 Found 915 hits with Last Name = 'seiler' and Initial = 'sm'
Found 915 hits with Last Name = 'seiler' and Initial = 'sm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50107460
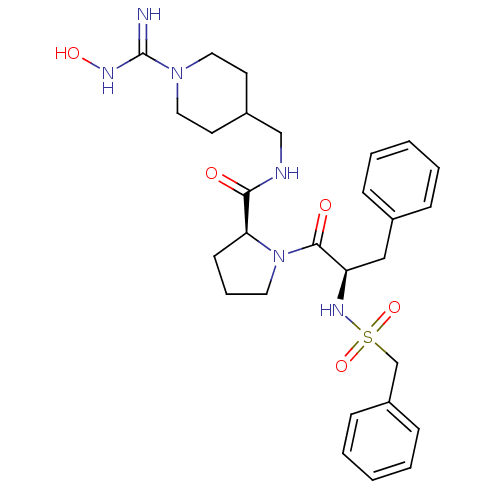
((S)-1-((R)-3-Phenyl-2-phenylmethanesulfonylamino-p...)Show SMILES ONC(=N)N1CCC(CNC(=O)[C@@H]2CCCN2C(=O)[C@@H](Cc2ccccc2)NS(=O)(=O)Cc2ccccc2)CC1 Show InChI InChI=1S/C28H38N6O5S/c29-28(31-37)33-16-13-22(14-17-33)19-30-26(35)25-12-7-15-34(25)27(36)24(18-21-8-3-1-4-9-21)32-40(38,39)20-23-10-5-2-6-11-23/h1-6,8-11,22,24-25,32,37H,7,12-20H2,(H2,29,31)(H,30,35)/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Competitive kinetic for thrombin inhibition Ki was determined |
Bioorg Med Chem Lett 12: 45-9 (2001)
BindingDB Entry DOI: 10.7270/Q2GX4C3P |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50280255

(7-Amino-3-{[(S)-1-((R)-2-amino-3-phenyl-propionyl)...)Show SMILES COC(=O)C(O)(OC)C(CCCCN)NC(=O)[C@@H]1CCCN1C(=O)[C@H](N)Cc1ccccc1 |r| Show InChI InChI=1S/C23H36N4O6/c1-32-22(30)23(31,33-2)19(12-6-7-13-24)26-20(28)18-11-8-14-27(18)21(29)17(25)15-16-9-4-3-5-10-16/h3-5,9-10,17-19,31H,6-8,11-15,24-25H2,1-2H3,(H,26,28)/t17-,18+,19?,23?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Overall Inhibitory constant of the compound against thrombin was determined |
Bioorg Med Chem Lett 2: 1607-1612 (1992)
Article DOI: 10.1016/S0960-894X(00)80440-0
BindingDB Entry DOI: 10.7270/Q2G44Q67 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002132
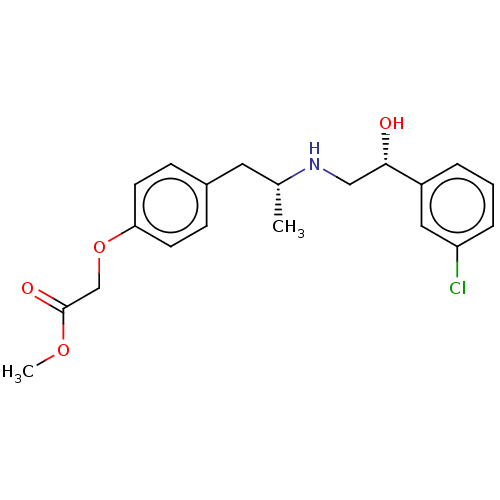
((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...)Show SMILES COC(=O)COc1ccc(C[C@@H](C)NC[C@H](O)c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C20H24ClNO4/c1-14(22-12-19(23)16-4-3-5-17(21)11-16)10-15-6-8-18(9-7-15)26-13-20(24)25-2/h3-9,11,14,19,22-23H,10,12-13H2,1-2H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-2 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50366780
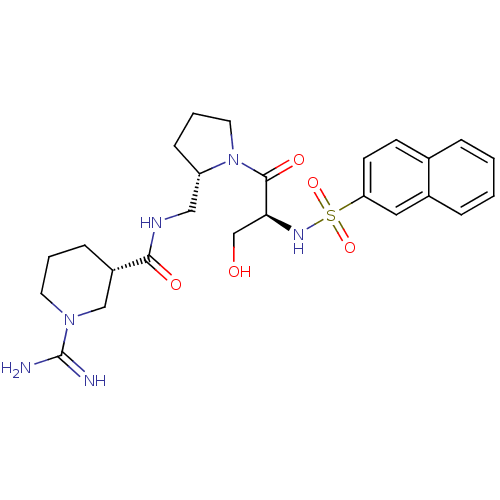
(BMS-189090 | CHEMBL138877)Show SMILES NC(=N)N1CCC[C@@H](C1)C(=O)NC[C@@H]1CCCN1C(=O)[C@H](CO)NS(=O)(=O)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C25H34N6O5S/c26-25(27)30-11-3-7-19(15-30)23(33)28-14-20-8-4-12-31(20)24(34)22(16-32)29-37(35,36)21-10-9-17-5-1-2-6-18(17)13-21/h1-2,5-6,9-10,13,19-20,22,29,32H,3-4,7-8,11-12,14-16H2,(H3,26,27)(H,28,33)/t19-,20-,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro reversible inhibition of thrombin catalytic activity |
Bioorg Med Chem Lett 12: 41-4 (2001)
BindingDB Entry DOI: 10.7270/Q2MP53T0 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.71 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50228863

((S)-1-((R)-2-Methylamino-3-phenyl-propionyl)-pyrro...)Show SMILES [#6]-[#7]-[#6@H](-[#6]-c1ccccc1)-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6]=O Show InChI InChI=1S/C21H32N6O3/c1-24-17(13-15-7-3-2-4-8-15)20(30)27-12-6-10-18(27)19(29)26-16(14-28)9-5-11-25-21(22)23/h2-4,7-8,14,16-18,24H,5-6,9-13H2,1H3,(H,26,29)(H4,22,23,25)/t16-,17+,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Concentration of the compound required to inhibit Human alpha-thrombin was determined |
Bioorg Med Chem Lett 12: 3183-6 (2002)
BindingDB Entry DOI: 10.7270/Q20P0ZBK |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50107463
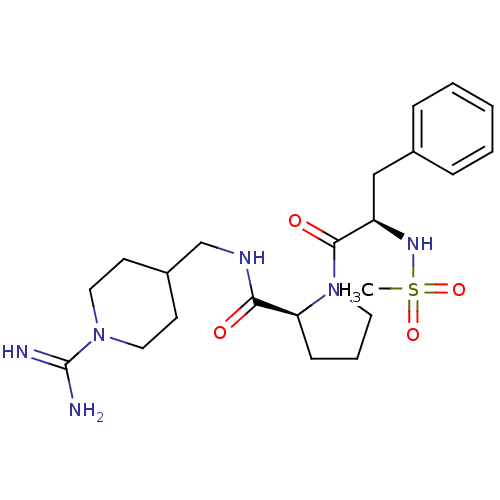
((S)-1-((R)-2-Methanesulfonylamino-3-phenyl-propion...)Show SMILES CS(=O)(=O)N[C@H](Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCC1CCN(CC1)C(N)=N Show InChI InChI=1S/C22H34N6O4S/c1-33(31,32)26-18(14-16-6-3-2-4-7-16)21(30)28-11-5-8-19(28)20(29)25-15-17-9-12-27(13-10-17)22(23)24/h2-4,6-7,17-19,26H,5,8-15H2,1H3,(H3,23,24)(H,25,29)/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Competitive kinetic for human alpha thrombin inhibition Ki was determined |
Bioorg Med Chem Lett 12: 45-9 (2001)
BindingDB Entry DOI: 10.7270/Q2GX4C3P |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50175075
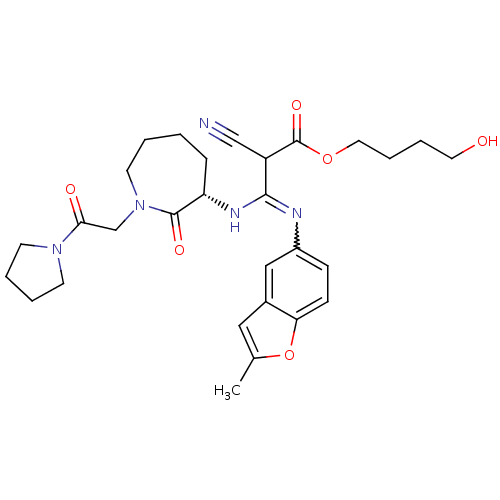
((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibitory activity against human FXa |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50039010
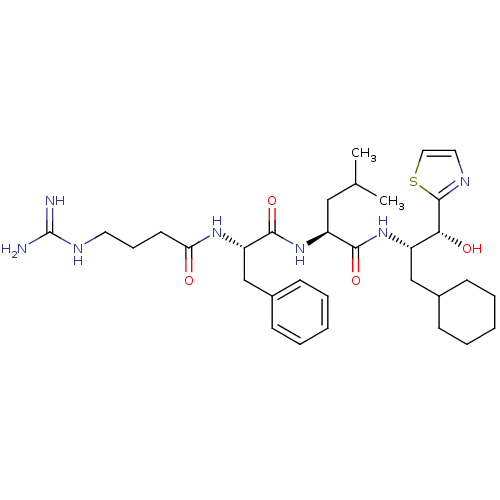
((S)-2-[(S)-2-(4-Guanidino-butyrylamino)-3-phenyl-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CCCNC(N)=N)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)c1nccs1 Show InChI InChI=1S/C32H49N7O4S/c1-21(2)18-25(29(42)38-24(19-22-10-5-3-6-11-22)28(41)31-35-16-17-44-31)39-30(43)26(20-23-12-7-4-8-13-23)37-27(40)14-9-15-36-32(33)34/h4,7-8,12-13,16-17,21-22,24-26,28,41H,3,5-6,9-11,14-15,18-20H2,1-2H3,(H,37,40)(H,38,42)(H,39,43)(H4,33,34,36)/t24-,25-,26-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity for thrombin was reported |
J Med Chem 37: 2122-4 (1994)
BindingDB Entry DOI: 10.7270/Q2FQ9VPM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289563
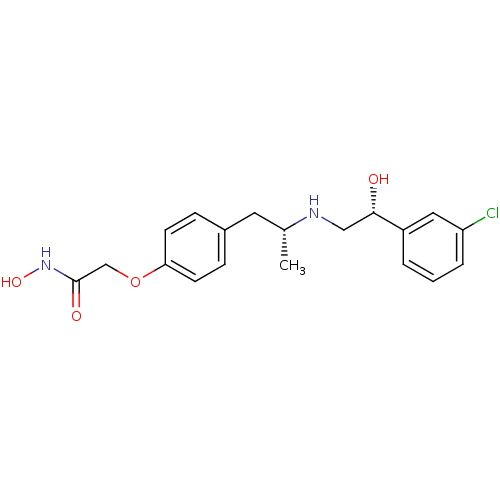
(2-(4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCC(=O)NO)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H23ClN2O4/c1-13(21-11-18(23)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)26-12-19(24)22-25/h2-8,10,13,18,21,23,25H,9,11-12H2,1H3,(H,22,24)/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-2 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287800
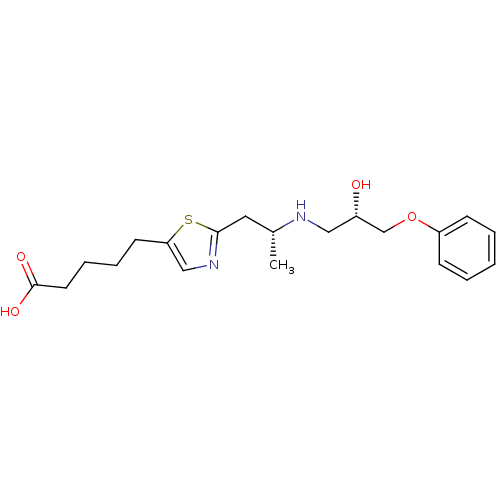
(5-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCCCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C20H28N2O4S/c1-15(21-12-16(23)14-26-17-7-3-2-4-8-17)11-19-22-13-18(27-19)9-5-6-10-20(24)25/h2-4,7-8,13,15-16,21,23H,5-6,9-12,14H2,1H3,(H,24,25)/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta2 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289567

(2-(4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCC(N)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H23ClN2O3/c1-13(22-11-18(23)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(21)24/h2-8,10,13,18,22-23H,9,11-12H2,1H3,(H2,21,24)/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-2 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002132

((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...)Show SMILES COC(=O)COc1ccc(C[C@@H](C)NC[C@H](O)c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C20H24ClNO4/c1-14(22-12-19(23)16-4-3-5-17(21)11-16)10-15-6-8-18(9-7-15)26-13-20(24)25-2/h3-9,11,14,19,22-23H,10,12-13H2,1-2H3/t14-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-1 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50287156

(2-Benzyl-1H-indole-5-carboxamidine | CHEMBL287401)Show InChI InChI=1S/C16H15N3/c17-16(18)12-6-7-15-13(9-12)10-14(19-15)8-11-4-2-1-3-5-11/h1-7,9-10,19H,8H2,(H3,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of human alpha-thrombin catalytic activity |
Bioorg Med Chem Lett 6: 1339-1344 (1996)
Article DOI: 10.1016/0960-894X(96)00229-6
BindingDB Entry DOI: 10.7270/Q2TM7B2Z |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289564
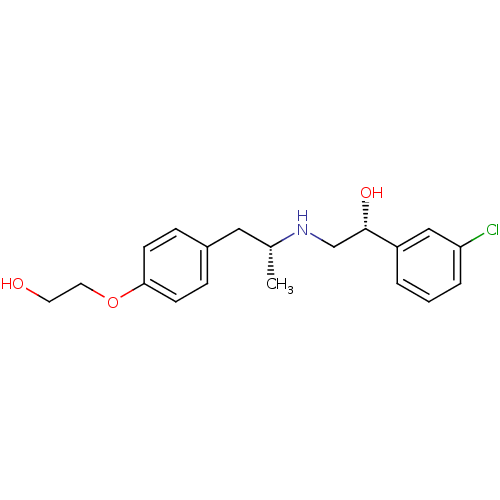
((R)-1-(3-Chloro-phenyl)-2-{(R)-2-[4-(2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCCO)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H24ClNO3/c1-14(11-15-5-7-18(8-6-15)24-10-9-22)21-13-19(23)16-3-2-4-17(20)12-16/h2-8,12,14,19,21-23H,9-11,13H2,1H3/t14-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-2 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287796

(CHEMBL308150 | ICI-215001 | {4-[2-((S)-2-Hydroxy-3...)Show InChI InChI=1S/C19H23NO6/c21-15(13-25-16-4-2-1-3-5-16)12-20-10-11-24-17-6-8-18(9-7-17)26-14-19(22)23/h1-9,15,20-21H,10-14H2,(H,22,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Beta-1 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligan... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289563

(2-(4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCC(=O)NO)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H23ClN2O4/c1-13(21-11-18(23)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)26-12-19(24)22-25/h2-8,10,13,18,21,23,25H,9,11-12H2,1H3,(H,22,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-1 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287800

(5-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCCCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C20H28N2O4S/c1-15(21-12-16(23)14-26-17-7-3-2-4-8-17)11-19-22-13-18(27-19)9-5-6-10-20(24)25/h2-4,7-8,13,15-16,21,23H,5-6,9-12,14H2,1H3,(H,24,25)/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta3 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287800

(5-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCCCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C20H28N2O4S/c1-15(21-12-16(23)14-26-17-7-3-2-4-8-17)11-19-22-13-18(27-19)9-5-6-10-20(24)25/h2-4,7-8,13,15-16,21,23H,5-6,9-12,14H2,1H3,(H,24,25)/t15-,16+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Beta-1 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligan... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287796

(CHEMBL308150 | ICI-215001 | {4-[2-((S)-2-Hydroxy-3...)Show InChI InChI=1S/C19H23NO6/c21-15(13-25-16-4-2-1-3-5-16)12-20-10-11-24-17-6-8-18(9-7-17)26-14-19(22)23/h1-9,15,20-21H,10-14H2,(H,22,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its ability to stimulate adenylyl cyclase activity in CHO Beta-3 adrenergic receptor cell membrane. |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289567

(2-(4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCC(N)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H23ClN2O3/c1-13(22-11-18(23)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(21)24/h2-8,10,13,18,22-23H,9,11-12H2,1H3,(H2,21,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-1 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002133
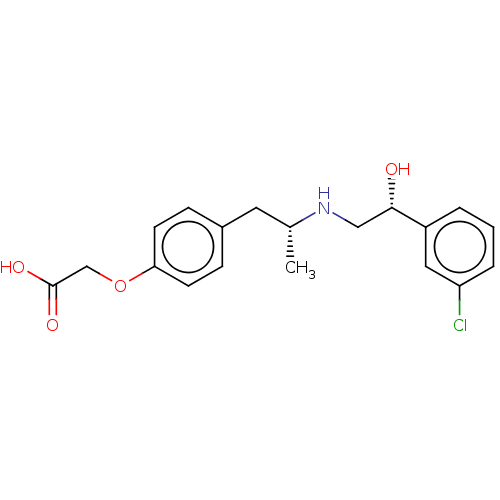
((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-2 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002133

((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta2 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287799
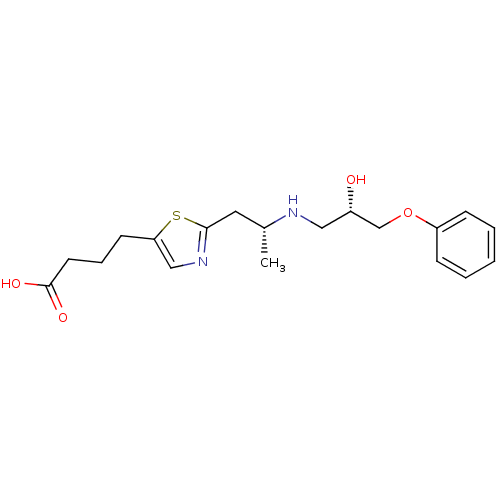
(4-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C19H26N2O4S/c1-14(10-18-21-12-17(26-18)8-5-9-19(23)24)20-11-15(22)13-25-16-6-3-2-4-7-16/h2-4,6-7,12,14-15,20,22H,5,8-11,13H2,1H3,(H,23,24)/t14-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta3 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50073045
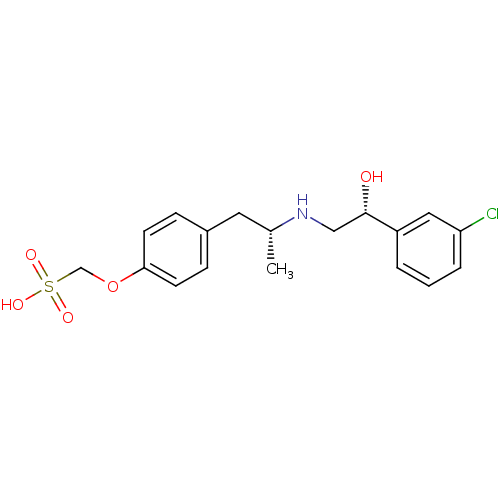
((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...)Show SMILES C[C@H](Cc1ccc(OCS(O)(=O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H22ClNO5S/c1-13(20-11-18(21)15-3-2-4-16(19)10-15)9-14-5-7-17(8-6-14)25-12-26(22,23)24/h2-8,10,13,18,20-21H,9,11-12H2,1H3,(H,22,23,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| 806 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Effective concentration for stimulation of adenylyl cyclase activity in CHO-beta3 AR expressing cell membranes |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002133

((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-3 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002133

((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta3 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002133

((+/-)-2-(4-((R)-2-((R)-2-(3-chlorophenyl)-2-hydrox...)Show SMILES C[C@H](Cc1ccc(OCC(O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H22ClNO4/c1-13(21-11-18(22)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(23)24/h2-8,10,13,18,21-22H,9,11-12H2,1H3,(H,23,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-1 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50002132

((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...)Show SMILES COC(=O)COc1ccc(C[C@@H](C)NC[C@H](O)c2cccc(Cl)c2)cc1 Show InChI InChI=1S/C20H24ClNO4/c1-14(22-12-19(23)16-4-3-5-17(21)11-16)10-15-6-8-18(9-7-15)26-13-20(24)25-2/h3-9,11,14,19,22-23H,10,12-13H2,1-2H3/t14-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-3 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287795
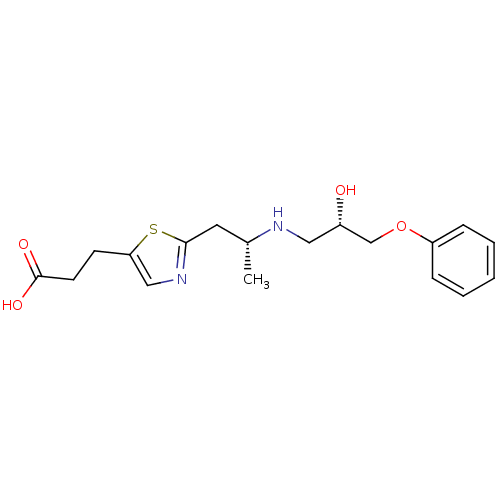
(3-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C18H24N2O4S/c1-13(9-17-20-11-16(25-17)7-8-18(22)23)19-10-14(21)12-24-15-5-3-2-4-6-15/h2-6,11,13-14,19,21H,7-10,12H2,1H3,(H,22,23)/t13-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta2 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289564

((R)-1-(3-Chloro-phenyl)-2-{(R)-2-[4-(2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCCO)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H24ClNO3/c1-14(11-15-5-7-18(8-6-15)24-10-9-22)21-13-19(23)16-3-2-4-17(20)12-16/h2-8,12,14,19,21-23H,9-11,13H2,1H3/t14-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-1 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human TPA by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287799

(4-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C19H26N2O4S/c1-14(10-18-21-12-17(26-18)8-5-9-19(23)24)20-11-15(22)13-25-16-6-3-2-4-7-16/h2-4,6-7,12,14-15,20,22H,5,8-11,13H2,1H3,(H,23,24)/t14-,15+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Beta-1 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligan... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287799

(4-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C19H26N2O4S/c1-14(10-18-21-12-17(26-18)8-5-9-19(23)24)20-11-15(22)13-25-16-6-3-2-4-7-16/h2-4,6-7,12,14-15,20,22H,5,8-11,13H2,1H3,(H,23,24)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta2 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289563

(2-(4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCC(=O)NO)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H23ClN2O4/c1-13(21-11-18(23)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)26-12-19(24)22-25/h2-8,10,13,18,21,23,25H,9,11-12H2,1H3,(H,22,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-3 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289564

((R)-1-(3-Chloro-phenyl)-2-{(R)-2-[4-(2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCCO)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H24ClNO3/c1-14(11-15-5-7-18(8-6-15)24-10-9-22)21-13-19(23)16-3-2-4-17(20)12-16/h2-8,12,14,19,21-23H,9-11,13H2,1H3/t14-,19+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-3 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50073045

((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...)Show SMILES C[C@H](Cc1ccc(OCS(O)(=O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H22ClNO5S/c1-13(20-11-18(21)15-3-2-4-16(19)10-15)9-14-5-7-17(8-6-14)25-12-26(22,23)24/h2-8,10,13,18,20-21H,9,11-12H2,1H3,(H,22,23,24)/t13-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-2 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287796

(CHEMBL308150 | ICI-215001 | {4-[2-((S)-2-Hydroxy-3...)Show InChI InChI=1S/C19H23NO6/c21-15(13-25-16-4-2-1-3-5-16)12-20-10-11-24-17-6-8-18(9-7-17)26-14-19(22)23/h1-9,15,20-21H,10-14H2,(H,22,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta2 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human alpha-thrombin by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287795

(3-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C18H24N2O4S/c1-13(9-17-20-11-16(25-17)7-8-18(22)23)19-10-14(21)12-24-15-5-3-2-4-6-15/h2-6,11,13-14,19,21H,7-10,12H2,1H3,(H,22,23)/t13-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Beta-1 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligan... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287795

(3-{2-[(R)-2-((S)-2-Hydroxy-3-phenoxy-propylamino)-...)Show SMILES C[C@H](Cc1ncc(CCC(O)=O)s1)NC[C@H](O)COc1ccccc1 Show InChI InChI=1S/C18H24N2O4S/c1-13(9-17-20-11-16(25-17)7-8-18(22)23)19-10-14(21)12-24-15-5-3-2-4-6-15/h2-6,11,13-14,19,21H,7-10,12H2,1H3,(H,22,23)/t13-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against beta3 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligand... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50358883

(CHEMBL1923468)Show SMILES Clc1ccc2cc(ccc2c1)S(=O)(=O)N[C@H]1CCCN(CC(=O)N2C[C@@H]3C[C@H](C2)c2cccc(=O)n2C3)C1=O |r| Show InChI InChI=1S/C28H29ClN4O5S/c29-22-8-6-20-13-23(9-7-19(20)12-22)39(37,38)30-24-3-2-10-31(28(24)36)17-27(35)32-14-18-11-21(16-32)25-4-1-5-26(34)33(25)15-18/h1,4-9,12-13,18,21,24,30H,2-3,10-11,14-17H2/t18-,21+,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 7a by Michaelis Menten equation analysis |
Bioorg Med Chem Lett 21: 7516-21 (2011)
Article DOI: 10.1016/j.bmcl.2011.06.098
BindingDB Entry DOI: 10.7270/Q2MK6DBS |
More data for this
Ligand-Target Pair | |
Beta-3 adrenergic receptor
(Homo sapiens (Human)) | BDBM50289567

(2-(4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-eth...)Show SMILES C[C@H](Cc1ccc(OCC(N)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C19H23ClN2O3/c1-13(22-11-18(23)15-3-2-4-16(20)10-15)9-14-5-7-17(8-6-14)25-12-19(21)24/h2-8,10,13,18,22-23H,9,11-12H2,1H3,(H2,21,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-3 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of t-PA |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50287798

(4-(2-{(R)-2-[2-(3-Chloro-phenyl)-2-hydroxy-ethylam...)Show SMILES C[C@H](Cc1ncc(CCCC(O)=O)s1)NCC(O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H23ClN2O3S/c1-12(20-11-16(22)13-4-2-5-14(19)9-13)8-17-21-10-15(25-17)6-3-7-18(23)24/h2,4-5,9-10,12,16,20,22H,3,6-8,11H2,1H3,(H,23,24)/t12-,16?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for its binding affinity against Beta-1 adrenergic receptor in CHO cell membrane using [125I]-iodocyanopindolol as the radioligan... |
Bioorg Med Chem Lett 6: 2253-2258 (1996)
Article DOI: 10.1016/0960-894X(96)00417-9
BindingDB Entry DOI: 10.7270/Q2RJ4JGC |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50073045

((4-{(R)-2-[(R)-2-(3-Chloro-phenyl)-2-hydroxy-ethyl...)Show SMILES C[C@H](Cc1ccc(OCS(O)(=O)=O)cc1)NC[C@H](O)c1cccc(Cl)c1 Show InChI InChI=1S/C18H22ClNO5S/c1-13(20-11-18(21)15-3-2-4-16(19)10-15)9-14-5-7-17(8-6-14)25-12-26(22,23)24/h2-8,10,13,18,20-21H,9,11-12H2,1H3,(H,22,23,24)/t13-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
| Article
| 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to bind to human Beta-1 adrenergic receptor using membranes of stably transfected CHO cells |
Bioorg Med Chem Lett 7: 1583-1588 (1997)
Article DOI: 10.1016/S0960-894X(97)00266-7
BindingDB Entry DOI: 10.7270/Q24749VQ |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM35283

(CHEMBL551991 | cyanoguanidine, 3)Show SMILES Cc1cc2cc(ccc2o1)N=C(NC#N)N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O |r,w:10.11| Show InChI InChI=1S/C23H28N6O3/c1-16-12-17-13-18(7-8-20(17)32-16)26-23(25-15-24)27-19-6-2-3-11-29(22(19)31)14-21(30)28-9-4-5-10-28/h7-8,12-13,19H,2-6,9-11,14H2,1H3,(H2,25,26,27)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of u-PA |
Bioorg Med Chem Lett 19: 4034-41 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.014
BindingDB Entry DOI: 10.7270/Q2TQ61KM |
More data for this
Ligand-Target Pair | |
Chymotrypsinogen B
(Homo sapiens (Human)) | BDBM50175075

((S,Z)-4-hydroxybutyl 2-cyano-3-(2-methylbenzofuran...)Show SMILES Cc1cc2cc(ccc2o1)N=C(N[C@H]1CCCCN(CC(=O)N2CCCC2)C1=O)C(C#N)C(=O)OCCCCO |w:10.11| Show InChI InChI=1S/C29H37N5O6/c1-20-16-21-17-22(9-10-25(21)40-20)31-27(23(18-30)29(38)39-15-7-6-14-35)32-24-8-2-3-13-34(28(24)37)19-26(36)33-11-4-5-12-33/h9-10,16-17,23-24,35H,2-8,11-15,19H2,1H3,(H,31,32)/t23?,24-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to chymotrypsin |
Bioorg Med Chem Lett 15: 5453-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.107
BindingDB Entry DOI: 10.7270/Q2KH0MW5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data