

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50131251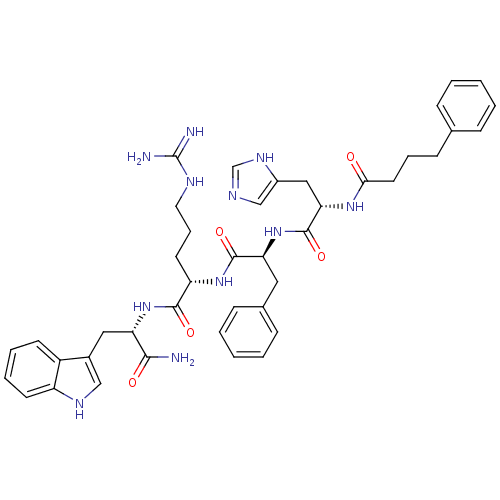 ((S)-5-Guanidino-2-{(S)-2-[(S)-3-(3H-imidazol-4-yl)...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (hMC1R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM23334 (3-(pyridin-3-yl)propyl (2S)-1-(3,3-dimethyl-2-oxop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 | Bioorg Med Chem Lett 12: 1429-33 (2002) BindingDB Entry DOI: 10.7270/Q23J3C9S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sigma non-opioid intracellular receptor 1 (RAT) | BDBM50248926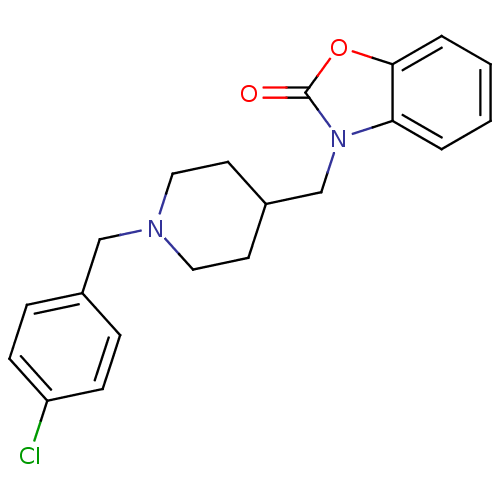 (3-((1-(4-chlorobenzyl)piperidin-4-yl)methyl)benzo[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Trieste Curated by ChEMBL | Assay Description Displacement of [3H](+)-pentazocine from sigma 1 receptor in rat liver membrane | Eur J Med Chem 44: 124-30 (2008) Article DOI: 10.1016/j.ejmech.2008.03.011 BindingDB Entry DOI: 10.7270/Q2639PHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50377898 (CHEMBL256109) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to human GnRHR | Bioorg Med Chem Lett 18: 3344-9 (2008) Article DOI: 10.1016/j.bmcl.2008.04.029 BindingDB Entry DOI: 10.7270/Q2CJ8FBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50377898 (CHEMBL256109) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to human GnRHR | Bioorg Med Chem Lett 18: 3344-9 (2008) Article DOI: 10.1016/j.bmcl.2008.04.029 BindingDB Entry DOI: 10.7270/Q2CJ8FBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50377895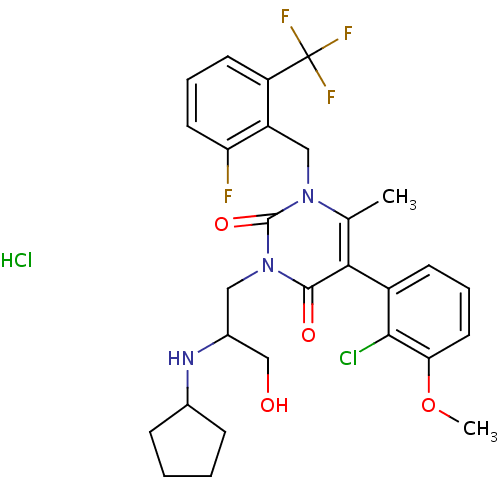 (CHEMBL556355) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to human GnRHR | Bioorg Med Chem Lett 18: 3344-9 (2008) Article DOI: 10.1016/j.bmcl.2008.04.029 BindingDB Entry DOI: 10.7270/Q2CJ8FBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189013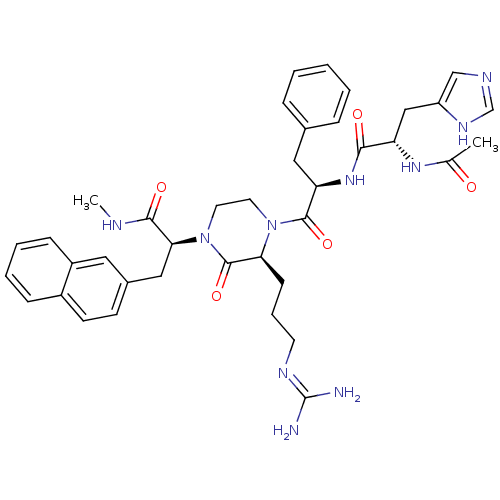 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189024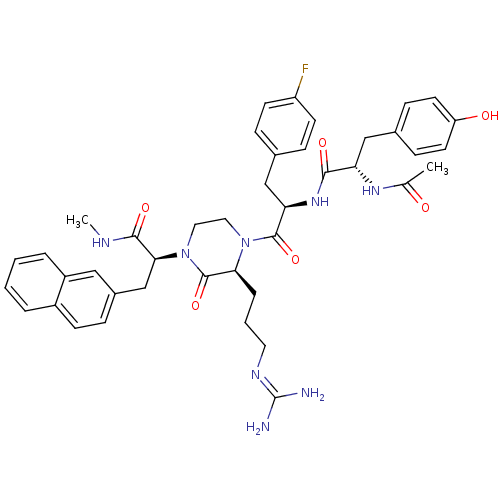 ((S)-2-[(S)-4-[(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50166441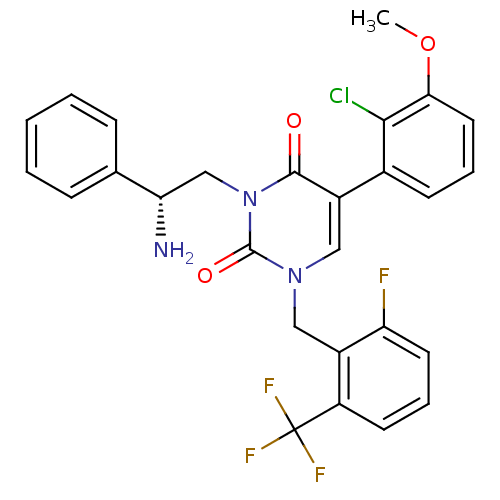 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin-releasing hormone receptor (GNRHR) using GnRH peptide as radioligand | Bioorg Med Chem Lett 15: 2519-22 (2005) Article DOI: 10.1016/j.bmcl.2005.03.057 BindingDB Entry DOI: 10.7270/Q2M044ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129410 (US8802673, 52) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM82411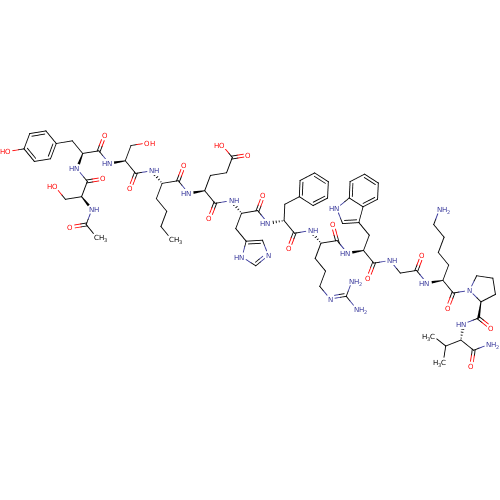 (CAS_75921-69-6 | NDP-MSH) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 1 receptor (hMC1R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50162026 (1-(2,6-Difluoro-benzyl)-5-(2-fluoro-3-methoxy-phen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity for Human gonadotropin-releasing hormone receptor | J Med Chem 48: 1169-78 (2005) Article DOI: 10.1021/jm049218c BindingDB Entry DOI: 10.7270/Q2MK6CD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129366 (US8802673, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129420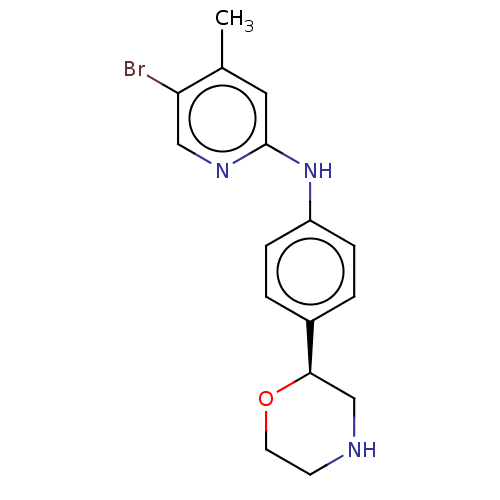 (US8802673, 62) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50162007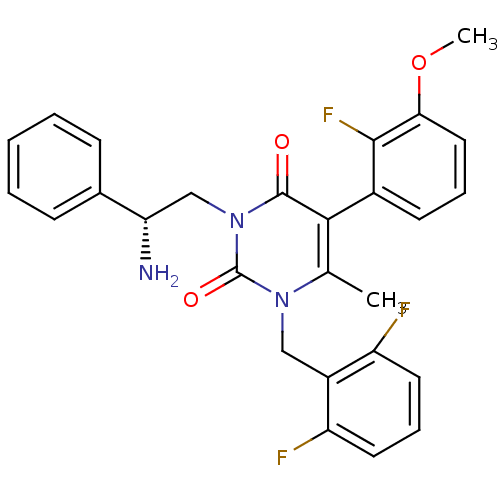 ((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin-releasing hormone receptor (GNRHR) using GnRH peptide as radioligand | Bioorg Med Chem Lett 15: 2519-22 (2005) Article DOI: 10.1016/j.bmcl.2005.03.057 BindingDB Entry DOI: 10.7270/Q2M044ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50162007 ((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to human GnRHR | Bioorg Med Chem Lett 18: 3344-9 (2008) Article DOI: 10.1016/j.bmcl.2008.04.029 BindingDB Entry DOI: 10.7270/Q2CJ8FBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50162007 ((R)-1-(2,6-difluorobenzyl)-3-(2-amino-2-phenylethy...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity for Human gonadotropin-releasing hormone receptor | J Med Chem 48: 1169-78 (2005) Article DOI: 10.1021/jm049218c BindingDB Entry DOI: 10.7270/Q2MK6CD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189010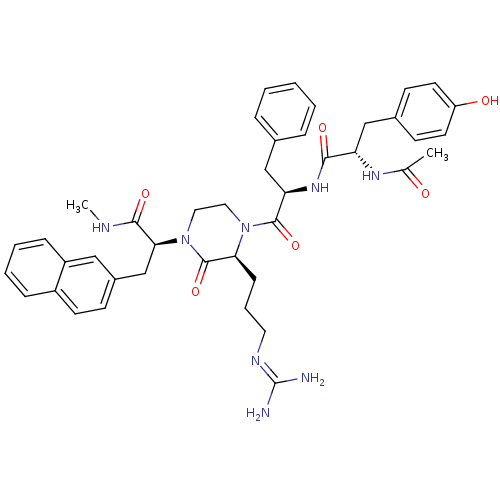 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129441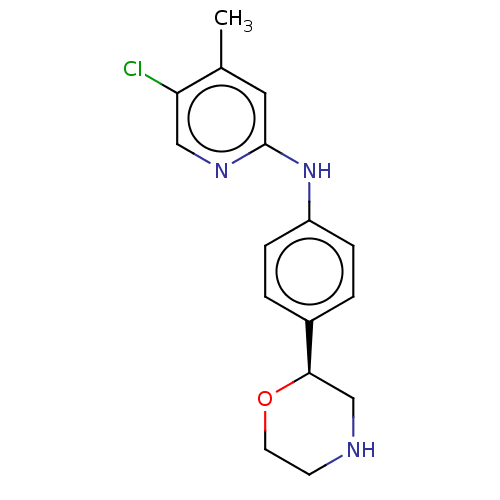 (US8802673, 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129522 (US8802673, 164) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.600 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50149255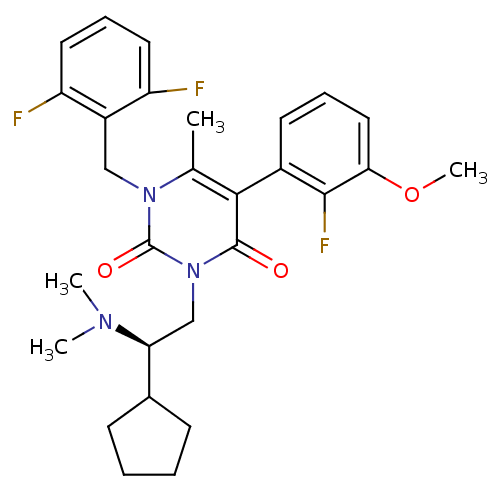 (3-((R)-2-Cyclopentyl-2-dimethylamino-ethyl)-1-(2,6...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences Inc. Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin releasing hormone receptor using des-Gly10[125I-Tyr5,D-Leu6,NMeLeu7,Pro9-NEt]GnRH as radioligand expresse... | J Med Chem 47: 3483-6 (2004) Article DOI: 10.1021/jm049791w BindingDB Entry DOI: 10.7270/Q2HT2NTX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50162008 ((R)-1-(2-chloro-6-fluorobenzyl)-3-(2-amino-2-pheny...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity for Human gonadotropin-releasing hormone receptor | J Med Chem 48: 1169-78 (2005) Article DOI: 10.1021/jm049218c BindingDB Entry DOI: 10.7270/Q2MK6CD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50377900 (CHEMBL259906) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity to human GnRHR | Bioorg Med Chem Lett 18: 3344-9 (2008) Article DOI: 10.1016/j.bmcl.2008.04.029 BindingDB Entry DOI: 10.7270/Q2CJ8FBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50423641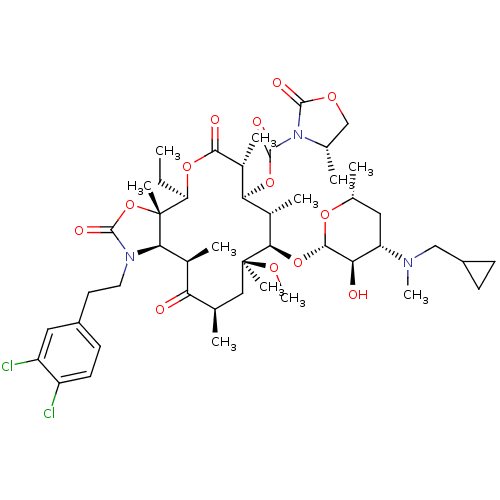 (A-198401 | CHEMBL303274) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Ability of compound to inhibit [125I-Tyr5,DLeu6,NMeLeu7,Pro9-NEt]GnRH agonist binding to the rat Gonadotropin-releasing hormone receptor was evaluate... | Bioorg Med Chem Lett 12: 2179-83 (2002) BindingDB Entry DOI: 10.7270/Q28S4P8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50166450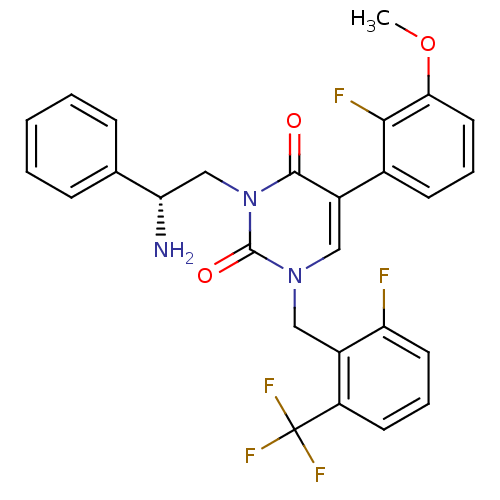 ((R)-1-(2-fluoro-6-(trifluoromethyl)benzyl)-3-(2-am...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin-releasing hormone receptor (GNRHR) using GnRH peptide as radioligand | Bioorg Med Chem Lett 15: 2519-22 (2005) Article DOI: 10.1016/j.bmcl.2005.03.057 BindingDB Entry DOI: 10.7270/Q2M044ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50191554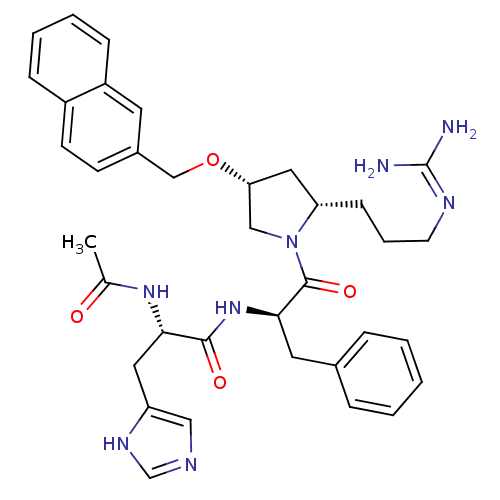 ((S)-2-acetamido-N-((R)-1-((2S,4R)-2-(3-guanidinopr...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of europium labeled NDP-alpha-MSH from human MC1R expressed in HEK293 cells | J Med Chem 49: 4745-61 (2006) Article DOI: 10.1021/jm060384p BindingDB Entry DOI: 10.7270/Q29W0F4J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM82411 (CAS_75921-69-6 | NDP-MSH) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human Melanocortin 3 receptor (hMC3R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50144640 (3-((R)-2-Amino-2-phenyl-ethyl)-1-(2-chloro-6-fluor...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin-releasing hormone receptor expressed in HEK293 cells using des-Gly10-[125I]Tyr,5 DLeu,6 NMeLeu,7 Pro9-NEt... | Bioorg Med Chem Lett 14: 2269-74 (2004) Article DOI: 10.1016/j.bmcl.2004.02.004 BindingDB Entry DOI: 10.7270/Q2736QCQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129402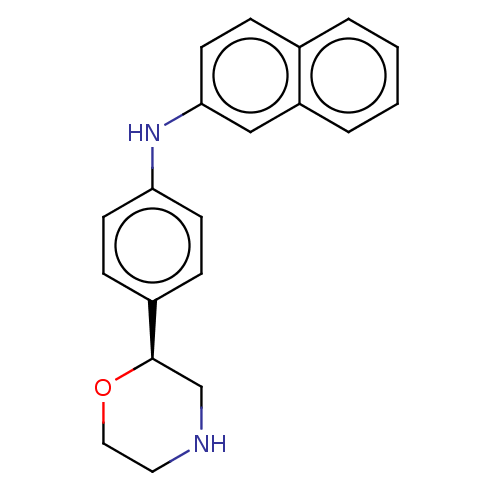 (US8802673, 44) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50357453 (CHEMBL1917719) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain membrane after 150 mins by liquid scintillation counting | Bioorg Med Chem 19: 6210-24 (2011) Article DOI: 10.1016/j.bmc.2011.09.016 BindingDB Entry DOI: 10.7270/Q2VH5P7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50357430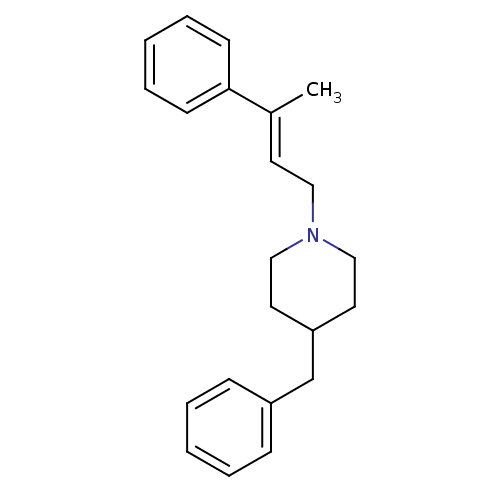 (CHEMBL1917711) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Displacement of [3H]-pentazocine from sigma 1 receptor in guinea pig brain cortical membrane after 120 mins by scintillation counting analysis | Eur J Med Chem 124: 649-665 (2016) Article DOI: 10.1016/j.ejmech.2016.08.067 BindingDB Entry DOI: 10.7270/Q2MS3VSX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129404 (US8802673, 46) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129440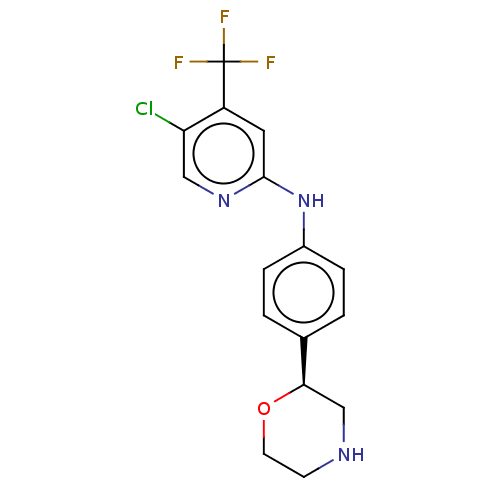 (US8802673, 82) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129457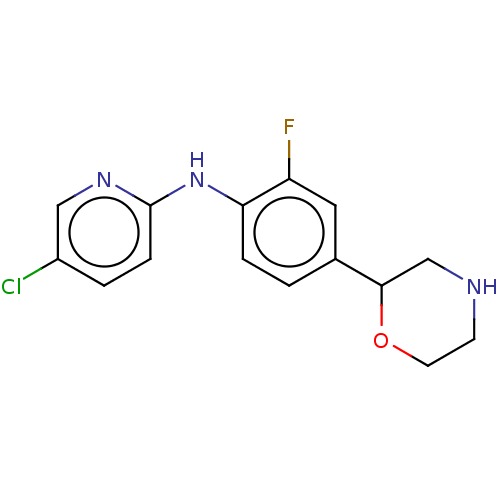 (US8802673, 99) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129412 (US8802673, 54) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM50189013 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC4R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129529 (US8802673, 171) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50357441 (CHEMBL1917705) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain membrane after 150 mins by liquid scintillation counting | Bioorg Med Chem 19: 6210-24 (2011) Article DOI: 10.1016/j.bmc.2011.09.016 BindingDB Entry DOI: 10.7270/Q2VH5P7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM129505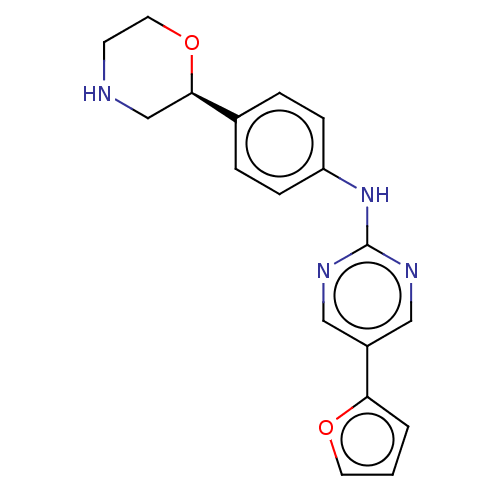 (US8802673, 147) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.900 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Hoffmann-La Roche Inc US Patent | Assay Description HEK-293 cells stably expressing rat TAAR1 were maintained at 37 C. and 5% CO2 in DMEM high glucose medium, containing fetal calf serum (10%, heat ina... | US Patent US8802673 (2014) BindingDB Entry DOI: 10.7270/Q2348J2G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM50166451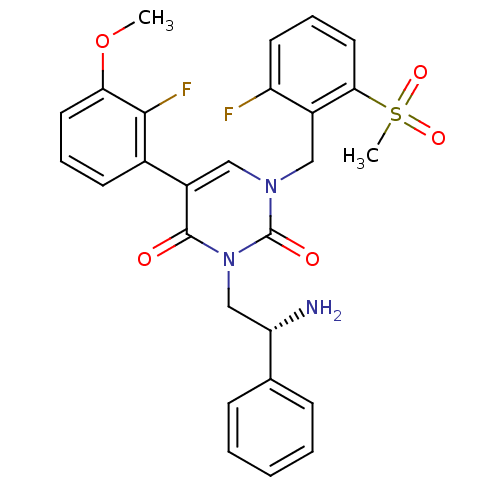 ((R)-1-(2-fluoro-6-(methylsulfonyl)benzyl)-3-(2-ami...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurocrine Biosciences, Inc. Curated by ChEMBL | Assay Description Binding affinity towards human gonadotropin-releasing hormone receptor (GNRHR) using GnRH peptide as radioligand | Bioorg Med Chem Lett 15: 2519-22 (2005) Article DOI: 10.1016/j.bmcl.2005.03.057 BindingDB Entry DOI: 10.7270/Q2M044ZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trace amine-associated receptor 1 (Mus musculus (Mouse)) | BDBM50336205 ((N-(3-Ethoxy-phenyl)-4-pyrrolidin-1-yl-3-trifluoro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd Curated by ChEMBL | Assay Description Inhibition of mouse TAAR1 | Bioorg Med Chem Lett 21: 1227-31 (2011) Article DOI: 10.1016/j.bmcl.2010.12.075 BindingDB Entry DOI: 10.7270/Q25T3KRG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 4 (Homo sapiens (Human)) | BDBM82411 (CAS_75921-69-6 | NDP-MSH) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cincinnati Curated by ChEMBL | Assay Description Binding affinity towards human melanocortin 4 receptor (hMC4R) | Bioorg Med Chem Lett 13: 2647-50 (2003) BindingDB Entry DOI: 10.7270/Q2474BD9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Cavia porcellus (Guinea pig)) | BDBM50357440 (CHEMBL1917704) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pavia Curated by ChEMBL | Assay Description Displacement of [3H]-(+)-pentazocine from sigma1 receptor in guinea pig brain membrane after 150 mins by liquid scintillation counting | Bioorg Med Chem 19: 6210-24 (2011) Article DOI: 10.1016/j.bmc.2011.09.016 BindingDB Entry DOI: 10.7270/Q2VH5P7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocyte-stimulating hormone receptor (Homo sapiens (Human)) | BDBM50189010 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC1R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189010 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(4-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanocortin receptor 3 (Homo sapiens (Human)) | BDBM50189013 ((S)-2-[(S)-4-{(R)-2-[(S)-2-acetylamino-3-(3H-imida...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Procter & Gamble Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity to human MC3R | Bioorg Med Chem Lett 16: 4668-73 (2006) Article DOI: 10.1016/j.bmcl.2006.05.087 BindingDB Entry DOI: 10.7270/Q2W66KDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50206981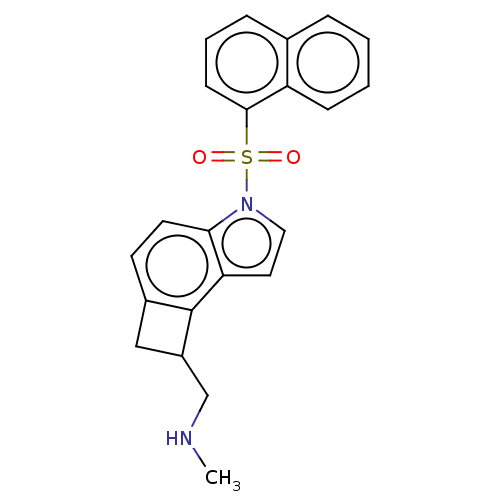 (CHEMBL3948819) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in CHO cell membranes by radioligand binding assay | Bioorg Med Chem 25: 38-52 (2017) Article DOI: 10.1016/j.bmc.2016.10.010 BindingDB Entry DOI: 10.7270/Q2VT1V3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidylprolyl isomerase (Gallus gallus) | BDBM50068597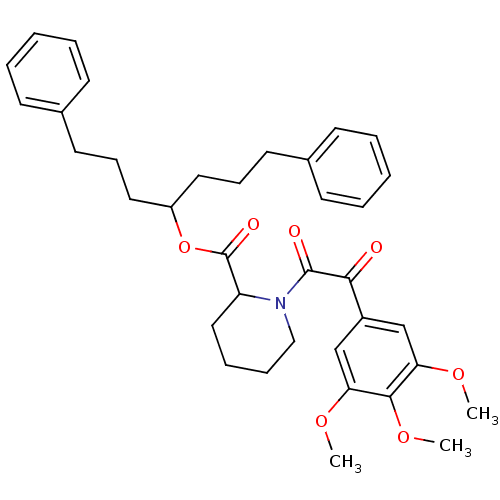 (1-[2-Oxo-2-(3,4,5-trimethoxy-phenyl)-acetyl]-piper...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of binding to FKBP12 receptor | Bioorg Med Chem Lett 7: 1785-1790 (1997) Article DOI: 10.1016/S0960-894X(97)00304-1 BindingDB Entry DOI: 10.7270/Q2PZ58TF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50113103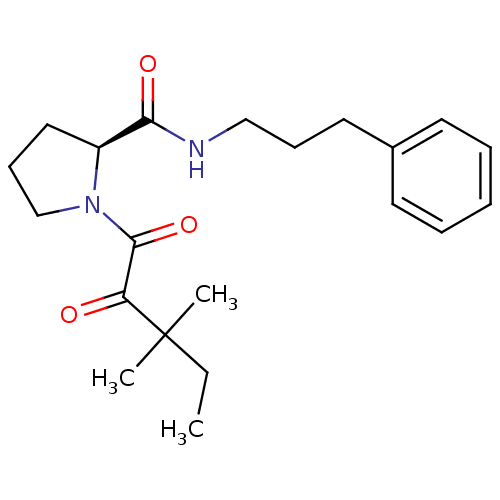 (1-(3,3-Dimethyl-2-oxo-pentanoyl)-pyrrolidine-2-car...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Guilford Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Ability to inhibit peptidyl-prolyl isomerase (PPIase, or rotamase) activity of FK506 binding protein 12 | Bioorg Med Chem Lett 12: 1429-33 (2002) BindingDB Entry DOI: 10.7270/Q23J3C9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50207038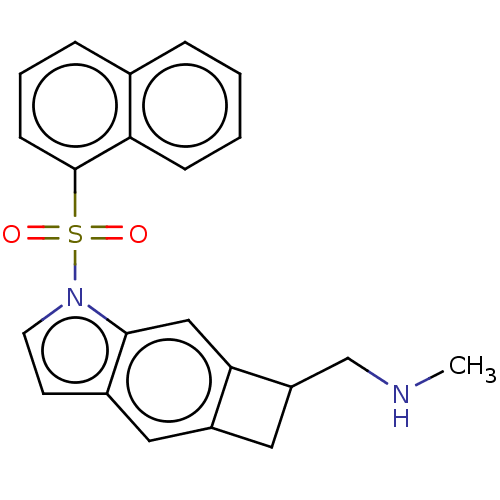 (CHEMBL3961596) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al Curated by ChEMBL | Assay Description Displacement of [3H]-LSD from human 5-HT6R expressed in CHO cell membranes by radioligand binding assay | Bioorg Med Chem 25: 38-52 (2017) Article DOI: 10.1016/j.bmc.2016.10.010 BindingDB Entry DOI: 10.7270/Q2VT1V3Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4685 total ) | Next | Last >> |