

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31149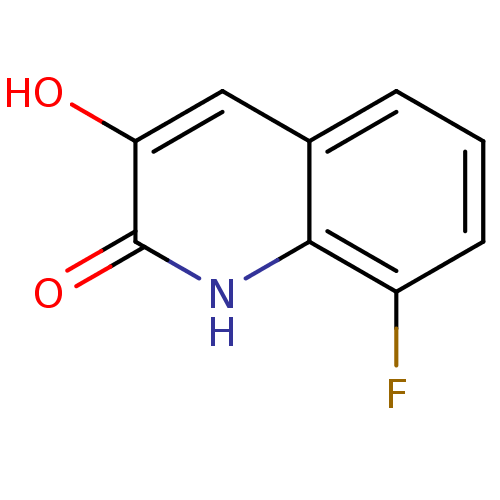 (3-hydroxyquinolin-2(1H)-one, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50081645 (CHEMBL3422237) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31173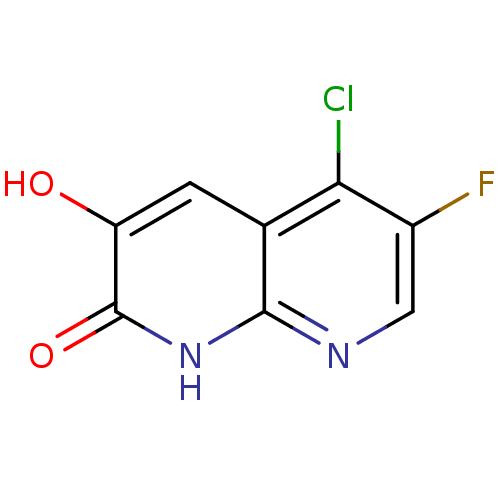 (naphthyridinone analog., 27) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136576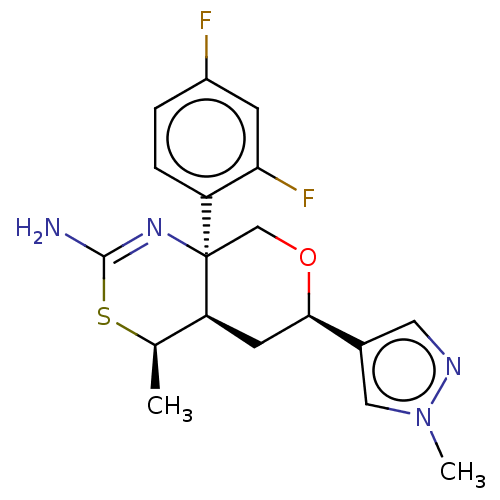 (US8865706, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM41536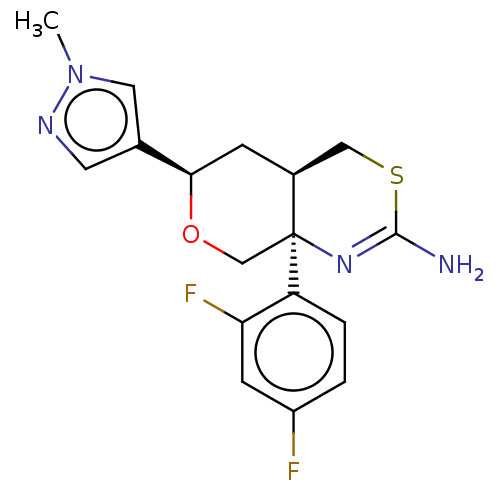 (US8865706, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50081646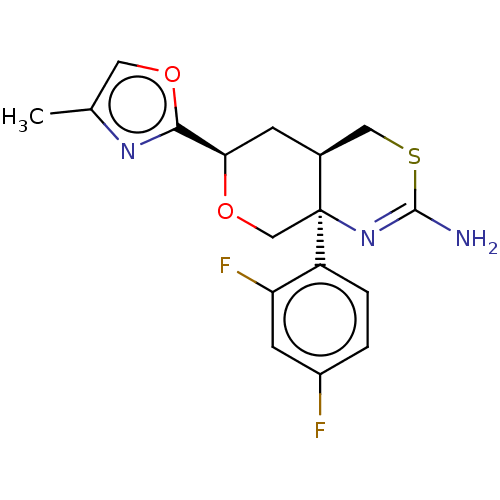 (CHEMBL3422236) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Rattus norvegicus (rat)) | BDBM31173 (naphthyridinone analog., 27) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31148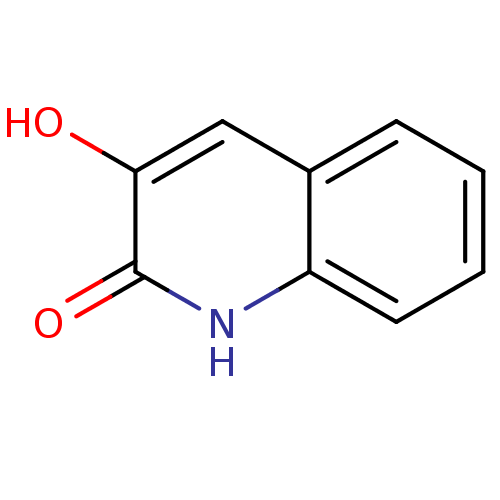 (3-hydroxyquinolin-2(1H)-one, 2 | US9701638, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31172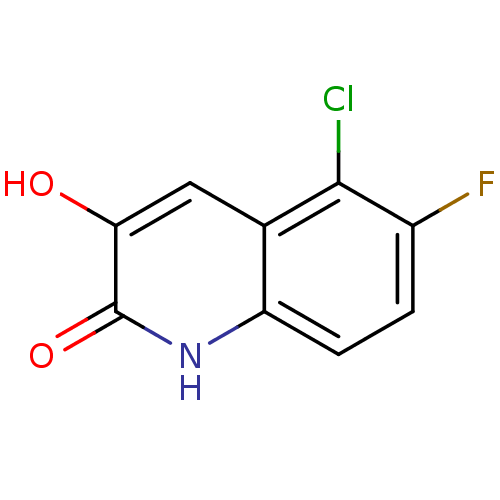 (3-hydroxyquinolin-2(1H)-one, 26) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM148176 (US8962616, 22 | US8962616, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136570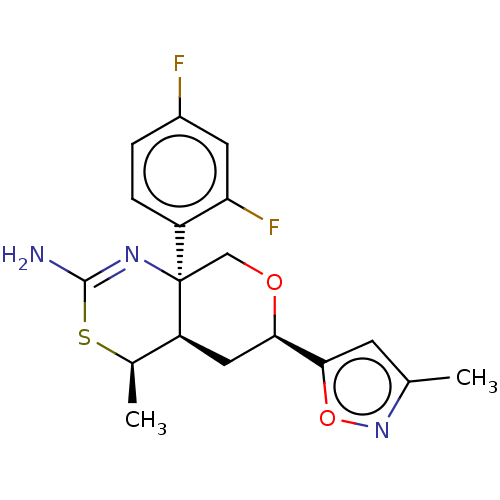 (US8865706, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136583 (US8865706, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31161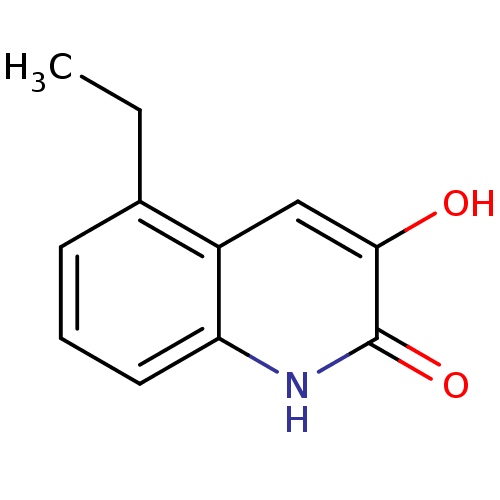 (3-hydroxyquinolin-2(1H)-one, 15) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31152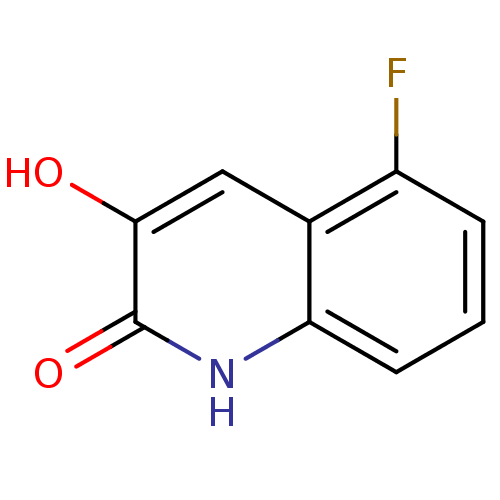 (3-hydroxyquinolin-2(1H)-one, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31164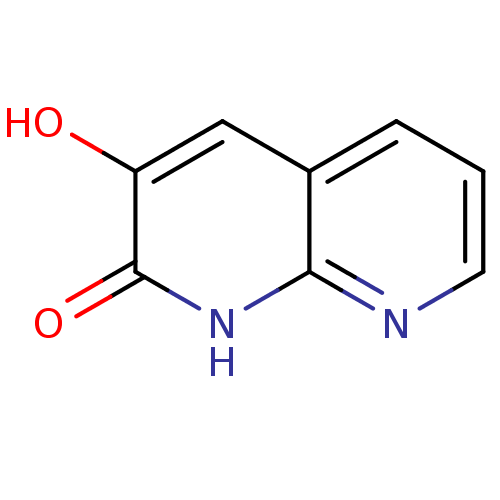 (naphthyridinone analog.,18) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31147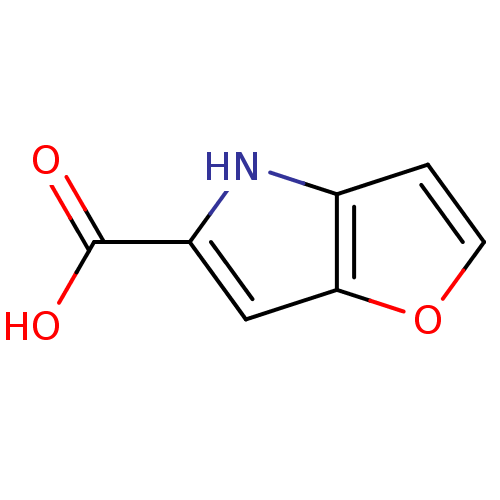 (4H-furo[3,2-b]pyrrole-5-carboxylic acid | 5-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31151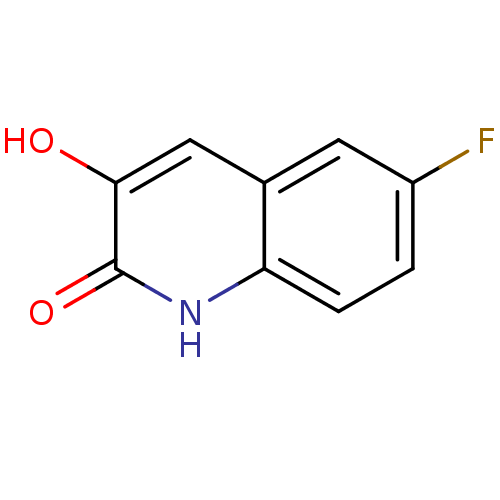 (3-hydroxyquinolin-2(1H)-one, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50081642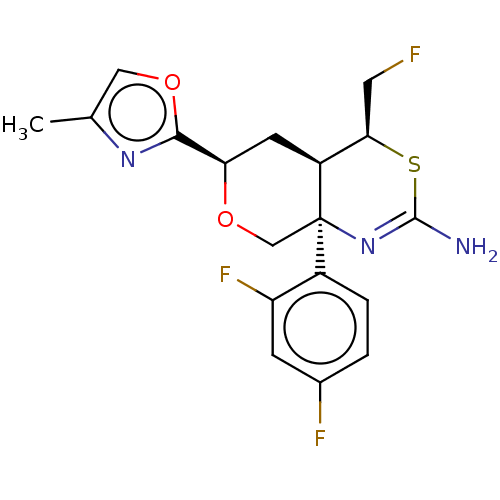 (CHEMBL3422244) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182352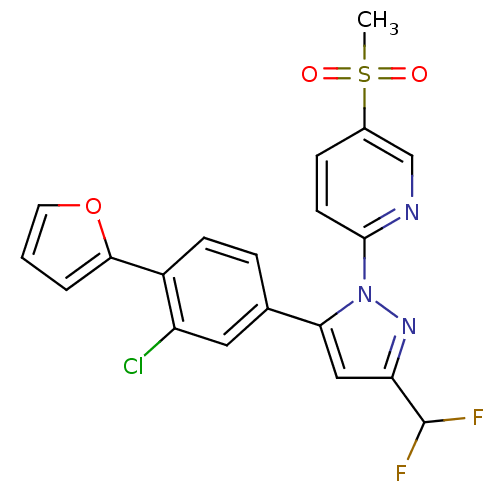 (2-(5-(3-chloro-4-(furan-2-yl)phenyl)-3-(difluorome...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182338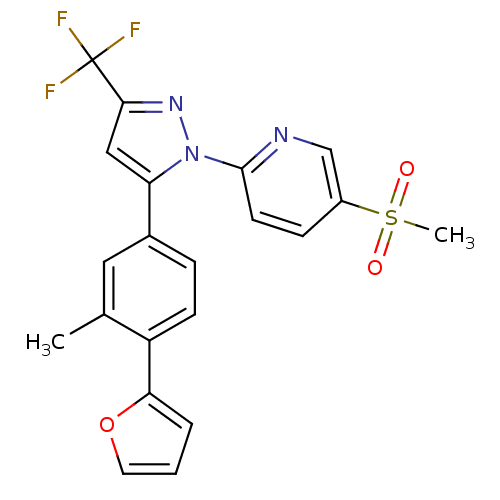 (2-(5-(4-(furan-2-yl)-3-methylphenyl)-3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31150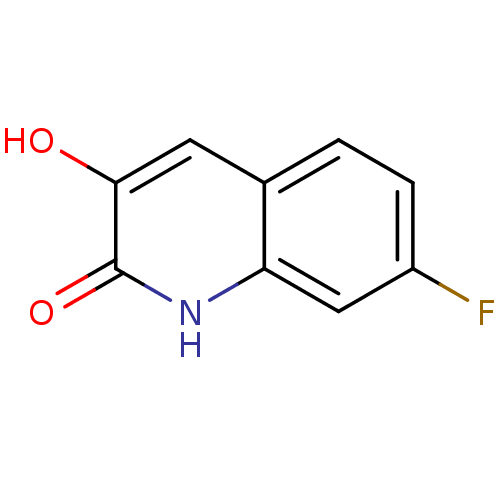 (3-hydroxyquinolin-2(1H)-one, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182347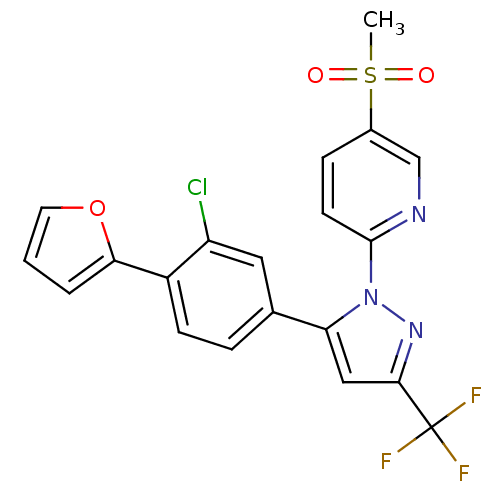 (2-(5-(3-chloro-4-(furan-2-yl)phenyl)-3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182339 (2-(furan-2-yl)-5-(1-(5-(methylsulfonyl)pyridin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136574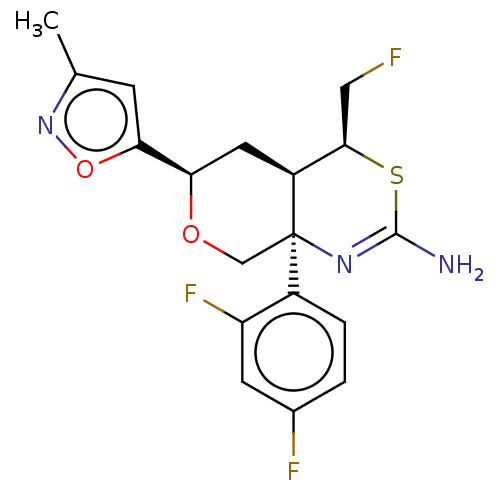 (US8865706, 13) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31160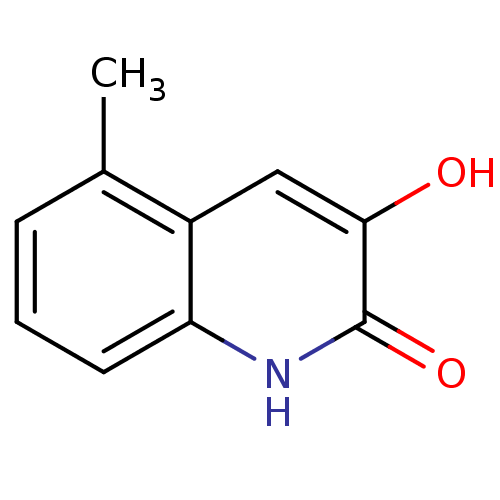 (3-hydroxyquinolin-2(1H)-one, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Prostaglandin G/H synthase 2 (Mus musculus (Mouse)) | BDBM50293282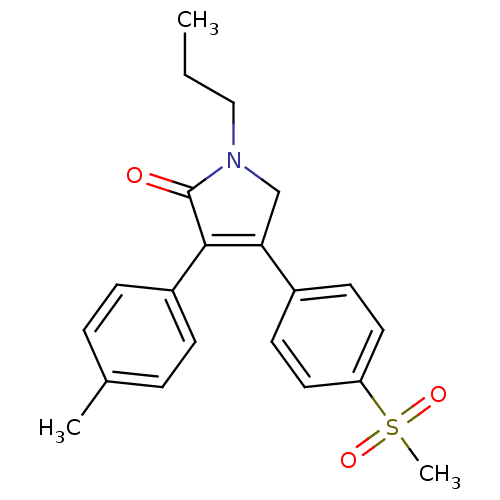 (CHEMBL504535 | Imrecoxib) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
PharmaPhase Co., Ltd Curated by ChEMBL | Assay Description Inhibition of LPS-induced COX-2 in peritoneal macrophage of C57BL/J6 mouse assessed as prostaglandin E2 formation preincubated for 1 hr followed by L... | Bioorg Med Chem 22: 2005-32 (2014) Article DOI: 10.1016/j.bmc.2014.02.017 BindingDB Entry DOI: 10.7270/Q2WS8VS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182353 (2-(3-(difluoromethyl)-5-(3-methyl-4-(thiazol-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182332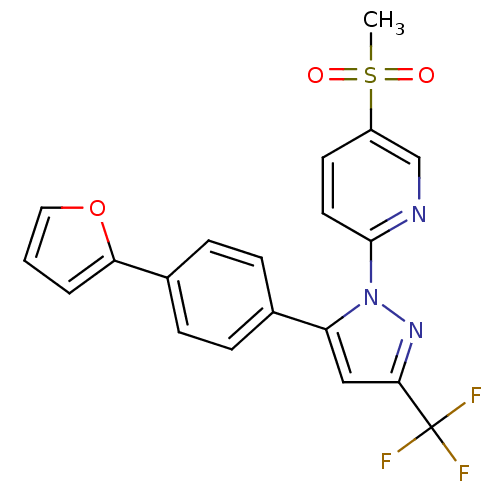 (2-(5-(4-(furan-2-yl)phenyl)-3-(trifluoromethyl)-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50396483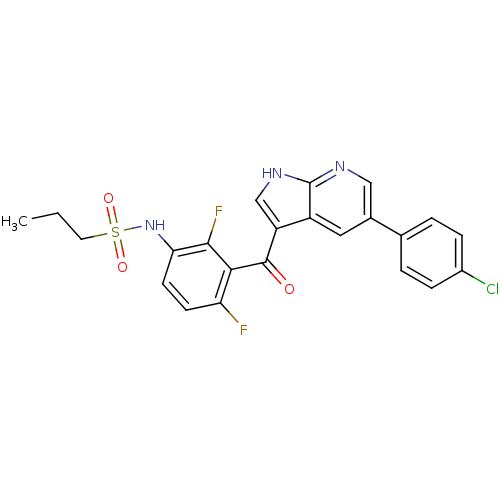 (PLX-4032 | RG 7204 | Ro 5185426 | US10570155, Vemu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenogen Pharma Group Curated by ChEMBL | Assay Description Inhibition of wild type BRAF (unknown origin) | Bioorg Med Chem 21: 2795-825 (2013) Article DOI: 10.1016/j.bmc.2013.02.061 BindingDB Entry DOI: 10.7270/Q2DV1M8C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM148173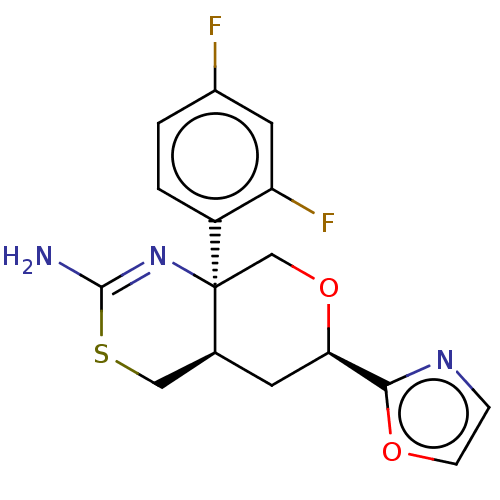 (US8962616, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50176545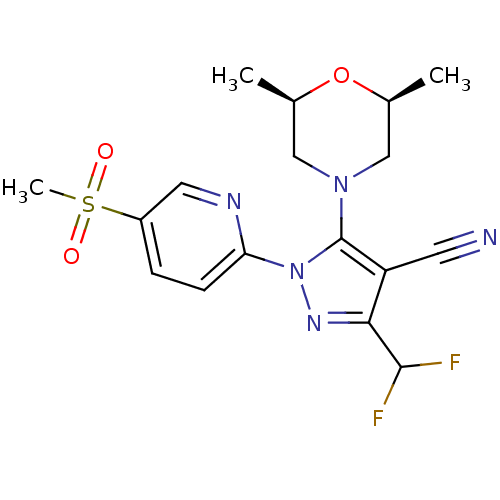 (3-(difluoromethyl)-5-((2R,6S)-2,6-dimethylmorpholi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against COX2 in feline whole blood assay | Bioorg Med Chem Lett 16: 288-92 (2005) Article DOI: 10.1016/j.bmcl.2005.10.006 BindingDB Entry DOI: 10.7270/Q2NZ876S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50176555 (3-(difluoromethyl)-5-(3-methylpiperidin-1-yl)-1-(5...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibitory activity against COX2 in canine whole blood | Bioorg Med Chem Lett 16: 288-92 (2005) Article DOI: 10.1016/j.bmcl.2005.10.006 BindingDB Entry DOI: 10.7270/Q2NZ876S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182345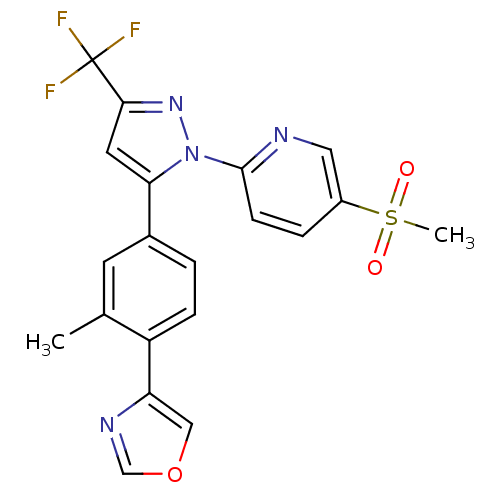 (2-(5-(3-methyl-4-(oxazol-4-yl)phenyl)-3-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31165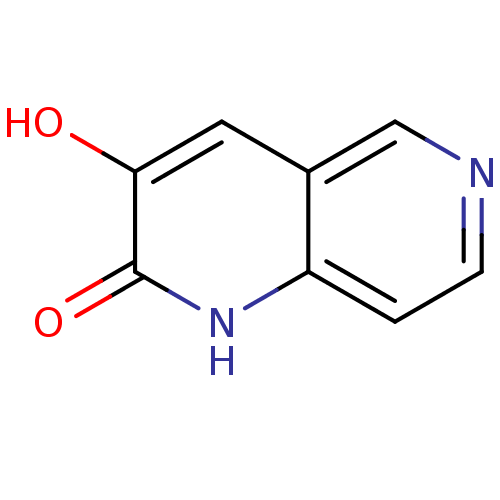 (naphthyridinone analog.,19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31153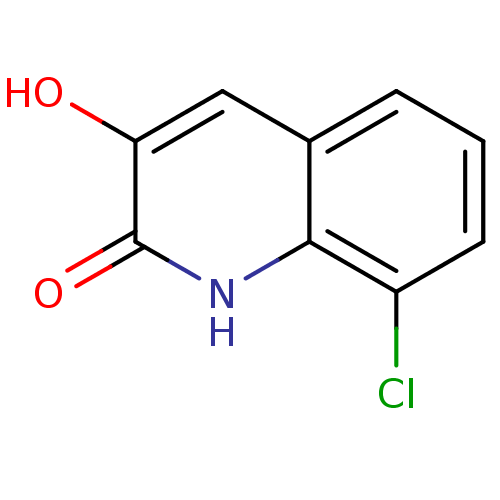 (3-hydroxyquinolin-2(1H)-one, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136576 (US8865706, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Homo sapiens (Human)) | BDBM31157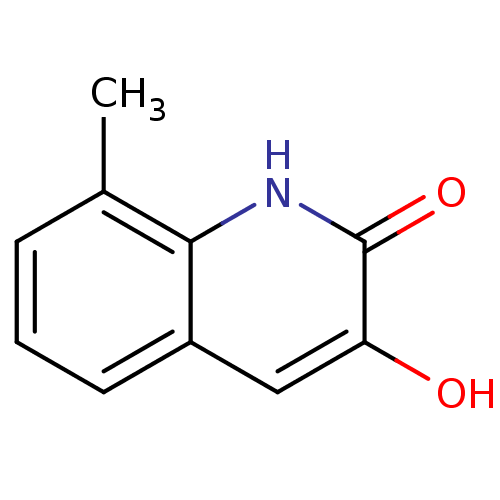 (3-hydroxyquinolin-2(1H)-one, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| D-amino-acid oxidase (Rattus norvegicus (rat)) | BDBM31156 (3-hydroxyquinolin-2(1H)-one, 10) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50179600 (5-(2-fluorobenzylthio)-1-(5-(methylsulfonyl)pyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 by canine whole blood assay | Bioorg Med Chem Lett 16: 1202-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.110 BindingDB Entry DOI: 10.7270/Q2FT8KKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137438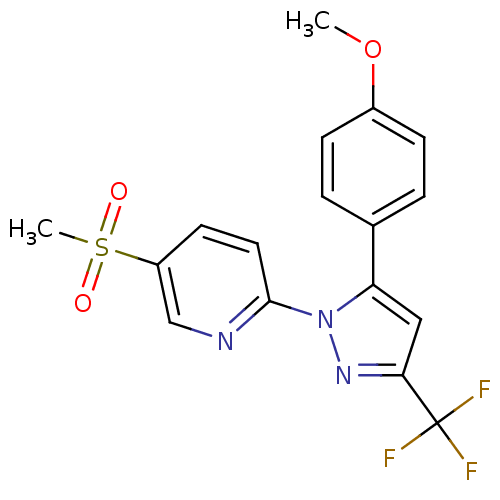 (5-Methanesulfonyl-2-[5-(4-methoxy-phenyl)-3-triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137423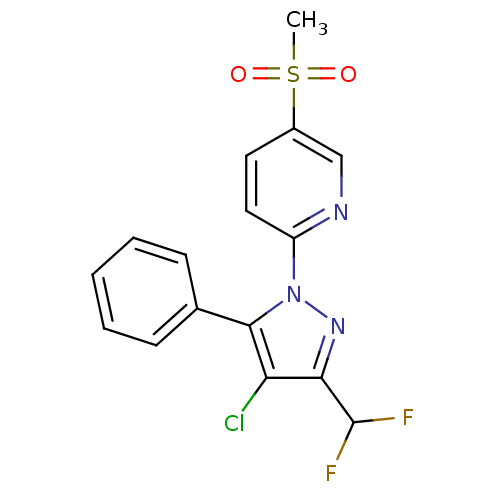 (2-(4-Chloro-3-difluoromethyl-5-phenyl-pyrazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50137442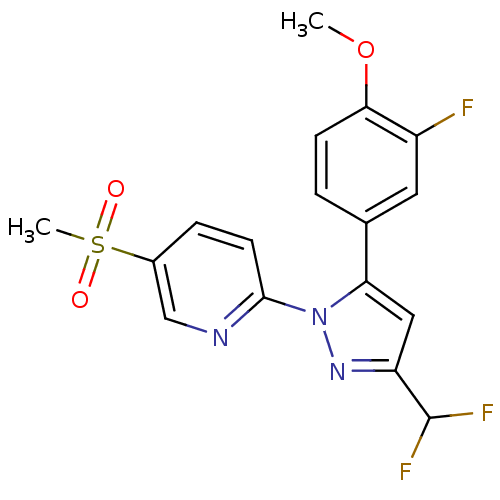 (2-[3-Difluoromethyl-5-(3-fluoro-4-methoxy-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 2. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182335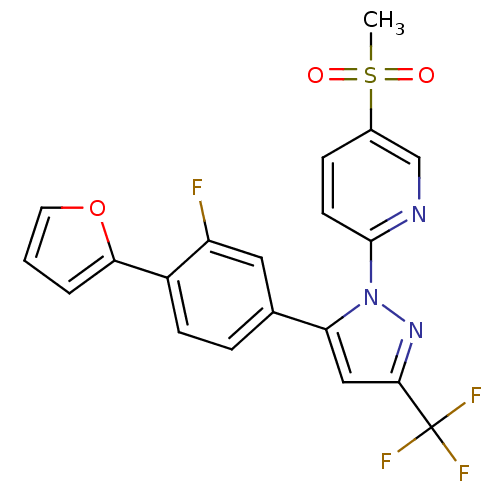 (2-(5-(3-fluoro-4-(furan-2-yl)phenyl)-3-(trifluorom...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D-amino-acid oxidase (Rattus norvegicus (rat)) | BDBM31172 (3-hydroxyquinolin-2(1H)-one, 26) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer | Assay Description Inhibitory effect of compounds was determined in a cell free fluorescence assay. The H2O2 generated from the degradation of D-serine was linked to ox... | J Med Chem 52: 3576-85 (2009) Article DOI: 10.1021/jm900128w BindingDB Entry DOI: 10.7270/Q2CZ35HM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Canis familiaris) | BDBM50137420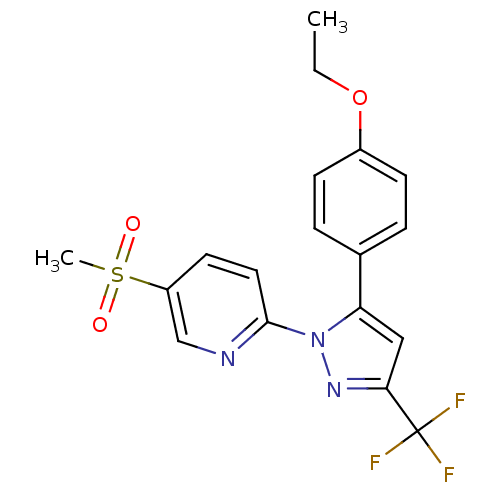 (2-[5-(4-Ethoxy-phenyl)-3-trifluoromethyl-pyrazol-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against cannine prostaglandin G/H synthase 1. | Bioorg Med Chem Lett 14: 95-8 (2003) BindingDB Entry DOI: 10.7270/Q25B01WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50182351 (2-(5-(3-chloro-4-(thiazol-4-yl)phenyl)-3-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Veterinary Medicine Research and Development Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 in canine whole blood | Bioorg Med Chem Lett 16: 2076-80 (2006) Article DOI: 10.1016/j.bmcl.2006.01.059 BindingDB Entry DOI: 10.7270/Q28K78PW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50179631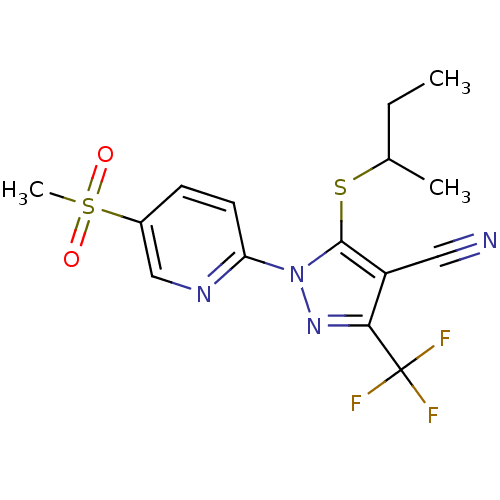 (5-(sec-butylthio)-1-(5-(methylsulfonyl)pyridin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 by canine whole blood assay | Bioorg Med Chem Lett 16: 1202-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.110 BindingDB Entry DOI: 10.7270/Q2FT8KKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Canis familiaris) | BDBM50179598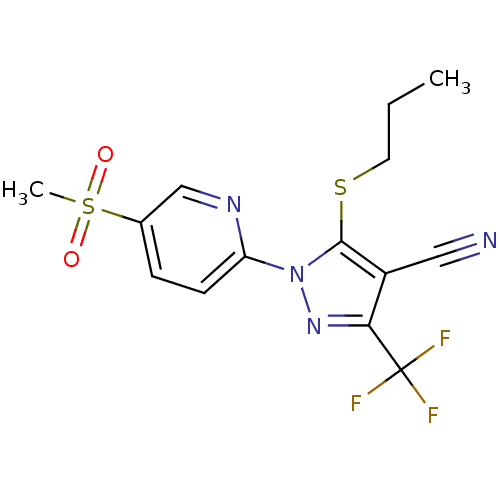 (1-(5-(methylsulfonyl)pyridin-2-yl)-5-(propylthio)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of COX2 by canine whole blood assay | Bioorg Med Chem Lett 16: 1202-6 (2006) Article DOI: 10.1016/j.bmcl.2005.11.110 BindingDB Entry DOI: 10.7270/Q2FT8KKM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM41536 (US8865706, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells overexpressing wild type human APP695 assessed as colorimetric reaction by Whole cell assay | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 424 total ) | Next | Last >> |