 Found 364 hits with Last Name = 'ruan' and Initial = 'f'
Found 364 hits with Last Name = 'ruan' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50071565
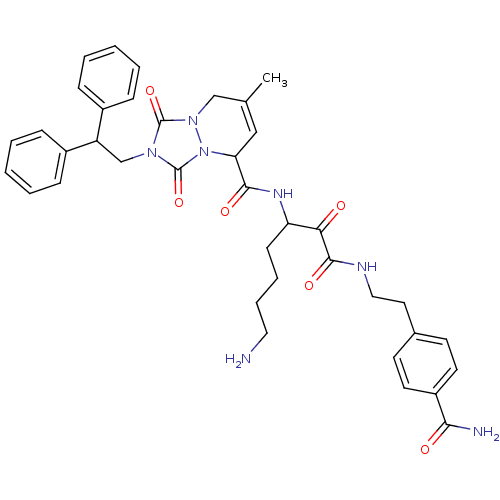
(2-(2,2-Diphenyl-ethyl)-7-methyl-1,3-dioxo-2,3,5,8-...)Show SMILES CC1=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc2ccc(cc2)C(N)=O)n2n(C1)c(=O)n(CC(c1ccccc1)c1ccccc1)c2=O |t:1| Show InChI InChI=1S/C38H43N7O6/c1-25-22-32(35(48)42-31(14-8-9-20-39)33(46)36(49)41-21-19-26-15-17-29(18-16-26)34(40)47)45-38(51)43(37(50)44(45)23-25)24-30(27-10-4-2-5-11-27)28-12-6-3-7-13-28/h2-7,10-13,15-18,22,30-32H,8-9,14,19-21,23-24,39H2,1H3,(H2,40,47)(H,41,49)(H,42,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071575

(2,2-Dibutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...)Show SMILES CCCCC1(CCCC)C(=O)N2CC(C)=CC(N2C1=O)C(=O)NC(CCCCN)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |c:14| Show InChI InChI=1S/C33H48N6O6/c1-4-6-16-33(17-7-5-2)31(44)38-21-22(3)20-26(39(38)32(33)45)29(42)37-25(10-8-9-18-34)27(40)30(43)36-19-15-23-11-13-24(14-12-23)28(35)41/h11-14,20,25-26H,4-10,15-19,21,34H2,1-3H3,(H2,35,41)(H,36,43)(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50071570

(8-Isobutyl-2-(4-methoxy-phenyl)-1,3-dioxo-2,3,5,8-...)Show SMILES COc1ccc(cc1)-n1c(=O)n2C(CC(C)C)C=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc3ccc(cc3)C(N)=O)n2c1=O |c:18| Show InChI InChI=1S/C34H43N7O7/c1-21(2)20-25-13-16-28(41-34(47)39(33(46)40(25)41)24-11-14-26(48-3)15-12-24)31(44)38-27(6-4-5-18-35)29(42)32(45)37-19-17-22-7-9-23(10-8-22)30(36)43/h7-16,21,25,27-28H,4-6,17-20,35H2,1-3H3,(H2,36,43)(H,37,45)(H,38,44) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the trypsin enzyme |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071573
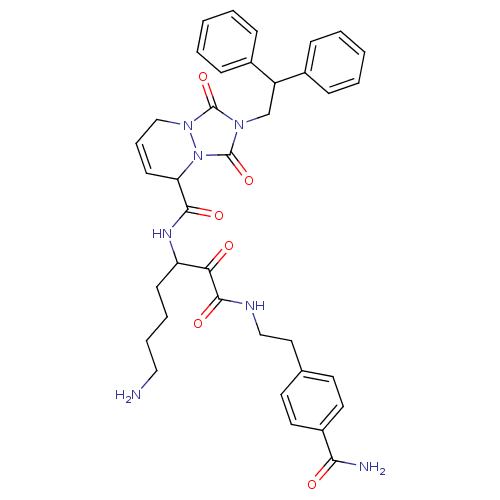
(2-(2,2-Diphenyl-ethyl)-1,3-dioxo-2,3,5,8-tetrahydr...)Show SMILES NCCCCC(NC(=O)C1C=CCn2n1c(=O)n(CC(c1ccccc1)c1ccccc1)c2=O)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |c:10| Show InChI InChI=1S/C37H41N7O6/c38-21-8-7-14-30(32(45)35(48)40-22-20-25-16-18-28(19-17-25)33(39)46)41-34(47)31-15-9-23-43-36(49)42(37(50)44(31)43)24-29(26-10-3-1-4-11-26)27-12-5-2-6-13-27/h1-6,9-13,15-19,29-31H,7-8,14,20-24,38H2,(H2,39,46)(H,40,48)(H,41,47) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50071571
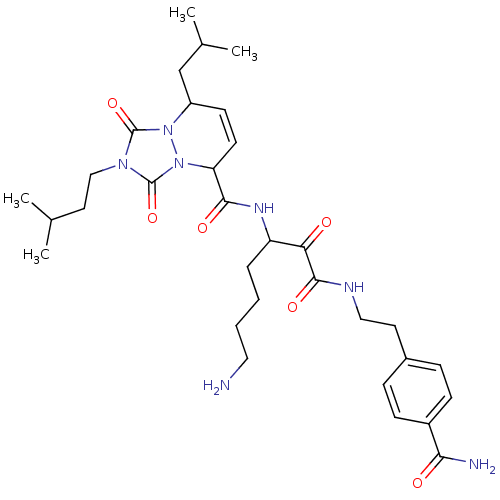
(8-Isobutyl-2-(3-methyl-butyl)-1,3-dioxo-2,3,5,8-te...)Show SMILES CC(C)CCn1c(=O)n2C(CC(C)C)C=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc3ccc(cc3)C(N)=O)n2c1=O |c:14| Show InChI InChI=1S/C32H47N7O6/c1-20(2)15-18-37-31(44)38-24(19-21(3)4)12-13-26(39(38)32(37)45)29(42)36-25(7-5-6-16-33)27(40)30(43)35-17-14-22-8-10-23(11-9-22)28(34)41/h8-13,20-21,24-26H,5-7,14-19,33H2,1-4H3,(H2,34,41)(H,35,43)(H,36,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the trypsin enzyme |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071568

(2-Amino-2-benzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrah...)Show SMILES CC1=CC(N2N(C1)C(=O)C(N)(Cc1ccccc1)C2=O)C(=O)NC(CCCCN)C(=O)C(=O)NCc1ccc(cc1)C(N)=O |t:1| Show InChI InChI=1S/C31H37N7O6/c1-19-15-24(38-30(44)31(34,29(43)37(38)18-19)16-20-7-3-2-4-8-20)27(41)36-23(9-5-6-14-32)25(39)28(42)35-17-21-10-12-22(13-11-21)26(33)40/h2-4,7-8,10-13,15,23-24H,5-6,9,14,16-18,32,34H2,1H3,(H2,33,40)(H,35,42)(H,36,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071567
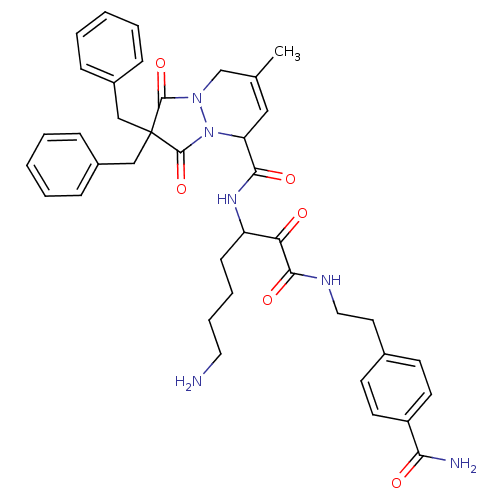
(2,2-Dibenzyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro...)Show SMILES CC1=CC(N2N(C1)C(=O)C(Cc1ccccc1)(Cc1ccccc1)C2=O)C(=O)NC(CCCCN)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |t:1| Show InChI InChI=1S/C39H44N6O6/c1-26-22-32(35(48)43-31(14-8-9-20-40)33(46)36(49)42-21-19-27-15-17-30(18-16-27)34(41)47)45-38(51)39(37(50)44(45)25-26,23-28-10-4-2-5-11-28)24-29-12-6-3-7-13-29/h2-7,10-13,15-18,22,31-32H,8-9,14,19-21,23-25,40H2,1H3,(H2,41,47)(H,42,49)(H,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071566
(2-Amino-8-benzyl-2-ethyl-1,3-dioxo-2,3,5,8-tetrahy...)Show SMILES CCC1(N)C(=O)N2C(Cc3ccccc3)C=CC(N2C1=O)C(=O)NC(CCCCN)C(=O)C(N)=O |c:16| Show InChI InChI=1S/C24H32N6O5/c1-2-24(27)22(34)29-16(14-15-8-4-3-5-9-15)11-12-18(30(29)23(24)35)21(33)28-17(10-6-7-13-25)19(31)20(26)32/h3-5,8-9,11-12,16-18H,2,6-7,10,13-14,25,27H2,1H3,(H2,26,32)(H,28,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071574

(2,2-Diisobutyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahyd...)Show SMILES CC(C)CC1(CC(C)C)C(=O)N2CC(C)=CC(N2C1=O)C(=O)NC(CCCCN)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |c:14| Show InChI InChI=1S/C33H48N6O6/c1-20(2)17-33(18-21(3)4)31(44)38-19-22(5)16-26(39(38)32(33)45)29(42)37-25(8-6-7-14-34)27(40)30(43)36-15-13-23-9-11-24(12-10-23)28(35)41/h9-12,16,20-21,25-26H,6-8,13-15,17-19,34H2,1-5H3,(H2,35,41)(H,36,43)(H,37,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Tryptase beta-2/delta/gamma
(Homo sapiens (Human)) | BDBM50216213

(CHEMBL306744)Show SMILES CC1=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc2ccc(cc2)C(N)=O)n2n(C1)c(=O)n(C(c1ccccc1)c1ccccc1)c2=O |t:1| Show InChI InChI=1S/C37H41N7O6/c1-24-22-30(44-37(50)43(36(49)42(44)23-24)31(26-10-4-2-5-11-26)27-12-6-3-7-13-27)34(47)41-29(14-8-9-20-38)32(45)35(48)40-21-19-25-15-17-28(18-16-25)33(39)46/h2-7,10-13,15-18,22,29-31H,8-9,14,19-21,23,38H2,1H3,(H2,39,46)(H,40,48)(H,41,47) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the tryptase |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50071564
(2,2-Diallyl-7-methyl-1,3-dioxo-2,3,5,8-tetrahydro-...)Show SMILES CC1=CC(N2N(C1)C(=O)C(CC=C)(CC=C)C2=O)C(=O)NC(CCCCN)C(=O)C(=O)NCCc1ccc(cc1)C(N)=O |t:1| Show InChI InChI=1S/C31H40N6O6/c1-4-14-31(15-5-2)29(42)36-19-20(3)18-24(37(36)30(31)43)27(40)35-23(8-6-7-16-32)25(38)28(41)34-17-13-21-9-11-22(12-10-21)26(33)39/h4-5,9-12,18,23-24H,1-2,6-8,13-17,19,32H2,3H3,(H2,33,39)(H,34,41)(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its binding affinity to the thrombin |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50071572
(8-{5-Amino-1-[2-(4-carbamoyl-phenyl)-ethylaminooxa...)Show SMILES COC(=O)C1C(CCc2ccccc2)=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc2ccc(cc2)C(N)=O)n2n1c(=O)n(Cc1cc(F)ccc1F)c2=O |c:14| Show InChI InChI=1S/C40H43F2N7O8/c1-57-38(54)33-27(15-12-24-7-3-2-4-8-24)22-32(48-39(55)47(40(56)49(33)48)23-28-21-29(41)16-17-30(28)42)36(52)46-31(9-5-6-19-43)34(50)37(53)45-20-18-25-10-13-26(14-11-25)35(44)51/h2-4,7-8,10-11,13-14,16-17,21-22,31-33H,5-6,9,12,15,18-20,23,43H2,1H3,(H2,44,51)(H,45,53)(H,46,52) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Kallikrein |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Kallikrein-1
(Homo sapiens (Human)) | BDBM50071563
(4-(2-{6-amino-2-[7-(3,4-dichlorobenzyl)-13,13-dime...)Show SMILES CC1(C)C2CC1C1=CC(C(=O)NC(CCCCN)C(=O)C(=O)NCCc3ccc(cc3)C(N)=O)n3n(C1C2)c(=O)n(Cc1ccc(Cl)c(Cl)c1)c3=O |t:7| Show InChI InChI=1S/C37H43Cl2N7O6/c1-37(2)23-16-25(37)24-18-30(46-36(52)44(35(51)45(46)29(24)17-23)19-21-8-11-26(38)27(39)15-21)33(49)43-28(5-3-4-13-40)31(47)34(50)42-14-12-20-6-9-22(10-7-20)32(41)48/h6-11,15,18,23,25,28-30H,3-5,12-14,16-17,19,40H2,1-2H3,(H2,41,48)(H,42,50)(H,43,49) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Molecumetics Ltd.
Curated by ChEMBL
| Assay Description
Compound was evaluated for its inhibitory activity against Kallikrein |
Bioorg Med Chem Lett 8: 2321-6 (1999)
BindingDB Entry DOI: 10.7270/Q29C6WKV |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326285
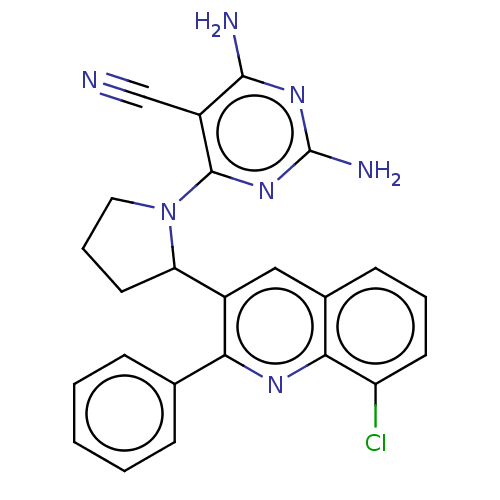
(US9637488, 68)Show SMILES Nc1nc(N)c(C#N)c(n1)N1CCCC1c1cc2cccc(Cl)c2nc1-c1ccccc1 Show InChI InChI=1S/C24H20ClN7/c25-18-9-4-8-15-12-16(20(29-21(15)18)14-6-2-1-3-7-14)19-10-5-11-32(19)23-17(13-26)22(27)30-24(28)31-23/h1-4,6-9,12,19H,5,10-11H2,(H4,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326282

(US9637488, 65)Show SMILES Cc1cccc2cc([C@H]3CCCN3c3ncnc(N)c3C#N)c(nc12)N1CCOCC1 Show InChI InChI=1S/C23H25N7O/c1-15-4-2-5-16-12-17(23(28-20(15)16)29-8-10-31-11-9-29)19-6-3-7-30(19)22-18(13-24)21(25)26-14-27-22/h2,4-5,12,14,19H,3,6-11H2,1H3,(H2,25,26,27)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326286
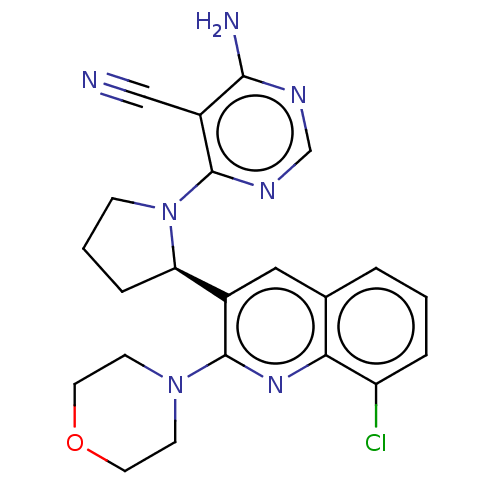
(US9637488, 69)Show SMILES Nc1ncnc(N2CCC[C@@H]2c2cc3cccc(Cl)c3nc2N2CCOCC2)c1C#N Show InChI InChI=1S/C22H22ClN7O/c23-17-4-1-3-14-11-15(22(28-19(14)17)29-7-9-31-10-8-29)18-5-2-6-30(18)21-16(12-24)20(25)26-13-27-21/h1,3-4,11,13,18H,2,5-10H2,(H2,25,26,27)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326284
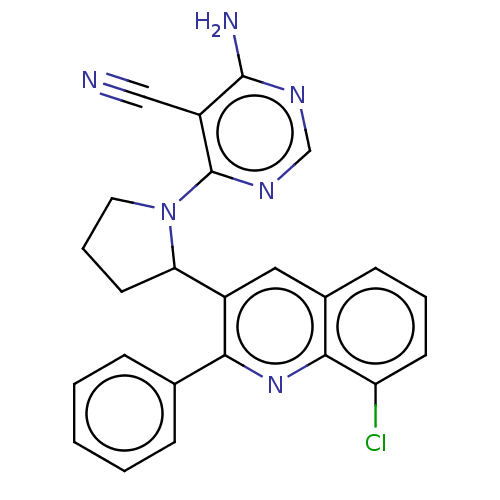
(US9637488, 67)Show SMILES Nc1ncnc(N2CCCC2c2cc3cccc(Cl)c3nc2-c2ccccc2)c1C#N Show InChI InChI=1S/C24H19ClN6/c25-19-9-4-8-16-12-17(21(30-22(16)19)15-6-2-1-3-7-15)20-10-5-11-31(20)24-18(13-26)23(27)28-14-29-24/h1-4,6-9,12,14,20H,5,10-11H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326267
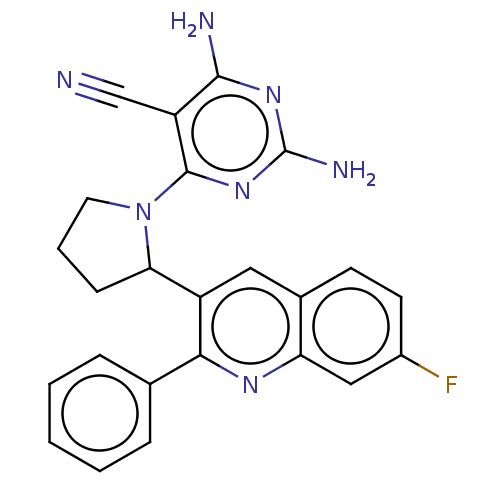
(US9637488, 27)Show SMILES Nc1nc(N)c(C#N)c(n1)N1CCCC1c1cc2ccc(F)cc2nc1-c1ccccc1 Show InChI InChI=1S/C24H20FN7/c25-16-9-8-15-11-17(21(29-19(15)12-16)14-5-2-1-3-6-14)20-7-4-10-32(20)23-18(13-26)22(27)30-24(28)31-23/h1-3,5-6,8-9,11-12,20H,4,7,10H2,(H4,27,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326278

(US9637488, 54)Show SMILES Nc1nc(N)c(C#N)c(n1)N1CCC[C@H]1c1cc2cccc(Cl)c2nc1-c1ccccc1 Show InChI InChI=1S/C24H20ClN7/c25-18-9-4-8-15-12-16(20(29-21(15)18)14-6-2-1-3-7-14)19-10-5-11-32(19)23-17(13-26)22(27)30-24(28)31-23/h1-4,6-9,12,19H,5,10-11H2,(H4,27,28,30,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326259
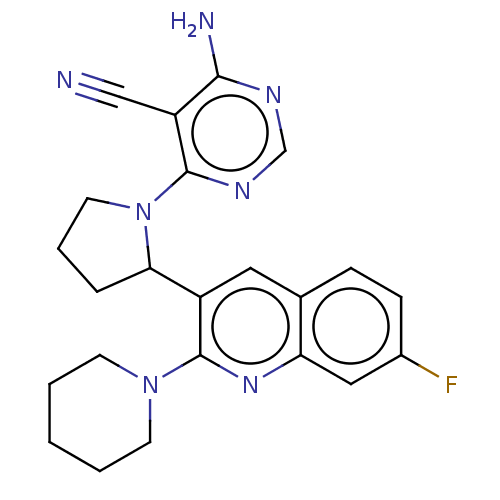
(US9637488, 18)Show SMILES Nc1ncnc(N2CCCC2c2cc3ccc(F)cc3nc2N2CCCCC2)c1C#N Show InChI InChI=1S/C23H24FN7/c24-16-7-6-15-11-17(23(29-19(15)12-16)30-8-2-1-3-9-30)20-5-4-10-31(20)22-18(13-25)21(26)27-14-28-22/h6-7,11-12,14,20H,1-5,8-10H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326262

(US9637488, 22 | US9637488, 24)Show SMILES Fc1ccc2cc(C3CCCN3c3ncnc4[nH]cnc34)c(nc2c1)-c1ccccc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326261

(US9637488, 21)Show SMILES Fc1ccc2cc(C3CCCN3c3ncnc4[nH]cnc34)c(nc2c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326258
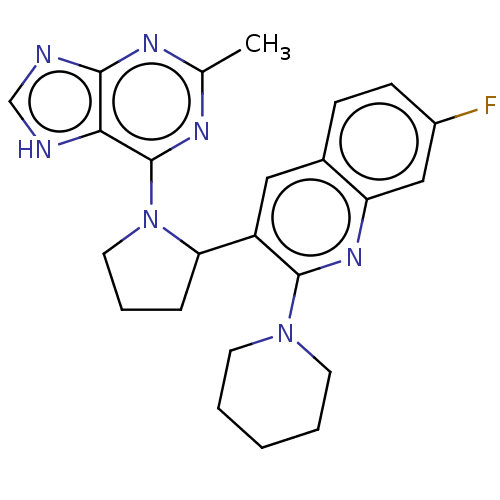
(US9637488, 17)Show SMILES Cc1nc(N2CCCC2c2cc3ccc(F)cc3nc2N2CCCCC2)c2[nH]cnc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326252

(4-amino-6-(2-(7-fluoro-2-morpholinoquinolin-3-yl)p...)Show SMILES Nc1ncnc(N2CCCC2c2cc3ccc(F)cc3nc2N2CCOCC2)c1C#N Show InChI InChI=1S/C22H22FN7O/c23-15-4-3-14-10-16(22(28-18(14)11-15)29-6-8-31-9-7-29)19-2-1-5-30(19)21-17(12-24)20(25)26-13-27-21/h3-4,10-11,13,19H,1-2,5-9H2,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326281

(US9637488, 64)Show SMILES Cc1cccc2cc([C@H]3CCCN3c3ncnc4nc[nH]c34)c(nc12)N1CCOCC1 Show InChI InChI=1S/C23H25N7O/c1-15-4-2-5-16-12-17(22(28-19(15)16)29-8-10-31-11-9-29)18-6-3-7-30(18)23-20-21(25-13-24-20)26-14-27-23/h2,4-5,12-14,18H,3,6-11H2,1H3,(H,24,25,26,27)/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326269
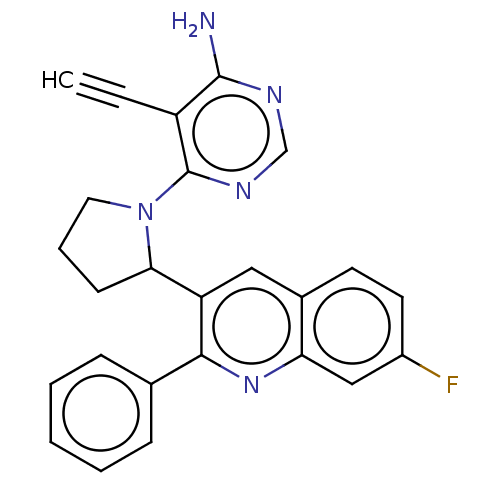
(US9637488, 29)Show SMILES Nc1ncnc(N2CCCC2c2cc3ccc(F)cc3nc2-c2ccccc2)c1C#C Show InChI InChI=1S/C25H20FN5/c1-2-19-24(27)28-15-29-25(19)31-12-6-9-22(31)20-13-17-10-11-18(26)14-21(17)30-23(20)16-7-4-3-5-8-16/h1,3-5,7-8,10-11,13-15,22H,6,9,12H2,(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326283

(US9637488, 66)Show SMILES Clc1cccc2cc(C3CCCN3c3ncnc4[nH]cnc34)c(nc12)N1CCOCC1 Show InChI InChI=1S/C22H22ClN7O/c23-16-4-1-3-14-11-15(21(28-18(14)16)29-7-9-31-10-8-29)17-5-2-6-30(17)22-19-20(25-12-24-19)26-13-27-22/h1,3-4,11-13,17H,2,5-10H2,(H,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326257
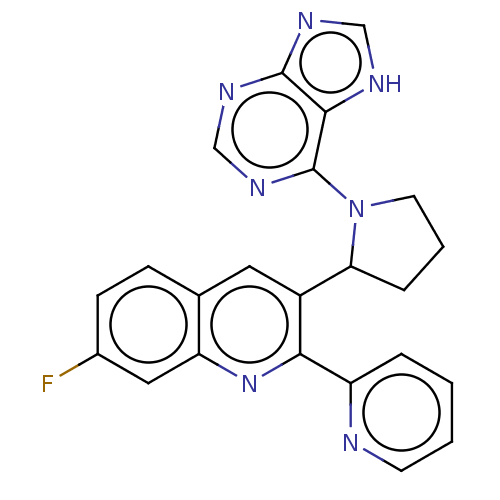
(US9637488, 16)Show SMILES Fc1ccc2cc(C3CCCN3c3ncnc4nc[nH]c34)c(nc2c1)-c1ccccn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM25045

(3-(4-morpholin-4-ylpyrido[2,3]furo[2,4-b]pyrimidin...)Show InChI InChI=1S/C19H16N4O3/c24-13-4-1-3-12(11-13)17-21-15-14-5-2-6-20-19(14)26-16(15)18(22-17)23-7-9-25-10-8-23/h1-6,11,24H,7-10H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326270
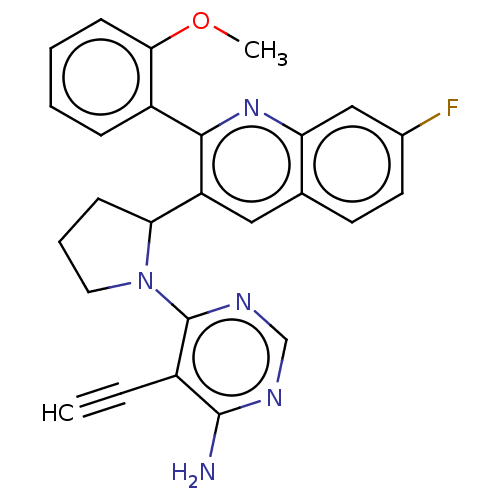
(US9637488, 30)Show SMILES COc1ccccc1-c1nc2cc(F)ccc2cc1C1CCCN1c1ncnc(N)c1C#C Show InChI InChI=1S/C26H22FN5O/c1-3-18-25(28)29-15-30-26(18)32-12-6-8-22(32)20-13-16-10-11-17(27)14-21(16)31-24(20)19-7-4-5-9-23(19)33-2/h1,4-5,7,9-11,13-15,22H,6,8,12H2,2H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326276

(US9637488, 44)Show SMILES Nc1ncnc(N2CCCCC2c2cc3ccc(F)cc3nc2N2CCOCC2)c1C#N Show InChI InChI=1S/C23H24FN7O/c24-16-5-4-15-11-17(23(29-19(15)12-16)30-7-9-32-10-8-30)20-3-1-2-6-31(20)22-18(13-25)21(26)27-14-28-22/h4-5,11-12,14,20H,1-3,6-10H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326272

(US9637488, 38 | US9637488, 43)Show SMILES Fc1ccc2cc(C3CCCCN3c3ncnc4[nH]cnc34)c(nc2c1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326272

(US9637488, 38 | US9637488, 43)Show SMILES Fc1ccc2cc(C3CCCCN3c3ncnc4[nH]cnc34)c(nc2c1)N1CCOCC1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150175
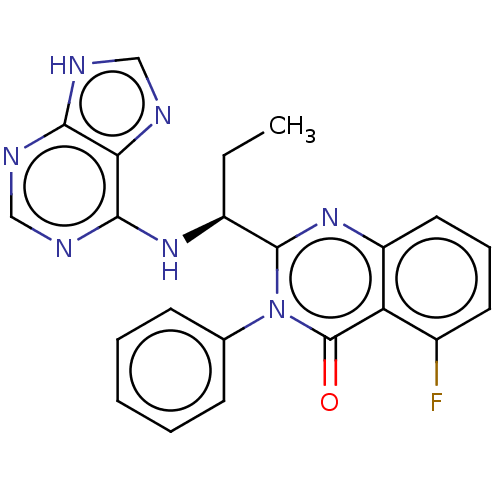
(US8980901, 107 | US9149477, Compound 107)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068
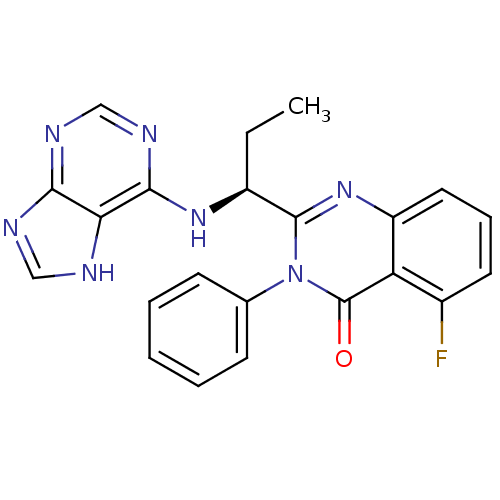
(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50403068

(CHEMBL2216870 | IDELALISIB | US9745321, CAL-101)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 |r| Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150175

(US8980901, 107 | US9149477, Compound 107)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(F)c2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H18FN7O/c1-2-15(28-20-18-19(25-11-24-18)26-12-27-20)21-29-16-10-6-9-14(23)17(16)22(31)30(21)13-7-4-3-5-8-13/h3-12,15H,2H2,1H3,(H2,24,25,26,27,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326263

(US9637488, 23 | US9637488, 25)Show SMILES COc1ccccc1-c1nc2cc(F)ccc2cc1C1CCCN1c1ncnc2[nH]cnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM182844

(US9149477, Compound 117)Show SMILES CC[C@H](Nc1ncnc2[nH]cnc12)c1nc2cccc(Cl)c2c(=O)n1-c1cc(F)cc(F)c1 Show InChI InChI=1S/C22H16ClF2N7O/c1-2-15(30-20-18-19(27-9-26-18)28-10-29-20)21-31-16-5-3-4-14(23)17(16)22(33)32(21)13-7-11(24)6-12(25)8-13/h3-10,15H,2H2,1H3,(H2,26,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150172

(US8980901, 117)Show SMILES C[C@H](Nc1ncnc2[nH]cnc12)c1nc2ccccc2c(=O)n1-c1cccc(F)c1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104486

(US8586597, 101 | USRE44599, 117)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(Cl)c2c(=O)n1-c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C22H16ClF2N7O/c1-2-15(30-20-18-19(27-9-26-18)28-10-29-20)21-31-16-5-3-4-14(23)17(16)22(33)32(21)13-7-11(24)6-12(25)8-13/h3-10,15H,2H2,1H3,(H2,26,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104486

(US8586597, 101 | USRE44599, 117)Show SMILES CC[C@H](Nc1ncnc2nc[nH]c12)c1nc2cccc(Cl)c2c(=O)n1-c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C22H16ClF2N7O/c1-2-15(30-20-18-19(27-9-26-18)28-10-29-20)21-31-16-5-3-4-14(23)17(16)22(33)32(21)13-7-11(24)6-12(25)8-13/h3-10,15H,2H2,1H3,(H2,26,27,28,29,30)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326279

(US9637488, 62)Show SMILES Fc1ccc2cc([C@H]3CCCN3c3ncnc4[nH]cnc34)c(nc2c1)-c1ccccn1 Show InChI InChI=1S/C23H18FN7/c24-15-7-6-14-10-16(20(30-18(14)11-15)17-4-1-2-8-25-17)19-5-3-9-31(19)23-21-22(27-12-26-21)28-13-29-23/h1-2,4,6-8,10-13,19H,3,5,9H2,(H,26,27,28,29)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12.8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326253
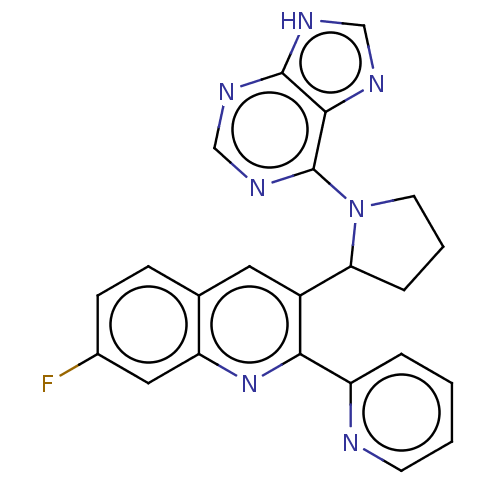
(3-(1-(9H-purin-6-yl)pyrrolidin-2-yl)-7-fluoro-2-(p...)Show SMILES Fc1ccc2cc(C3CCCN3c3ncnc4[nH]cnc34)c(nc2c1)-c1ccccn1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326263

(US9637488, 23 | US9637488, 25)Show SMILES COc1ccccc1-c1nc2cc(F)ccc2cc1C1CCCN1c1ncnc2[nH]cnc12 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM150171

(US8980901, 98)Show SMILES CC(Nc1ncnc2[nH]cc(C)c12)c1nc2cccc(C)c2c(=O)n1-c1cccc(F)c1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Icos Corporation
US Patent
| Assay Description
Using the method described in Example 2, compounds of the invention were
tested for inhibitory activity and potency against PI3Kdelta, and for
sele... |
US Patent US8980901 (2015)
BindingDB Entry DOI: 10.7270/Q28C9V0N |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104485

(US8586597, 99 | USRE44599, 98)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2ccccc2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H19N7O/c1-2-16(27-20-18-19(24-12-23-18)25-13-26-20)21-28-17-11-7-6-10-15(17)22(30)29(21)14-8-4-3-5-9-14/h3-13,16H,2H2,1H3,(H2,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Compounds of the invention were tested for inhibitory activity and potency against PI3Kδ. |
US Patent US8586597 (2013)
BindingDB Entry DOI: 10.7270/Q2RV0M9J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM182845

(US9149477, Compound 98)Show SMILES CCC(Nc1ncnc2[nH]cnc12)c1nc2ccccc2c(=O)n1-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Using the method described in US9149477, Example 2, compounds of the invention were tested for inhibitory activity and potency against PI3Kδ, an... |
US Patent US9149477 (2015)
BindingDB Entry DOI: 10.7270/Q2RR1X11 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM326262

(US9637488, 22 | US9637488, 24)Show SMILES Fc1ccc2cc(C3CCCN3c3ncnc4[nH]cnc34)c(nc2c1)-c1ccccc1F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
US Patent
| Assay Description
Exemplary compounds of the invention were tested their inhibitory activity or potency against PI3Kδ in 10-dose IC50 mode with 3-fold serial dilu... |
US Patent US9637488 (2017)
BindingDB Entry DOI: 10.7270/Q2D220Q6 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM104485

(US8586597, 99 | USRE44599, 98)Show SMILES CCC(Nc1ncnc2nc[nH]c12)c1nc2ccccc2c(=O)n1-c1ccccc1 Show InChI InChI=1S/C22H19N7O/c1-2-16(27-20-18-19(24-12-23-18)25-13-26-20)21-28-17-11-7-6-10-15(17)22(30)29(21)14-8-4-3-5-9-14/h3-13,16H,2H2,1H3,(H2,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ICOS Corporation
US Patent
| Assay Description
Biochemical assay using PI3K delta. |
US Patent USRE44599 (2013)
BindingDB Entry DOI: 10.7270/Q2736PJJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data