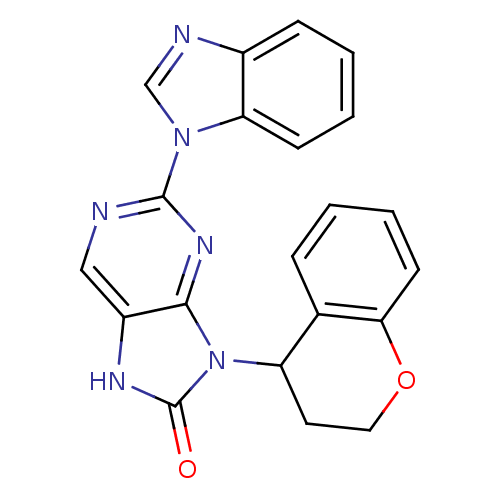
((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(chroman-4-yl...)Show SMILES O=c1[nH]c2cnc(nc2n1C1CCOc2ccccc12)-n1cnc2ccccc12 Show InChI InChI=1S/C21H16N6O2/c28-21-24-15-11-22-20(26-12-23-14-6-2-3-7-17(14)26)25-19(15)27(21)16-9-10-29-18-8-4-1-5-13(16)18/h1-8,11-12,16H,9-10H2,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50311650
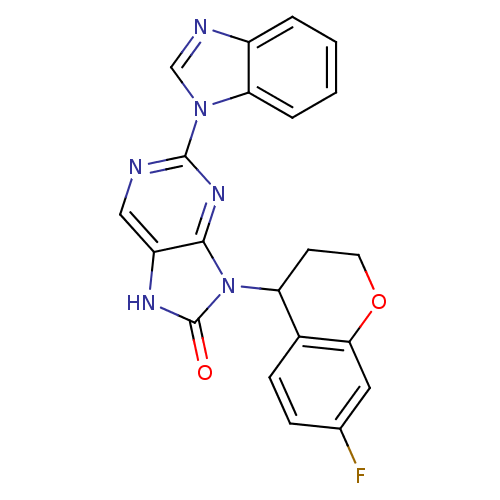
((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(7-fluorochro...)Show SMILES Fc1ccc2C(CCOc2c1)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccccc12 Show InChI InChI=1S/C21H15FN6O2/c22-12-5-6-13-16(7-8-30-18(13)9-12)28-19-15(25-21(28)29)10-23-20(26-19)27-11-24-14-3-1-2-4-17(14)27/h1-6,9-11,16H,7-8H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 95 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM50311647

((+/-)-2-(1H-benzo[d]imidazol-1-yl)-9-(5-fluorochro...)Show SMILES Fc1cccc2OCCC(c12)n1c2nc(ncc2[nH]c1=O)-n1cnc2ccccc12 Show InChI InChI=1S/C21H15FN6O2/c22-12-4-3-7-17-18(12)16(8-9-30-17)28-19-14(25-21(28)29)10-23-20(26-19)27-11-24-13-5-1-2-6-15(13)27/h1-7,10-11,16H,8-9H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK2 (532-1132) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50325879

(CHEMBL1224370 | N-(3-fluorobenzyl)-1-((2'-methoxyb...)Show SMILES COc1ccccc1-c1cccc(Cn2cnc3ccc(cc23)C(=O)NCc2cccc(F)c2)c1 Show InChI InChI=1S/C29H24FN3O2/c1-35-28-11-3-2-10-25(28)22-8-4-7-21(14-22)18-33-19-32-26-13-12-23(16-27(26)33)29(34)31-17-20-6-5-9-24(30)15-20/h2-16,19H,17-18H2,1H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CC55940 human cannabinoid CB1 receptor expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 20: 5449-53 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.091
BindingDB Entry DOI: 10.7270/Q2SN0958 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Homo sapiens (Human)) | BDBM50033289
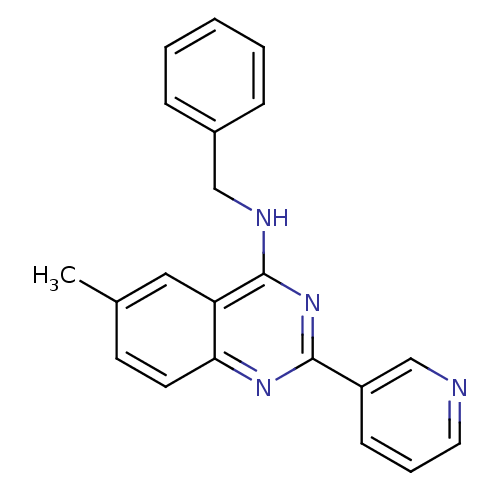
(Benzyl-(6-methyl-2-pyridin-3-yl-quinazolin-4-yl)-a...)Show InChI InChI=1S/C21H18N4/c1-15-9-10-19-18(12-15)21(23-13-16-6-3-2-4-7-16)25-20(24-19)17-8-5-11-22-14-17/h2-12,14H,13H2,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
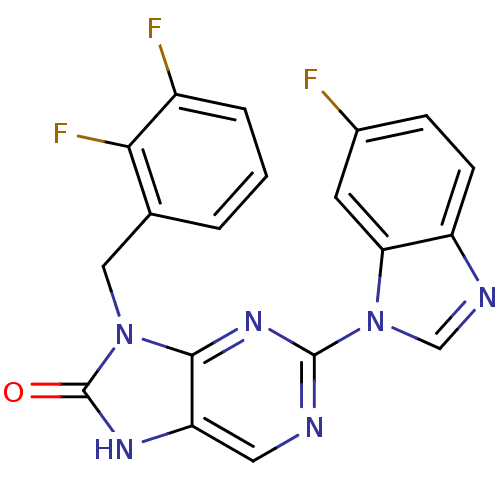
(Homo sapiens (Human)) | BDBM50311641
(9-(2,3-difluorobenzyl)-2-(6-fluoro-1H-benzo[d]imid...)Show SMILES Fc1ccc2ncn(-c3ncc4[nH]c(=O)n(Cc5cccc(F)c5F)c4n3)c2c1 Show InChI InChI=1S/C19H11F3N6O/c20-11-4-5-13-15(6-11)28(9-24-13)18-23-7-14-17(26-18)27(19(29)25-14)8-10-2-1-3-12(21)16(10)22/h1-7,9H,8H2,(H,25,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Ligand Pharmaceuticals, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human JAK3 (508-1124) by time resolved fluorescence assay |
Bioorg Med Chem Lett 19: 6788-92 (2009)
Article DOI: 10.1016/j.bmcl.2009.09.080
BindingDB Entry DOI: 10.7270/Q24B31G4 |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
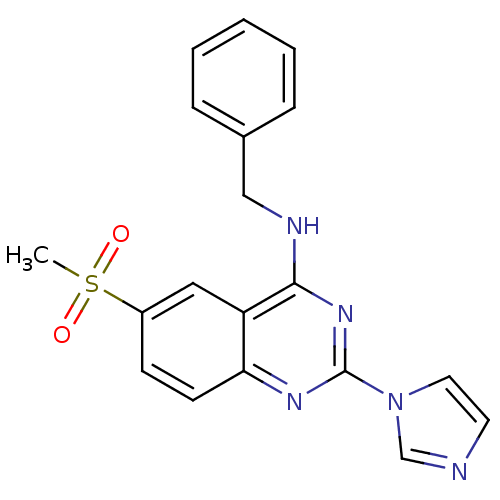
(Homo sapiens (Human)) | BDBM50033301
(Benzyl-(2-imidazol-1-yl-6-methanesulfonyl-quinazol...)Show SMILES CS(=O)(=O)c1ccc2nc(nc(NCc3ccccc3)c2c1)-n1ccnc1 Show InChI InChI=1S/C19H17N5O2S/c1-27(25,26)15-7-8-17-16(11-15)18(21-12-14-5-3-2-4-6-14)23-19(22-17)24-10-9-20-13-24/h2-11,13H,12H2,1H3,(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Biofor Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against phosphodiesterase 5 from human platelets |
J Med Chem 38: 3547-57 (1995)
BindingDB Entry DOI: 10.7270/Q2F190CB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data