

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Platelet-derived growth factor receptor alpha (Homo sapiens (Human)) | BDBM50336457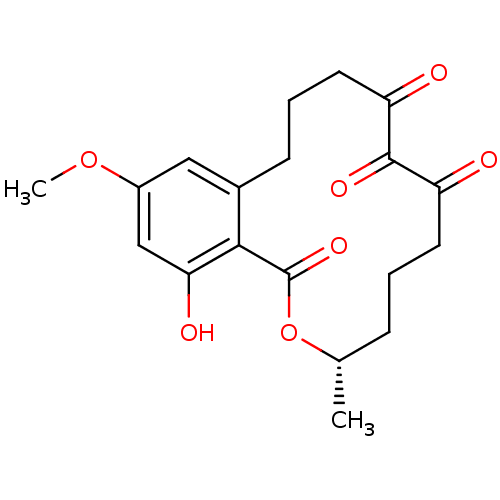 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Curated by ChEMBL | Assay Description Inhibition of human recombinant PDGFRalpha in cell free system after 60 mins | Bioorg Med Chem Lett 21: 1167-70 (2011) Article DOI: 10.1016/j.bmcl.2010.12.100 BindingDB Entry DOI: 10.7270/Q23T9HH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50336457 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR2 expressed in Sf9 cells after 60 mins | Bioorg Med Chem Lett 21: 1167-70 (2011) Article DOI: 10.1016/j.bmcl.2010.12.100 BindingDB Entry DOI: 10.7270/Q23T9HH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 3 (Homo sapiens (Human)) | BDBM50336457 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Curated by ChEMBL | Assay Description Inhibition of human recombinant VEGFR3 in cell free system after 90 mins | Bioorg Med Chem Lett 21: 1167-70 (2011) Article DOI: 10.1016/j.bmcl.2010.12.100 BindingDB Entry DOI: 10.7270/Q23T9HH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27359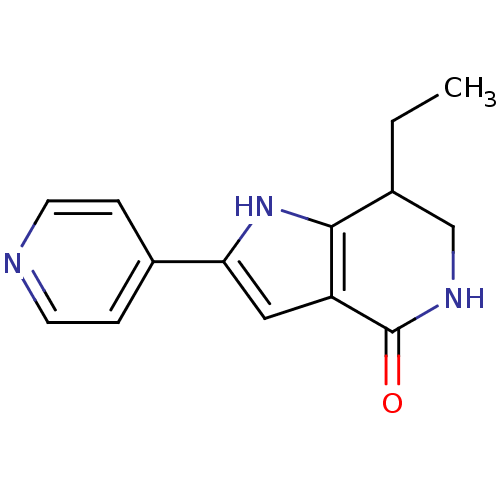 (7-ethyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27362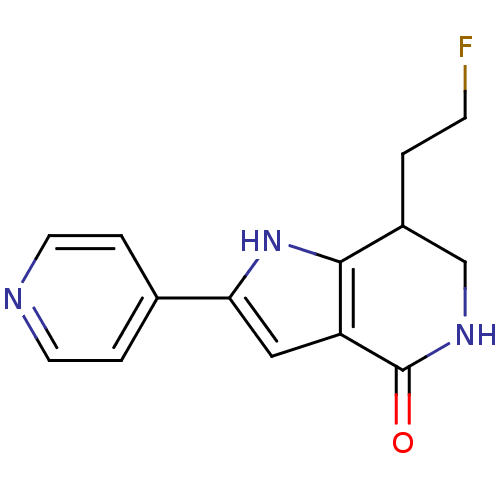 (7-(2-fluoroethyl)-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27391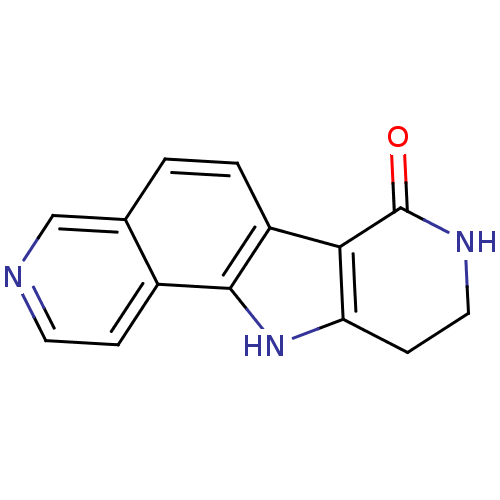 (5,13,17-triazatetracyclo[8.7.0.0^{2,7}.0^{11,16}]h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27380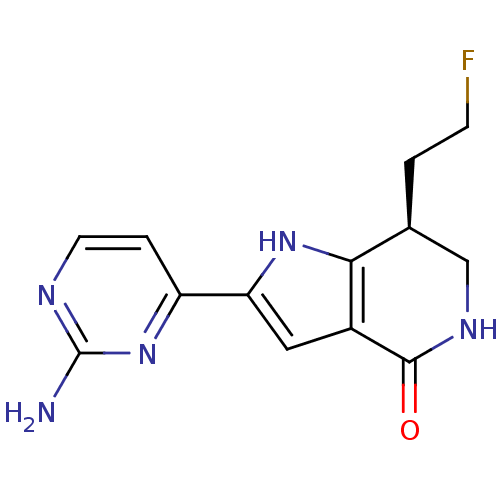 ((7S)-2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50329419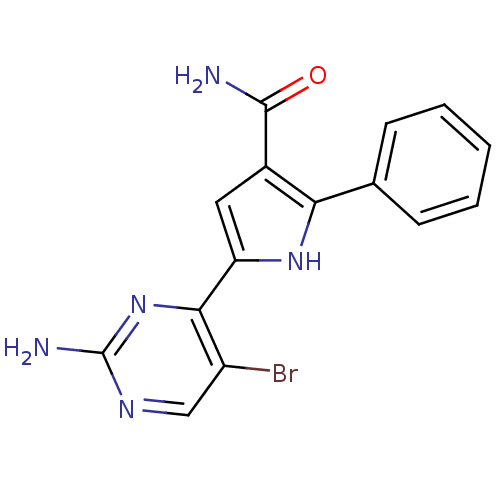 (5-(2-amino-5-bromopyrimidin-4-yl)-2-p-tolyl-1H-pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Cdc7 | J Med Chem 53: 7296-315 (2010) Article DOI: 10.1021/jm100504d BindingDB Entry DOI: 10.7270/Q24T6JM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1 (Homo sapiens (Human)) | BDBM50336457 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Curated by ChEMBL | Assay Description Inhibition of human recombinant MEK1 expressed in insect cells after 20 mins | Bioorg Med Chem Lett 21: 1167-70 (2011) Article DOI: 10.1016/j.bmcl.2010.12.100 BindingDB Entry DOI: 10.7270/Q23T9HH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27371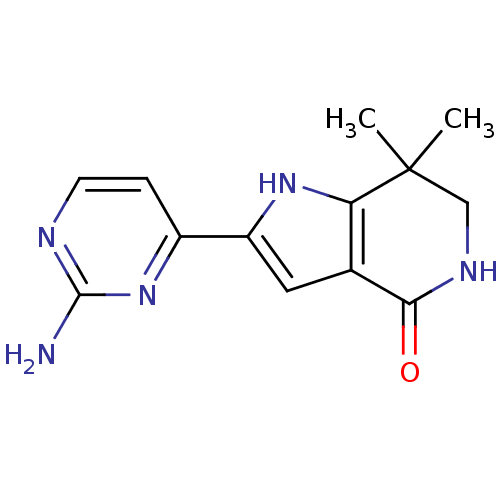 (2-(2-aminopyrimidin-4-yl)-7,7-dimethyl-1H,4H,5H,6H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27370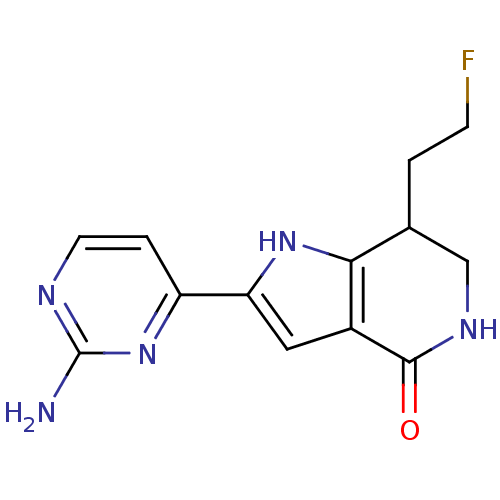 (2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1H,4H,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27413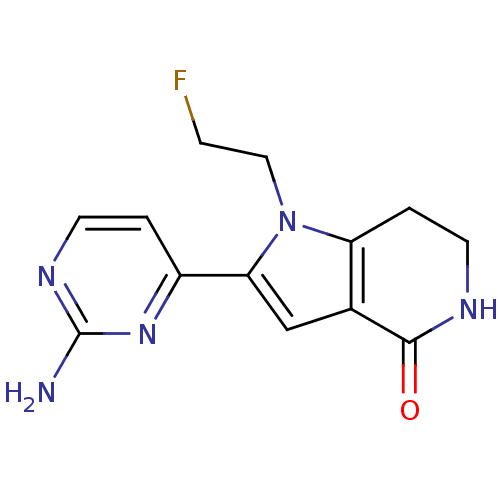 (2-(2-aminopyrimidin-4-yl)-1-(2-fluoroethyl)-1H,4H,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27361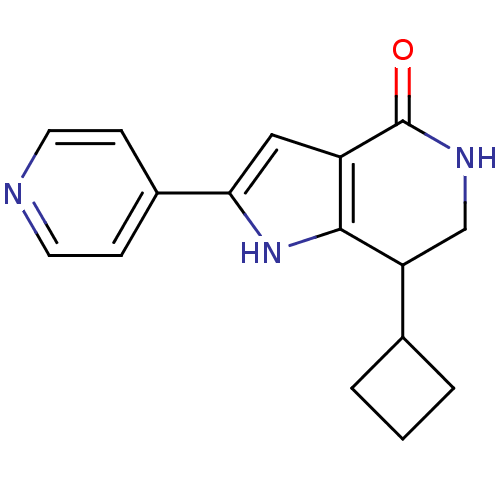 (7-cyclobutyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27348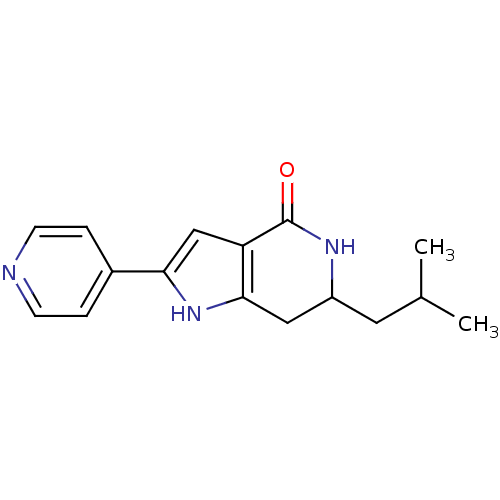 (6-(2-methylpropyl)-2-(pyridin-4-yl)-1H,4H,5H,6H,7H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27363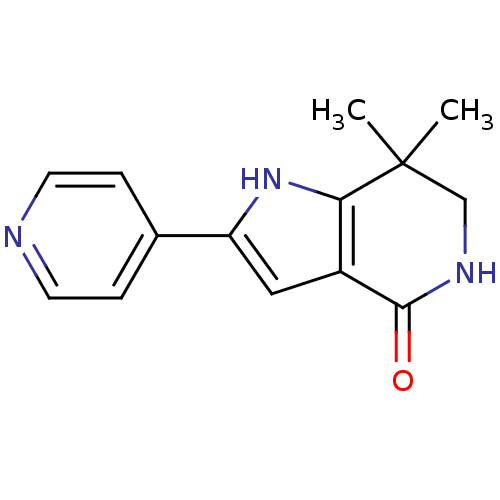 (7,7-dimethyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27390 (2-(2-amino-5-bromopyrimidin-4-yl)-1H,4H,5H,6H,7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27368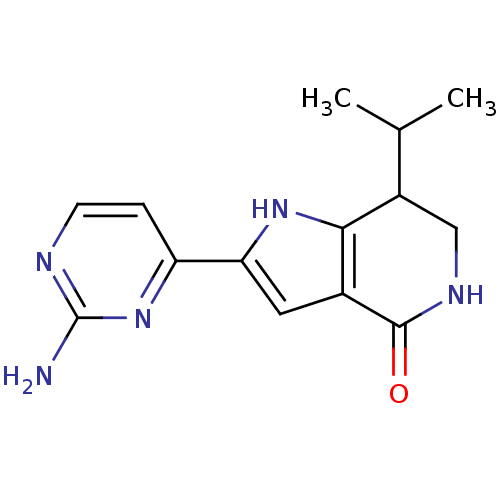 (2-(2-aminopyrimidin-4-yl)-7-(propan-2-yl)-1H,4H,5H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27412 (2-(2-aminopyrimidin-4-yl)-1-(cyclopropylmethyl)-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27377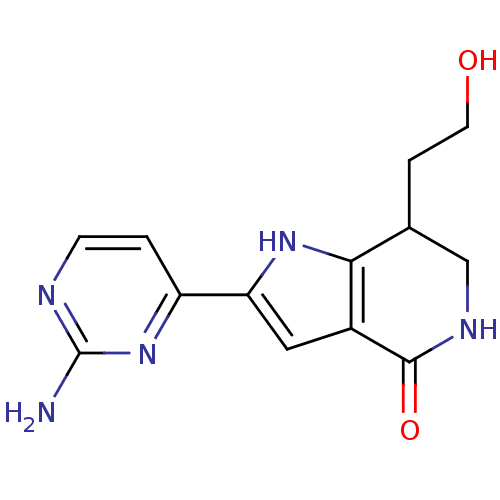 (2-(2-aminopyrimidin-4-yl)-7-(2-hydroxyethyl)-1H,4H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50279340 ((Z)-2-[(Furan-2-ylmethyl)-amino]-5-[1-(1H-pyrrolo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Cdc7 (unknown origin)-mediated phosphorylation of Mcm2 | J Med Chem 52: 4380-90 (2009) Article DOI: 10.1021/jm900248g BindingDB Entry DOI: 10.7270/Q2FF3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50336457 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Curated by ChEMBL | Assay Description Inhibition of human recombinant FLT-3 kinase expressed in insect cells after 90 mins | Bioorg Med Chem Lett 21: 1167-70 (2011) Article DOI: 10.1016/j.bmcl.2010.12.100 BindingDB Entry DOI: 10.7270/Q23T9HH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27379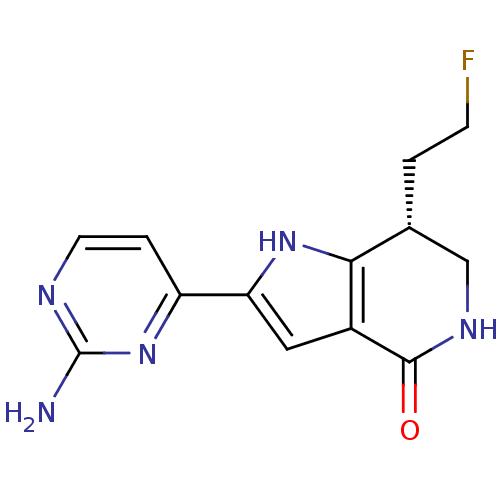 ((7R)-2-(2-aminopyrimidin-4-yl)-7-(2-fluoroethyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27347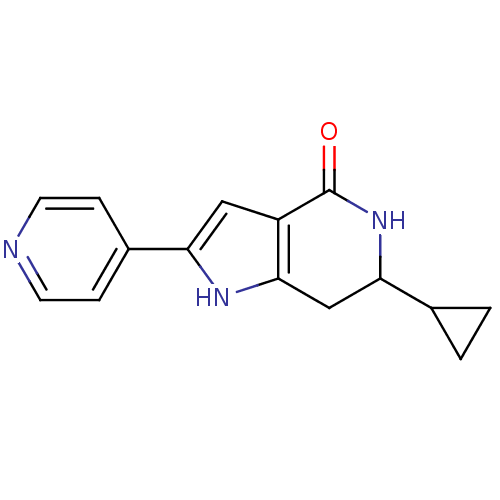 (6-cyclopropyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27406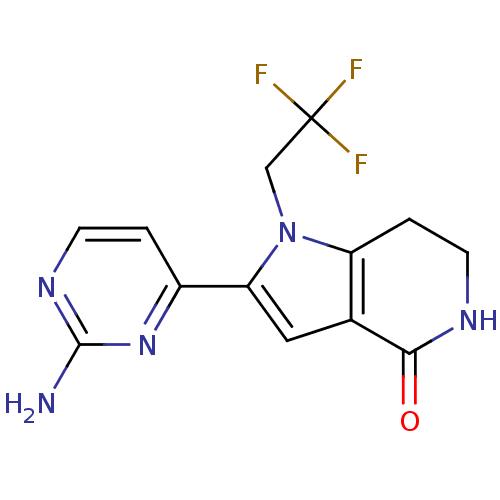 (2-(2-aminopyrimidin-4-yl)-1-(2,2,2-trifluoroethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27372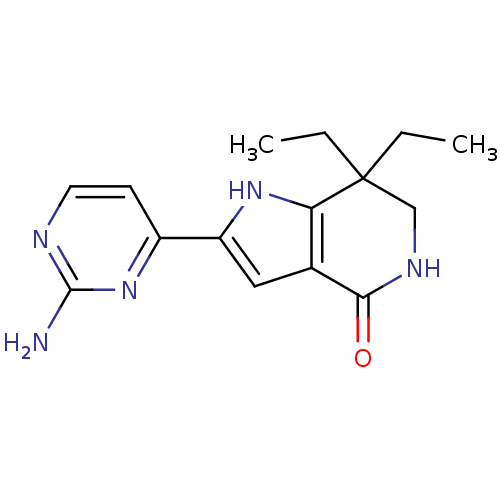 (2-(2-aminopyrimidin-4-yl)-7,7-diethyl-1H,4H,5H,6H,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27369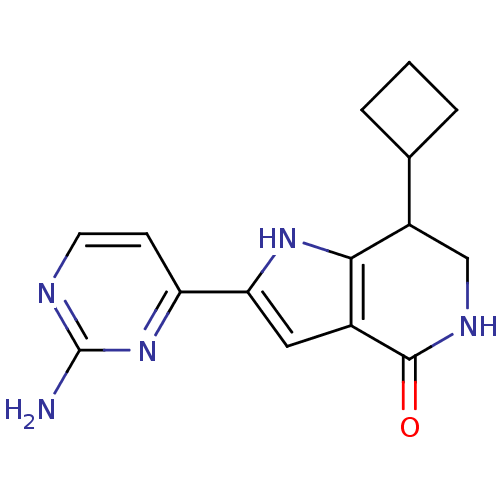 (2-(2-aminopyrimidin-4-yl)-7-cyclobutyl-1H,4H,5H,6H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27367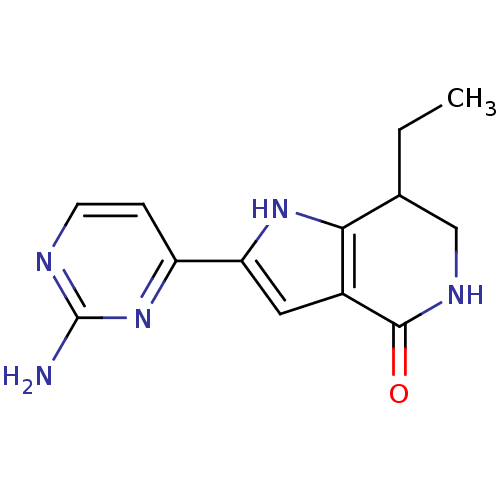 (2-(2-aminopyrimidin-4-yl)-7-ethyl-1H,4H,5H,6H,7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50329420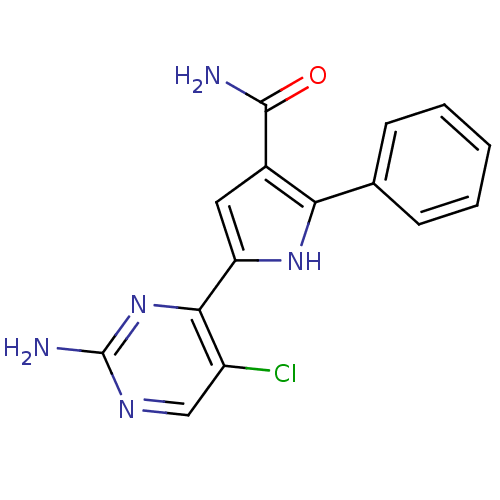 (5-(2-amino-5-chloropyrimidin-4-yl)-2-p-tolyl-1H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Cdc7 | J Med Chem 53: 7296-315 (2010) Article DOI: 10.1021/jm100504d BindingDB Entry DOI: 10.7270/Q24T6JM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27364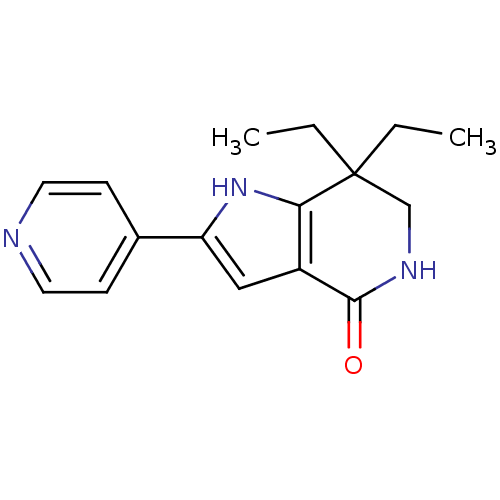 (7,7-diethyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27346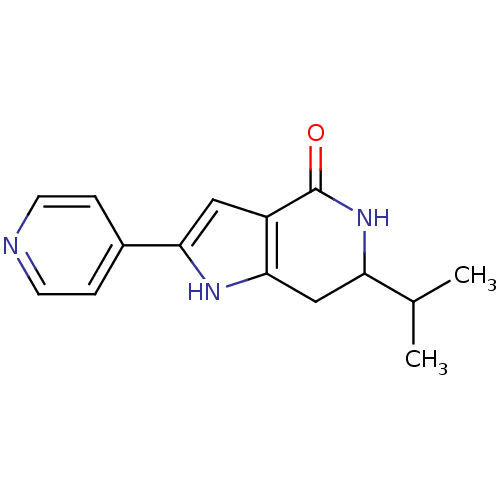 (6-(propan-2-yl)-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27366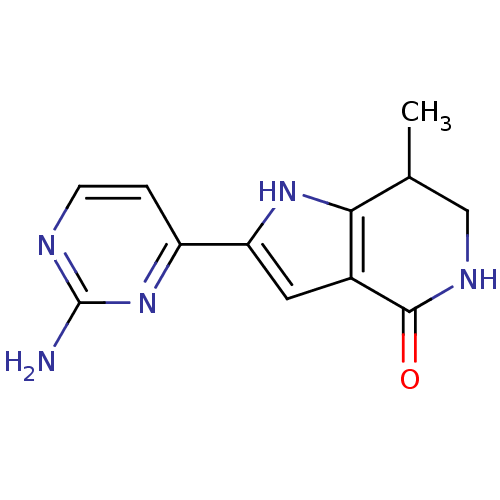 (2-(2-aminopyrimidin-4-yl)-7-methyl-1H,4H,5H,6H,7H-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27351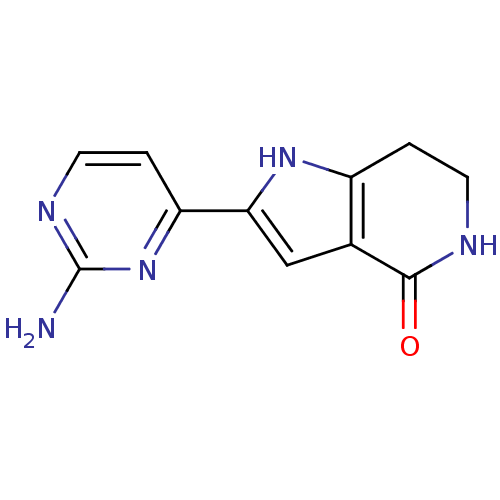 (2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27375 (2-(2-aminopyrimidin-4-yl)-7-[2-(benzyloxy)ethyl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27351 (2-(2-aminopyrimidin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27421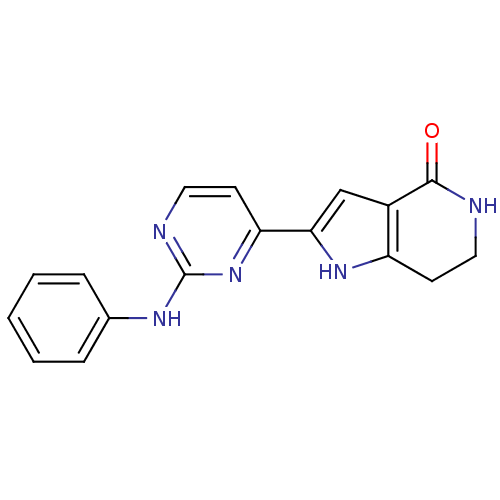 (2-[2-(phenylamino)pyrimidin-4-yl]-1H,4H,5H,6H,7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50329416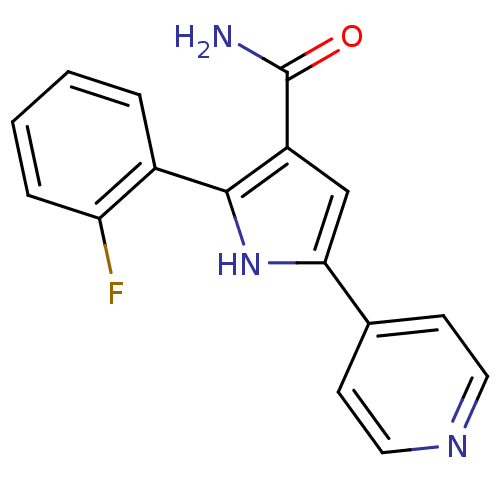 (2-(2-Fluoro-phenyl)-5-pyridin-4-yl-1H-pyrrole-3-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Cdc7 | J Med Chem 53: 7296-315 (2010) Article DOI: 10.1021/jm100504d BindingDB Entry DOI: 10.7270/Q24T6JM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27404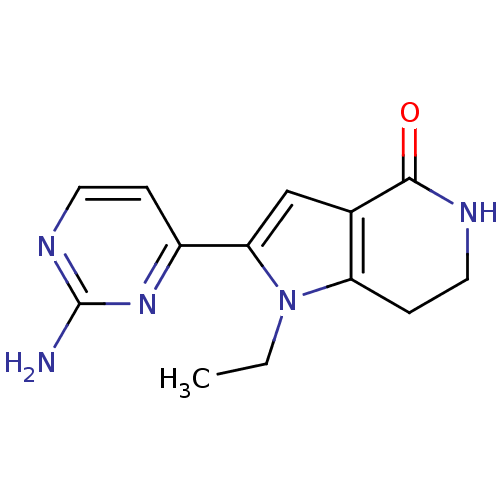 (2-(2-aminopyrimidin-4-yl)-1-ethyl-1H,4H,5H,6H,7H-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27355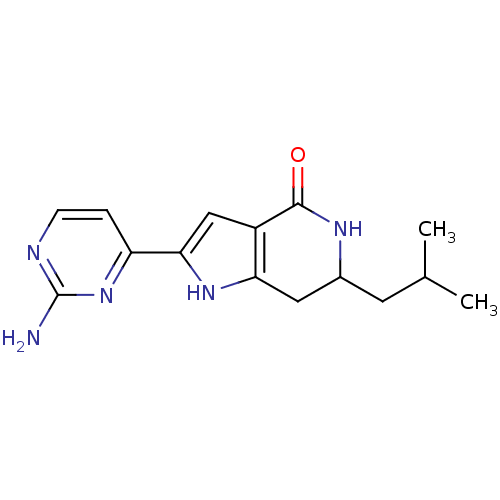 (2-(2-aminopyrimidin-4-yl)-6-(2-methylpropyl)-1H,4H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50329417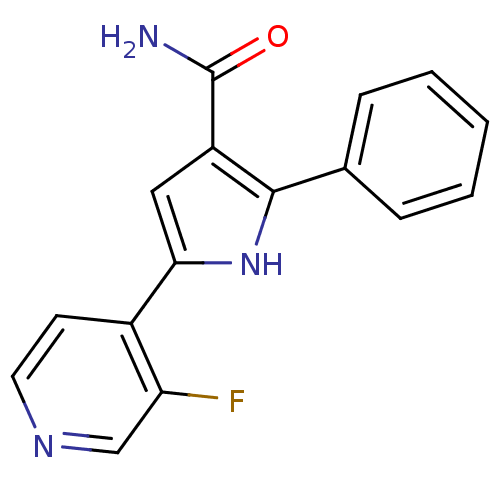 (5-(3-Fluoro-pyridin-4-yl)-2-phenyl-1H-pyrrole-3-ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Cdc7 | J Med Chem 53: 7296-315 (2010) Article DOI: 10.1021/jm100504d BindingDB Entry DOI: 10.7270/Q24T6JM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50329418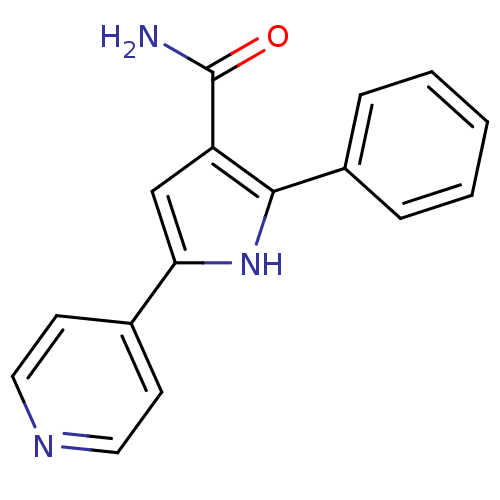 (2-Phenyl-5-pyridin-4-yl-1H-pyrrole-3-carboxamide |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Cdc7 | J Med Chem 53: 7296-315 (2010) Article DOI: 10.1021/jm100504d BindingDB Entry DOI: 10.7270/Q24T6JM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27382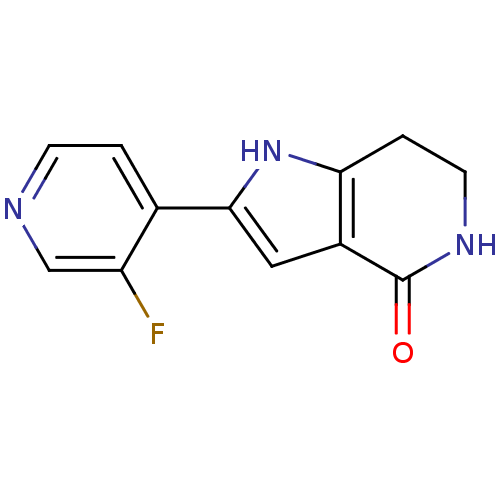 (2-(3-fluoropyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50279338 ((Z)-2-(Benzylamino)-5-(1H-pyrrolo[2,3-b]pyridin-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Cdc7 (unknown origin)-mediated phosphorylation of Mcm2 | J Med Chem 52: 4380-90 (2009) Article DOI: 10.1021/jm900248g BindingDB Entry DOI: 10.7270/Q2FF3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50336457 ((3S,5Z,8S,9R,11E)-8,9,16-trihydroxy-14-methoxy-3-m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
National University of Ireland Curated by ChEMBL | Assay Description Inhibition of human recombinant c-kit kinase in cell free system after 30 mins | Bioorg Med Chem Lett 21: 1167-70 (2011) Article DOI: 10.1016/j.bmcl.2010.12.100 BindingDB Entry DOI: 10.7270/Q23T9HH5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27344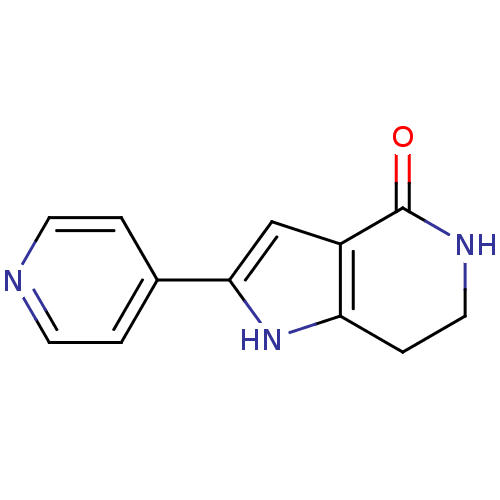 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM50279339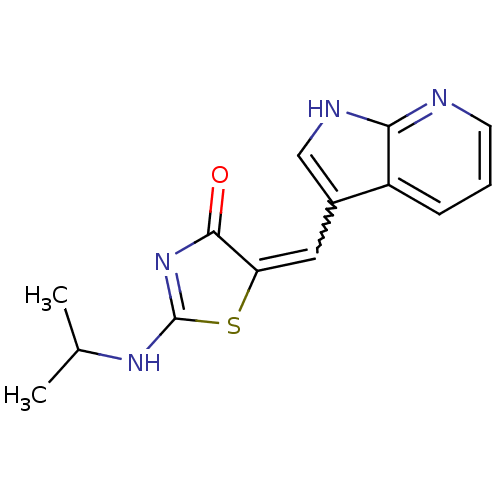 ((Z)-2-Isopropylamino-5-[1-(1H-pyrrolo[2,3-b]pyridi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Curated by ChEMBL | Assay Description Inhibition of Cdc7 (unknown origin)-mediated phosphorylation of Mcm2 | J Med Chem 52: 4380-90 (2009) Article DOI: 10.1021/jm900248g BindingDB Entry DOI: 10.7270/Q2FF3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27344 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 51: 487-501 (2008) Article DOI: 10.1021/jm700956r BindingDB Entry DOI: 10.7270/Q247485B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM27344 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl Curated by ChEMBL | Assay Description Inhibition of Cdc7 | J Med Chem 53: 7296-315 (2010) Article DOI: 10.1021/jm100504d BindingDB Entry DOI: 10.7270/Q24T6JM3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27374 (2-(2-aminopyrimidin-4-yl)-7-(3,3,3-trifluoropropyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase [58-574]/Protein DBF4 homolog A (Homo sapiens (Human)) | BDBM27358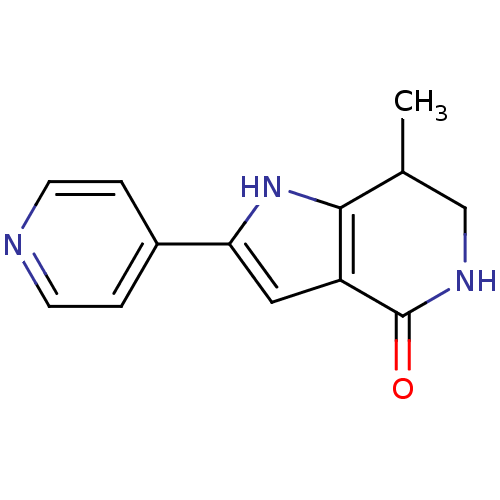 (7-methyl-2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.9 | 25 |
Nerviano Medical Sciences Srl | Assay Description The potency of the compound toward kinase activity was determined using a Dowex resin-based assay. The substrate was phosphorylated by kinase in the ... | J Med Chem 52: 293-307 (2009) Article DOI: 10.1021/jm800977q BindingDB Entry DOI: 10.7270/Q27W69JX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cell division cycle 7-related protein kinase (Homo sapiens (Human)) | BDBM27344 (2-(pyridin-4-yl)-1H,4H,5H,6H,7H-pyrrolo[3,2-c]pyri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Nerviano Medical Sciences Oncology Curated by ChEMBL | Assay Description Inhibition of cdc7 | Nat Chem Biol 4: 357-65 (2008) Article DOI: 10.1038/nchembio.90 BindingDB Entry DOI: 10.7270/Q2DV1KVK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 367 total ) | Next | Last >> |